Abstract
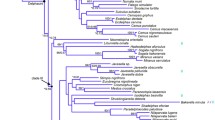
The Felidae family represents a challenge for molecular phylogenetic reconstruction because it consists of 38 living species that evolved from a relatively recent common ancestor (10–15 million years ago). We have determined mitochondrial DNA sequences from two genes that evolve at relatively rapid evolutionary rates, 16S rRNA (379 bp) and NADH dehydrogenase subunit 5 (NADH-5, 318 bp), from multiple individuals of 35 species. Based on separate and combined gene analyses using minimum evolution, maximum parsimony, and maximum likelihood phylogenetic methods, we recognized eight significant clusters or species clades that likely reflect separate monophyletic evolutionary radiations in the history of this family. The clusters include (1) ocelot lineage, (2) domestic cat lineage, (3) Panthera genus, (4) puma group, (5) Lynx genus, (6) Asian leopard cat group, (7) caracal group, and (8) bay cat group. The results confirm and extend previously hypothesized associations in most cases, but in others, e.g., the bay cat group, suggest novel phylogenetic relationships. The results are compared and evaluated with molecular, cytogenetic, and morphological data to derive a phylogenetic synthesis of field evolutionary history.
Similar content being viewed by others
References
Adams DB (1979) The cheetah: native American. Science 205:1155–1158
Avise JC (1994) Molecular markers, natural history and evolution. Chapman and Hall, New York
Benveniste RE, Todaro GJ (1974) Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci USA 70:3316–3320
Benveniste RE, Sherr CJ, Todaro GJ (1975) Evolution of type C viral genes: origin of feline leukemia virus. Science 190:886–888
Benveniste RE (1985) The contributions of retroviruses to the study of mammalian evolution. In: Maclntyre RJ (ed) Molecular evolutionary genetics. Plenum Press, New York, pp 359–417
Berta A (1983) A new species of small cat (Felidae) from the late Pliocene-early Pleistocene Uquian of Argentinian. J Mammal 64: 720–725
Clutton-Brock J (1987) A natural history of domesticated animals. Cambridge University Press, Cambridge, England
Collier GE, O’Brien SJ (1985) A molecular phylogeny of the Felidae: immunological distance. Evolution 39:437–487
Engelke DR, Hoener PA, Collins FS (1988) Direct sequencing of enzymatically amplified human genomic DNA. Proc Natl Acad Sci USA 85:544–548
Ewer RF (ed) (1973) The carnivores. Cornell University Press, Ithaca, NY 409 pp.
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Felsenstein J (1993) Phylogeny inference package (PHYLIP). Version 3.5. University of Washington, Seattle
Ficcarelli G (1984). The Villafranchian cheetahs from Tuscany and remarks on the dispersal and evolution of the genus Acinonyx. Palaeontographia Italica 73:94–103
Glass GE, Martin LD (1978) A multivariate comparison of some extant and fossil Felidae, Carnivora. Carnivora 1:80–88
Hemmer H (1978) The evolutionary systematics of living Felidae: present status and current problems. Carnivore 1:71–79
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Hillis DM (1995) Approaches for assessing phylogenetic accuracy. Syst Biol 44:3–16
Hoelzel AR, Green A (1992) Analysis of population-level variation by sequencing PCR-amplified DNA. In: Hoelzel AR (ed) Molecular genetic analysis of populations, a practical approach. IRL Press, Oxford pp. 159–187
Huelsenbeck JP, Hillis DM (1993) Success of phylogenetic methods in the four-taxon case. Syst Biol 42:247–264
Huelsenbeck JP, Bull JJ, Cunningham CW (1996) Combining data in phylogenetic analysis. Trends Ecol Evol 11:152–157
Hunt MH (1996) Biogeography of the order Carnivora. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution, vol 2. Cornell University Press, Ithaca, NY, pp. 485–541
Ikemura T (1985) Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol 2:13–34
Janczewski DN, Modi WS, Stephens JC, O’Brien SJ (1995) Molecular evolution of mitochondrial 12S RNA and cytochrome b sequences in the Pantherine lineage of Felidae. Mol Biol Evol 12:690–707
Johnson WE, Dratch PA, Martenson JS, O’Brien SJ (1996) Resolution of recent radiations within three evolutionary lineages of Felidae using mitochondrial restriction fragment length polymorphism variation. J Mammal Evol 3:97–120
Johnson WE, Culver M, Warte A, O’Brien SJ (submitted) Tracking the evolution of the elusive Andean Mountain Cat (Oreailurus jacobita) from mitochondrial DNA. J Heredity
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kluge AG (1989) A concern for evidence and a phylogenetic hypothesis of relationships among Epiarates (Boidae, Serpentes) Syst Zool 38:7–25
Kumar S, Tamura K, Nei M (1993) MEGA: Molecular evolutionary genetics analysis, version 1.01. The Pennsylvania State University, University Park, PA
Kurten B (1968) Pleistocene mammals of Europe. Aldine Press, Chicago
Kurten B, Anderson E (1980) In: Pleistocene mammals of North America. Columbia University Press, New York, pp. 108–118
Leyhausen P (1979) Cat behavior. Garland Press, New York
Lopez JV, Yuhki N, Masuda R, Modi W, O’Brien SJ (1994) Numt, a recent transfer and tandem amplification of mitochondrial DNA to the nuclear genome of the domestic cat. J Mol Evol 39:174–190
Lopez JV, Cevario S, O’Brien SJ (1996) Complete nucleotide sequences of the domestic cat (Felis catus) mitochondrial genome and a transposed mtDNA tandem repeat (Numt) in the nuclear genome. Genomics 33:229–246
Lopez JV, Culver M, Stephens JC, Johnson WE, O’Brien SJ (submitted) Rates of nuclear and cytoplasmic mitochondrial DNA sequence divergence in mammals. Mol Biol Evol
MacFadden BJ, Galiano H (1981) Late Hemphillian cat (Mammalia: Felidae) from the Bone Valley Formation of central Florida. J Paleontolo 55:218–226
Martin LD (1980) Functional morphology and the evolution of cats. Trans Neb Acad Sci 13:141–154
Martin LD (1989) Fossil history of the terrestrial Carnivora. In: Gittleman JL (ed) Carnivore behavior, ecology, and evolution. Cornell University Press, Cornell, NY, pp 536–568
Masuda RM, Lopez JV, O’Brien SJ, Pecon Slattery J, Yuhki N, O’Brien SJ (in press) Molecular phylogeny of mitochondrial cytochrome b and 12S rRNA sequences in the Felidae: ocelot and domestic cat lineages. Mol Phyl Evol
Modi WS, Nash WG, Ferrari AC, O’Brien SJ (1987) Cytogenetic methodologies for gene mapping and comparative analyses in mammalian cell culture systems. Gene Anal Tech 4:75–85
Modi WS, O’Brien SJ (1988) Quantitative cladistic analysis of chromosomal banding among species in three orders of mammals: hominoid primates, felids and arvicolid rodents. In: Gustafson JP, Appels R (eds) Chromosome structure and function. Plenum Press, New York
Needleman SB, Wunsch CD (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–453
Neff NA (1982) The big cats: the paintings of Guy Coheleach. Abrams Press, New York
Neigel JE, Avise JC (1986) Phylogenetic relationships of mitochondrial DNA under various demographic models of speciation. In: Karlin S, Nevo E (eds) Evolutionary processes and theory. Academic Press, Orlando, FL
Nowak RM (1991) Walker’s mammals of the world, vol 2. John Hopkins University Press, Baltimore
O’Brien SJ, Collier GE, Benveniste RE, Nash WG, Newman AK, Simonson JM, Eichelberger MA, Seal US, Janssen D, Bush M, Wildt DE (1987) Setting the molecular clock in Felidae: the great cats, Panthern. In: Tilson RL, Seal US (eds) Tigers of the world. Noyes, NJ, pp 10–27
Orr PC (1969) Felis trumani, a new radiocarbon dated cat skull from Crypt Cave, Nevada. Santa Barbara Mus Nat Hist Bull 2:1–8
Patterson B, Pascual R (1972) The fossil mammal fauna of South American. In: Keast A, Erk C, Glass B (eds) Evolution, mammals and southern continents. State University of New York Press, Albany, NY, pp 247–309
Pecon Slattery J, Johnson WE, Goldman D, O’Brien SJ (1994) Phylogenetic reconstruction of South American felids defined by protein electrophoresis. J Mol Evol 39:296–305
Peters G, Hast MH (1994) Hyoid structure, laryngeal anatomy, and vocalization in fields (Mammalia: Carnivora: Felidae). Z Saugetierkunde 59:87–104
Pocock, RI (1932) The marbled cat (Pardofelis marmorata) and some other Oriental species, with the definition of a new genus of the Felidae. Proc Zool Soc London 132:741–766
Randi E, Ragni B (1991) Genetic variability and biochemical systematics of domestic and wild cat populations (Felis silvestris: Felidae). J Mamm 72:79–88
Reeves RH, O’Brien SJ (1984) Molecular genetic characterization of the RD-114 gene family of endogenous feline retroviral sequences of the domestic cat. J Virol 52:164–171
Saiki RK, Scharf S, Fallona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of B-globin genomic sequences and restriction site analysis for diagnosis of sickle-cell anemia. Science 230:1350–1354
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–426
Salles LO (1992) Felid phylogenetics: extant taxa and skull morphology (Felidae, Aeluroidae). Am Mus Novit 3047:1–67
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning, a lab manual. 2nd ed. Cold Spring Harbor Laboratory publications, Cold Spring Harbor, NY
Savage DE, Russell DE (1983) Mammalian paleofaunas of the world. Addison-Wesley, London
Sharp PM, Cowe E, Higgins DG, Shields DC, Wolfe KH, Wright F (1988) Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisae, Schizosaccharomyces pombe, Drosophila melanogaster, and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res 16:8207–8211
Sibley SG, Ahlquist JE (1987) DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol 26:99–121
Sourdis J, Nei M (1988) Relative efficiencies of the maximum parsimony and distance-matrix methods in obtaining the correct tree. Mol Biol Evol 5:298–311
Swofford DL (1993) Phylogenetic analysis using parsimony (PAUP), version 3.1.1. University of Illinois, Champaign
Turner A (1985) Extinction, speciation and dispersal in African larger carnivores from the late Miocene to Recent. S Afr J Sci 81:256–257
Turner A (1987) New fossil carnivore remains from the Sterkfontein hominid site (Mammalia: Camivora). Ann Transvall Mus 34:319–347
Umbgrove JHF (1949) Structural history of the East Indies. Cambridge University Press, Cambridge, England
Van Valkenburgh B, Grady F, Kurten B (1990) The Plio-Pleistocene cheetah-like cat Miracinonyx inexpectatus of North America. J Vert Paleo 10:434–454
Werdelin L (1985) Small pleistocene felines of North America. J Vert Paleo 5:194–210
Wozencraft C (1993) Order Carnivora, Family Felidae. In: Wilson DE, Reeder DA (eds) Mammal species of the world. Smithonsian Institution Press, 2nd edition. Washington, DC, pp 288–299
Wu CI (1991) Inference of species phylogeny in relation to segregation of ancient polymorphisms. Genetics 127:429–435
Wurster-Hill DH, Centerwall WR (1982) The interrelationships of chromosome banding patterns in Procyonids, Viverrids and Felids. Cytogenet Cell Genet 34:178–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, W.E., O’Brien, S.J. Phylogenetic reconstruction of the felidae using 16S rRNA and NADH-5 mitochondrial genes. J Mol Evol 44 (Suppl 1), S98–S116 (1997). https://doi.org/10.1007/PL00000060
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/PL00000060