-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Fine, Ken Eames, David L. Heymann, “Herd Immunity”: A Rough Guide, Clinical Infectious Diseases, Volume 52, Issue 7, 1 April 2011, Pages 911–916, https://doi.org/10.1093/cid/cir007
Close - Share Icon Share
Abstract
The term “herd immunity” is widely used but carries a variety of meanings [1–7]. Some authors use it to describe the proportion immune among individuals in a population. Others use it with reference to a particular threshold proportion of immune individuals that should lead to a decline in incidence of infection. Still others use it to refer to a pattern of immunity that should protect a population from invasion of a new infection. A common implication of the term is that the risk of infection among susceptible individuals in a population is reduced by the presence and proximity of immune individuals (this is sometimes referred to as “indirect protection” or a “herd effect”). We provide brief historical, epidemiologic, theoretical, and pragmatic public health perspectives on this concept.
HISTORY
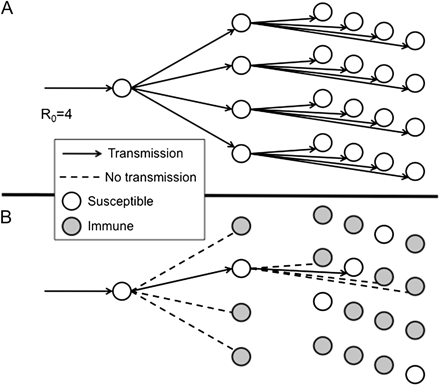
Though coined almost a century ago [8], the term “herd immunity” was not widely used until recent decades, its use stimulated by the increasing use of vaccines, discussions of disease eradication, and analyses of the costs and benefits of vaccination programs. An important milestone was the recognition by Smith in 1970 [9] and Dietz in 1975 [10] of a simple threshold theorem—that if immunity (ie, successful vaccination) were delivered at random and if members of a population mixed at random, such that on average each individual contacted R0 individuals in a manner sufficient to transmit the infection [11, 12], then incidence of the infection would decline if the proportion immune exceeded (R0 − 1)/R0, or 1 –1/R0. This is illustrated in Figures 1 and 2.
Diagram illustrating transmission of an infection with a basic reproduction number R0 = 4 (see Table 1). A, Transmission over 3 generations after introduction into a totally susceptible population (1 case would lead to 4 cases and then to 16 cases). B, Expected transmissions if (R0 − 1)/R0 = 1 − 1/R0 = ¾ of the population is immune. Under this circumstance, all but 1 of the contacts for each case s immune, and so each case leads to only 1 successful transmission of the infection. This implies constant incidence over time. If a greater proportion are immune, then incidence will decline. On this basis, (R0 − 1)/R0 is known as the “herd immunity threshold.”
Definitions of Terms
| Term | Symbolic Expression | Definition |
| Basic reproduction number | R0 | Number of secondary cases generated by a typical infectious individual when the rest of the population is susceptible (ie, at the start of a novel outbreak) |
| Critical vaccination level | Vc | Proportion of the population that must be vaccinated to achieve herd immunity threshold, assuming that vaccination takes place at random |
| Vaccine effectiveness against transmission | E | Reduction in transmission of infection to and from vaccinated compared with control individuals in the same population (analogous to conventional vaccine efficacy but measuring protection against transmission rather than protection against disease). |
| Term | Symbolic Expression | Definition |
| Basic reproduction number | R0 | Number of secondary cases generated by a typical infectious individual when the rest of the population is susceptible (ie, at the start of a novel outbreak) |
| Critical vaccination level | Vc | Proportion of the population that must be vaccinated to achieve herd immunity threshold, assuming that vaccination takes place at random |
| Vaccine effectiveness against transmission | E | Reduction in transmission of infection to and from vaccinated compared with control individuals in the same population (analogous to conventional vaccine efficacy but measuring protection against transmission rather than protection against disease). |
Definitions of Terms
| Term | Symbolic Expression | Definition |
| Basic reproduction number | R0 | Number of secondary cases generated by a typical infectious individual when the rest of the population is susceptible (ie, at the start of a novel outbreak) |
| Critical vaccination level | Vc | Proportion of the population that must be vaccinated to achieve herd immunity threshold, assuming that vaccination takes place at random |
| Vaccine effectiveness against transmission | E | Reduction in transmission of infection to and from vaccinated compared with control individuals in the same population (analogous to conventional vaccine efficacy but measuring protection against transmission rather than protection against disease). |
| Term | Symbolic Expression | Definition |
| Basic reproduction number | R0 | Number of secondary cases generated by a typical infectious individual when the rest of the population is susceptible (ie, at the start of a novel outbreak) |
| Critical vaccination level | Vc | Proportion of the population that must be vaccinated to achieve herd immunity threshold, assuming that vaccination takes place at random |
| Vaccine effectiveness against transmission | E | Reduction in transmission of infection to and from vaccinated compared with control individuals in the same population (analogous to conventional vaccine efficacy but measuring protection against transmission rather than protection against disease). |
Simple threshold concept of herd immunity. A, Relationship between the herd immunity threshold, (R0 – 1)/R0 = 1 − 1/R0,and basic reproduction number, R0, in a randomly mixing homogeneous population. Note the implications of ranges of R0, which can vary considerably between populations [12], for ranges of immunity coverage required to exceed the threshold. B, Cumulative lifetime incidence of infection in unvaccinated individuals as a function of the level of random vaccine coverage of an entire population, as predicted by a simple susceptible-infected-recovered model for a ubiquitous infection with R0 = 3 [13]. This assumes a 100% effective vaccine (E = 1). Note that the expected cumulative incidence is 0 if coverage is maintained above VC = 1− 1/R0 = 67%.
Though an important paper by Fox et al in 1971 [1] argued that emphasis on simple thresholds was not appropriate for public health, because of the importance of population heterogeneity, assumptions of homogeneous mixing and simple thresholds have persisted. A large theoretical literature shows how to derive R0 for different infections, often implying that the 1 − 1/R0 threshold be used as a target for immunization coverage and that its achievement can lead to eradication of target infections [3, 12, 14].
EPIDEMIOLOGIC PERSPECTIVE
Many examples of herd immunity have been described, illustrating the importance of indirect protection for predicting the short- and long-term impact of vaccination programs, for justifying them economically, and for understanding the nature of the immunity induced by various vaccines.
Among the classic examples was the recognition that periodic epidemics of ubiquitous childhood infections such as measles, mumps, rubella, pertussis, chickenpox, and polio, arose because of the accrual of a critical number of susceptible individuals in populations and that epidemics could be delayed or averted by maintaining numbers of susceptible individuals below this critical density (ie, by maintaining the proportion immune above some threshold) [15, 16].
Impressive examples of indirect protection have been observed after the introduction of conjugate vaccines against pneumococcal and Haemophilus infections. Reductions in disease incidence among cohorts too old to have been vaccinated have been responsible for one- to two-thirds of the total disease reduction attributable to these vaccines in some populations. These are due to the ability of conjugate vaccines to protect vaccinees not only against disease but also against nasal carriage, and hence infectiousness [7].
Selective vaccination of groups that are important in transmission can slow transmission in general populations or reduce incidence among population segments that may be at risk of severe consequences of infection. Schools play an important role in community transmission of influenza viruses, and thus there has been discussion of slowing transmission either by closing schools or by vaccinating schoolchildren. Selective vaccination of schoolchildren against influenza was policy in Japan during the 1990s and was shown to have reduced morbidity and mortality among the elderly [17]. Analogous issues relate to vaccination against rubella and human papillomavirus (HPV) in males; for each of these examples the consequences of infection (with rubella or HPV) in males are relatively minor, so the policy issue becomes whether vaccination of males is warranted to protect females, and many societies have decided in favor for rubella but not for HPV [18].
A particularly interesting example of using vaccines to reduce transmission is the potential for “transmission blocking vaccines” for malaria. These vaccines would not protect the individual recipient against infection or disease, but would produce antibodies that block life cycle stages of the malaria parasite in the mosquito [19]. Recent work has shown the biologic feasibility of such vaccines, and models have shown their potential contribution to reducing overall transmission in malaria-endemic communities. They would thus provide the first example of a vaccine that in theory would provide no direct benefit to the recipient.
Finally we may refer to eradication programs based on vaccines—globally successful in the case of smallpox and rinderpest, and at least regionally successful to date in the case of wild polio virus. The Americas have been free of wild polio virus circulation for almost 20 years, though the thresholds for herd immunity have proved more elusive in parts of Asia and Africa. Each of these programs has used a combination of routine vaccination, itself successful in some populations, supplemented by campaigns in high-risk regions and populations in order to stop the final chains of transmission.
Such examples illustrate how the direct effect of immunity (ie, successful vaccination) in reducing infection or infectiousness in certain individuals can decrease the risk of infection among those who remain susceptible in the population. Importantly, it is a vaccine's effect on transmission that is responsible for the indirect effect. If the only effect of a vaccine were to prevent disease but not to alter either the risk of infection or infectiousness, then there would be no indirect effect, and no herd immunity. It was once wrongly argued, for example, that inactivated polio vaccines protected only against paralysis and not against infection. We now know that this is wrong, and that inactivated polio vaccines can decrease both infection risk and infectiousness, as demonstrated in several countries that interrupted wild poliovirus transmission using only these vaccines [20].
The magnitude of the indirect effect of vaccine-derived immunity is a function of the transmissibility of the infectious agent, the nature of the immunity induced by the vaccine, the pattern of mixing and infection transmission in populations, and the distribution of the vaccine—and, more importantly, of immunity—in the population. The nuances of immunity and the complexity of population heterogeneity make prediction difficult, but our understanding of these effects has grown in recent years, associated with 3 particular developments: (1) the accumulation of experience with a variety of vaccines in different populations, (2) the development of ever more sophisticated models capable of exploring heterogeneous mixing within populations, and (3) the development of analytic methods to measure indirect protection in the context of vaccine trials and observational studies, by comparing the risks of infection among individuals as a function of the vaccination status of their household or village contacts [21].
THEORETICAL DEVELOPMENTS
Much of the early theoretical work on herd immunity assumed that vaccines induce solid immunity against infection and that populations mix at random, consistent with the simple herd immunity threshold for random vaccination of Vc = (1−1/R0), using the symbol Vc for the critical minimum proportion to be vaccinated (assuming 100% vaccine effectiveness). More recent research has addressed the complexities of imperfect immunity, heterogeneous populations, nonrandom vaccination, and “freeloaders” [13, 22]
Imperfect Immunity
If vaccination does not confer solid immunity against infection to all recipients, the threshold level of vaccination required to protect a population increases. If vaccination protects only a proportion E among those vaccinated (E standing for effectiveness against infection transmission, in the field), then the critical vaccination coverage level should be Vc=(1− 1/R0)/E. We can see from this that if E is <(1− 1/R0) it would be impossible to eliminate an infection even by vaccinating the whole population. Similarly, waning vaccine-induced immunity demands higher levels of coverage or regular booster vaccination. Important among illustrations of this principle are the shifts to multiple doses (up to 20) and to monovalent vaccines in the effort to eliminate polio in India, where the standard trivalent oral polio vaccines and regimens produce low levels of protection [23].
Heterogeneous Populations—Nonrandom Mixing
Modeling heterogeneous populations requires knowledge—or assumptions—about how different groups interact. The dynamics of infection within each group depend on the rate of acquisition of infection from all other groups. In simple random models, all mixing behavior is captured by a single parameter, but in heterogeneous populations this must be replaced by an array of parameters that describe how each group interacts with each other group. Evaluating this contact matrix may be impracticable, or impossible, and so approximations are often used. Recent questionnaire studies have collected detailed data about levels of interactions between different age groups, allowing evidence-based parameterization of age-structured models with complex mixing [24]. Similarly, spatially explicit models can be parameterized using transport data [25].
Although the mathematics to describe heterogeneous mixing are complex, the critical threshold remains: Vc = (1− 1/R0)/E, except that R0 is no longer a simple function of the average number of contacts of individuals. Instead, R0 is a measure of the average number of secondary cases generated by a “typical” infectious person [14]. This average depends on how the various groups interact and can be calculated from a matrix describing how infection spreads within and between groups. Interactions are often observed to be more frequent within than between groups [24], in which case the most highly connected groups will dominate transmission, resulting in a higher value of R0, and a larger vaccination threshold than would be obtained by assuming that all individuals display average behavior.
Nonrandom Vaccination
If vaccination coverage differs between groups in a population, and these groups differ in their risk behavior, the simple results no longer follow. To illustrate this, consider a population consisting of 2 groups, high and low risk, and suppose that each high-risk case infects 5 high-risk individuals and each low-risk case infects 1 low-risk individual. Here, R0 = 5, so Vc = 80%. Because the high-risk group is responsible for any increase in incidence, outbreaks could in theory be prevented by vaccinating 80% of the high-risk group alone, thus <80% of the entire population. In general, if highly transmitting groups can be preferentially vaccinated, lower values of coverage than predicted using random vaccination models can suffice to protect the entire population.
Although nonrandom vaccination may offer theoretical opportunities for more cost-effective interventions, it raises problems in practice. If those at greatest risk are the least likely to be vaccinated—perhaps because both are associated with poor socioeconomic conditions—extra resources are required to ensure sufficient coverage in the disadvantaged communities.
A nonrandom distribution of vaccine can be ineffective even in a behaviorally homogeneous population, if it results in clusters of unvaccinated individuals; such groups are vulnerable to outbreaks. Clusters may emerge because of spatial patchiness but may also arise because of social segregation. This nonrandom mixing can in theory be described through the use of network models that include more detail information about who mixes with whom [26]. Social clustering among parents who decide not to vaccinate their children can result in groups of children in which vaccination levels are well below the herd immunity threshold [27]. The same effect is found in religious communities that eschew vaccination [28, 29]; though they form only a small proportion of the population, the fact that they often mix selectively with other members of the same community means that they are at an elevated risk of infection.
“Freeloaders”
When vaccination has costs to the individual—side effects, time, money, inconvenience—individual decisions about whether to be vaccinated are based on a complex balancing of perceived costs of vaccination and disease. A high level of vaccine uptake in the community may mean that the chance of contracting an infection is close to 0. From the point of view of an individual, therefore, the ideal (selfish) strategy is that everyone else should be directly protected by vaccination, allowing the exceptional freeloaders to benefit from the indirect protection this provides.
Exploring this idea, vaccine choices can be considered using tools from mathematical game theory [30, 31], which show that when coverage is close to Vc, or when vaccination is perceived to carry a risk similar to or greater than the infection, the incentive for a logical individual to receive a vaccine is lowered [32]. One observes this in the declining measles and pertussis vaccine coverage in several countries with low disease incidence, after media scares about vaccines [33]. People are in effect performing complex cost-benefit analyses, based on imperfect assumptions (for example a failure to appreciate the complex relationship between age and clinical severity of infections), when deciding whether or not to have themselves or their children vaccinated. It is not surprising that a sustained low incidence of infection, caused in large part by successful vaccination programs, makes the maintenance of high vaccination levels difficult, especially in the face of questioning or negative media attention.
PUBLIC HEALTH PRACTICE
Theory provides a useful background, but managers of vaccination programs face many nontheoretical problems in attempting to protect populations.
Managers must be wary of target thresholds for vaccination, insofar as thresholds are based on assumptions that greatly simplify the complexity of actual populations. In most circumstances, the sensible public health practice is to aim for 100% coverage, with all the doses recommended, recognizing that 100% is never achievable, hoping to reach whatever is the “real” herd immunity threshold in the population concerned.
Monitoring of coverage is itself a problem. Managers can rarely be totally confident of the immunity coverage actually attained, given the problems of avoidance of vaccine by some population subsets, ineffective or poorly administered vaccine, vaccination outside the recommended schedule, delays and inaccurate (sometimes even falsified) statistics, as well as population movements. In some populations particular problems are raised by private sector vaccine providers, if they do not provide data to national statistics. Another difficulty is raised by campaigns, carried out widely in recent years for polio and measles, that may keep no records of individual vaccinations, only total numbers of doses administered [34]. Among the important insights of the smallpox program was the recognition that it is often the same people who receive multiple (unnecessary) vaccinations, whereas others are repeatedly left out. Sound knowledge of one's population is a requirement for sound policy.
Maintenance of high coverage is particularly difficult as the diseases decline in frequency, and as populations become more sophisticated and more likely to question recommendations. The growth of antivaccine sentiment in many societies is a complicated issue, whether based on religious views, libertarian philosophies, or frank misinformation (of which there is an increasing amount, readily available on the Web). The recent epidemic of pertussis in California is the latest in a long list of examples of the difficulty of maintaining high vaccine coverage and to strike the appropriate message for the public [35].
Other problems arise because herd immunity is not the same as biologic (immunologic) immunity; individuals protected only by indirect herd effects remain fully susceptible to infection, should they ever be exposed. This has advantages, in protecting individuals with contraindications to vaccination or those who for other reasons miss vaccination, but it also has its disadvantages. Measles and mumps outbreaks among university students, and pertussis in adults, are among examples of the consequences of accumulation of susceptible individuals who have not been protected by vaccination, and escaped infection because of a herd immunity effect earlier in their lives [36]. Sometimes infection later in life causes more serious disease, a particular problem with rubella, which has its most severe consequences in the first trimester of pregnancy. In at least one instance, herd immunity and associated delays in infection of unvaccinated individuals led to increased congenital rubella syndrome [37]. This means that there is a need for immunization programs to maintain high vaccine coverage, together with surveillance and outbreak response capabilities, as numbers of susceptible individuals accumulate in older age groups. Herd immunity implies a lasting programmatic responsibility to the public.
Though there has been a tendency to emphasize the eradication implications of herd immunity in much of the theoretical literature, eradication programs are the exception in public health, because most programs aim at disease reduction to some “tolerable” level. Both eradication and control strategies aim at protecting the maximum number of individuals at risk, typically with a combination of high routine coverage and supplemental targeted vaccination of high risk populations. For meningitis epidemics, for example, once the reported number of cases exceeds 10 per 100,000, containment is often begun by mass vaccination campaigns that are first limited to the areas where transmission is known to occur and then expanded to other areas thought to be at risk [38]. These strategies require timely understanding of where transmission is occurring, and thus surveillance is critical.
Mass campaigns for eradication and containment are costly and require detailed planning. These are massive logistic undertakings, often implying severe disruption to routine health services. They have engendered considerable antipathy in some populations and are not to be undertaken lightly. Vaccination activities could be made more cost-effective if there were better tools to determine immunity levels and to understand transmission dynamics. It is important to recognize that models are just tautologies of their assumptions, and sound field epidemiology is essential to provide appropriate data on which to base these assumptions.
Finally, there are ethical and legal consequences of herd protection. Insofar as vaccination is encouraged in part to provide indirect protection to unvaccinated individuals, there is the implication of risk—albeit a very small risk—being imposed on certain individuals for the benefit of other individuals. This may have implications—different in different cultural, ethical, or legal contexts—for government liability in circumstances of adverse events to vaccines. Viewed from this perspective we find that indirect protection, the basis of “herd immunity,” raises many interesting and important issues about individual and public values. Indeed, one might argue that herd immunity, in the final analysis, is about protecting society itself.
Financial support: P.F. has had travel costs paid by Fondation Merieux to conferences to lecture on herd immunity. He has also received travel/meeting reimbursement from World Health Organization, Fondation Merieux, PATH, Bill and Melinda Gates Foundation for attending meetings related to this topic.
Potential conflicts of interest: All authors: no conflicts.




![Simple threshold concept of herd immunity. A, Relationship between the herd immunity threshold, (R0 – 1)/R0 = 1 − 1/R0,and basic reproduction number, R0, in a randomly mixing homogeneous population. Note the implications of ranges of R0, which can vary considerably between populations [12], for ranges of immunity coverage required to exceed the threshold. B, Cumulative lifetime incidence of infection in unvaccinated individuals as a function of the level of random vaccine coverage of an entire population, as predicted by a simple susceptible-infected-recovered model for a ubiquitous infection with R0 = 3 [13]. This assumes a 100% effective vaccine (E = 1). Note that the expected cumulative incidence is 0 if coverage is maintained above VC = 1− 1/R0 = 67%.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/52/7/10.1093/cid/cir007/2/m_cidcir007f02_ht.gif?Expires=1716388517&Signature=Pv3N6GCJsRw3QWdzmI6cPt4zmElIzTSTmPDQZkd-heQZGCeMWBgKQz77hDu-ADxapphWBFgh4tcpHDUeXdYrdL8CrtHJgBci6cFVBWpV0wMH2ApV1o~D6eTTjc-49iEo9Ody3Zbc4X5w3FGuI-iOvjJ9slGF4JFZCny2WYSNv9jV738oMu~oRhaXutSiveQ87AAI9w8tpOt9SE3zEAytnXeUb96piT~PtlGpIfQTPhdxbp3UuR56wBmuc~wQ2BKTI--uVrWnSJfhnJsEI8V-ePjJGhhZr0yM1Xv~iW-2HuazI8ROJY3OTlV~Ob3V3ajKkA2Z3KcYnQG3M5ioGqYTiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments