Abstract
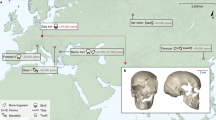
Clovis, with its distinctive biface, blade and osseous technologies, is the oldest widespread archaeological complex defined in North America, dating from 11,100 to 10,700 14C years before present (bp) (13,000 to 12,600 calendar years bp)1,2. Nearly 50 years of archaeological research point to the Clovis complex as having developed south of the North American ice sheets from an ancestral technology3. However, both the origins and the genetic legacy of the people who manufactured Clovis tools remain under debate. It is generally believed that these people ultimately derived from Asia and were directly related to contemporary Native Americans2. An alternative, Solutrean, hypothesis posits that the Clovis predecessors emigrated from southwestern Europe during the Last Glacial Maximum4. Here we report the genome sequence of a male infant (Anzick-1) recovered from the Anzick burial site in western Montana. The human bones date to 10,705 ± 35 14C years bp (approximately 12,707–12,556 calendar years bp) and were directly associated with Clovis tools. We sequenced the genome to an average depth of 14.4× and show that the gene flow from the Siberian Upper Palaeolithic Mal’ta population5 into Native American ancestors is also shared by the Anzick-1 individual and thus happened before 12,600 years bp. We also show that the Anzick-1 individual is more closely related to all indigenous American populations than to any other group. Our data are compatible with the hypothesis that Anzick-1 belonged to a population directly ancestral to many contemporary Native Americans. Finally, we find evidence of a deep divergence in Native American populations that predates the Anzick-1 individual.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
Sequence Read Archive
Data deposits
Sequence data (fastq files) for Anzick-1 is available for download through NCBI SRA accession number SRX381032. Additionally, alignments and genotype calls are available for download at http://www.cbs.dtu.dk/suppl/clovis/. Raw reads (fastq files) and alignments (BAM files) for the two modern genomes sequenced in this study are available for demographic research under data access agreement with E.W. The Cervus elaphus sequences are available under GenBank accessions KF906070, KF906071 and KF906072.
References
Waters, M. R. & Stafford Jr, T. W. Redefining the age of Clovis: implications for the peopling of the Americas. Science 315, 1122–1126 (2007)
Goebel, T., Waters, M. R. & O’Rourke, D. H. The late Pleistocene dispersal of modern humans in the Americas. Science 319, 1497–1502 (2008)
Meltzer, D. J. First Peoples in a New World (Univ. California Press, 2009)
Stanford, D. J. & Bradley, B. A. Across Atlantic Ice: the Origin of America’s Clovis Culture (Univ. California Press, 2012)
Raghavan, M. et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505, 87–91 (2014)
Lahren, L. & Bonnichsen, R. Bone foreshafts from a Clovis burial in southwestern Montana. Science 186, 147–150 (1974)
Owsley, D. W. & Hunt, D. Clovis and early Archaic crania from the Anzick site (24PA506), Park County, Montana. Plains Anthropol. 46, 115–124 (2001)
Lahren, L. A. Homeland (Cayuse Press, 2006)
Bradley, B. A., Collins, M. B. & Hemmings, A. Clovis Technology (Archaeological Series 17, 2010)
Morrow, J. E. & Fiedel, S. J. in Paleoindian Archaeology: a Hemispheric Perspective (eds Morrow, J. E. & Fiedel, S. J. ) 123–138 (Univ. Press of Florida, 2006)
Perego, U. A. et al. Distinctive Paleo-Indian migration routes from Beringia marked by two rare mtDNA haplogroups. Curr. Biol. 19, 1–8 (2009)
Kemp, B. M. et al. Genetic analysis of early holocene skeletal remains from Alaska and its implications for the settlement of the Americas. Am. J. Phys. Anthropol. 132, 605–621 (2007)
van Oven, M. & Kayser, M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum. Mutat. 30, E386–E394 (2009)
Behar, D. M. et al. A ‘Copernican’ reassessment of the human mitochondrial DNA tree from its root. Am. J. Hum. Genet. 90, 675–684 (2012)
Pruvost, M. et al. Freshly excavated fossil bones are best for amplification of ancient DNA. Proc. Natl Acad. Sci. USA 104, 739–744 (2007)
Allentoft, M. E. et al. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proc. R. Soc. Lond. B 279, 4724–4733 (2012)
Briggs, A. W. et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proc. Natl Acad. Sci. USA 104, 14616–14621 (2007)
Fu, Q. et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr. Biol. 23, 553–559 (2013)
Rasmussen, M. et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature 463, 757–762 (2010)
Poznik, G. D. et al. Sequencing Y chromosomes resolves discrepancy in time to common ancestor of males versus females. Science 341, 562–565 (2013)
Patterson, N. et al. Ancient admixture in human history. Genetics 192, 1065–1093 (2012)
Reich, D. et al. Reconstructing Native American population history. Nature 488, 370–374 (2012)
Pickrell, J. K. & Pritchard, J. K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8, e1002967 (2012)
Straus, L. G., Meltzer, D. J. & Goebel, T. Ice Age Atlantis? Exploring the Solutrean-Clovis ‘connection’. World Archaeol. 37, 507–532 (2005)
Fagundes, N. J. R. et al. Mitochondrial population genomics supports a single pre-Clovis origin with a coastal route for the peopling of the Americas. Am. J. Hum. Genet. 82, 583–592 (2008)
Jantz, R. L. & Owsley, D. W. Variation among early North American crania. Am. J. Phys. Anthropol. 114, 146–155 (2001)
Steele, D. G. & Powell, J. F. in Who Were the First Americans: Proceedings of the 58th Annual Biology Colloquium 97–126 (Center for the Study of the First Americans, Oregon State Univ., 1999)
Dillehay, T. D. et al. Monte Verde: seaweed, food, medicine, and the peopling of South America. Science 320, 784–786 (2008)
Kemp, B. M. & Schurr, T. G. in Human Variation in the Americas (ed. Auerbach, B. M. ) 12–50 (Southern Illinois Univ., 2010)
Acknowledgements
We thank the Danish National High-throughput DNA Sequencing Centre for help with sequencing, B. Henn and J. Kidd for assistance with Human Genome Diversity Project data, J. Keene for help with illustrations, M. Li, P. L. F. Johnson and M. Stoneking for help with the mtDNA analysis, L. A. Lahren for input to the site description and for establishing contact with the Native American groups, and J. E. Morrow, S. Fiedel and E. Lorenzen for comments on the manuscript. GeoGenetics were supported by the Lundbeck Foundation and the Danish National Research Foundation (DNRF94). M.D. was supported by the US National Science Foundation (grant DBI-1103639). A.-S.M. was supported by the Swiss National Science foundation. G.D.P. was supported by National Science Foundation (NSF) graduate research fellowship DGE-1147470. M.M., M.K., K.T. and L.S. were supported by the European Regional Development Fund through the Centre of Excellence in Genomics to Estonian Biocentre and University of Tartu, Estonian Basic Research (grant SF0270177As08) and Estonian Science Foundation (grant 8973). Computations in Uppsala were performed on resources provided by SNIC-UPPMAX (project b2012063) and in Tartu using the High Performance Computing Centre of the University of Tartu. A.E., V.M.W., M.C.L., F.B. and A.M. were supported by the Biotechnology and Biological Sciences Research Council (grant P25032 and BB/H005854/1). We thank the North Star Archaeological Research Program, Center for the Study of the First Americans, Texas A&M University, E. Hill, and Stafford Research, Inc. for funding some of the project.
Author information
Authors and Affiliations
Contributions
E.W., S.L.A., M.R.W. and T.W.S. conceived the project. E.W. headed the project. E.W. and M.R. designed the research project setup. R.N. supervised the bioinformatical and population genetic analyses with input from M.J. S.M.D., R.S.M. and T.L.P. helped with ethics and contact to local communities. S.L.A. and M.R.W. provided access to the Anzick-1 sample and the archaeological context, with input from S.S.W. and D.J.M. T.W.S. performed AMS 14C dating, stable isotope analyses and provided geochemical and geoarchaeological assessments. S.L.A. and J.S. performed initial mtDNA screening experiments. Elk extracts were processed by P.D.H. and I.B. Ancient DNA extractions and library constructs for shotgun sequencing and preparation for sequencing was done by M.R. O.E.C. prepared the two modern genomes. M.R. and S.R. did initial bioinformatics and mapping of the ancient sample. Mapping of modern samples, and genotype calls was done by S.R., with input from T.S.K., A.E., V.M.W. and M.C.L. S.R., T.S.-P. and S.B. provided super computing resources. O.E.C. and S.R. did phasing and ancestry painting, with input from A.E., V.M.W. and M.C.L. M.E.A. and M.C. did half-life estimates, with input on geology from T.W.S. DNA damage patterns were done by M.R. and L.O. mtDNA consensus and damage estimate was done by A.-S.M. I.M. and A.A. performed the X-chromosome contamination estimates, error rate estimates and D-statistic analyses on genomic sequence data. G.D.P. conducted Y-chromosome analysis with input from C.D.B. M.M. did ADMIXTURE analysis. K.T., M.K. and M.M. did mtDNA characterization. P.S. did f3-statistics on SNP array data and tested Native American population models using D-statistics. M.D. performed TreeMix analysis and genome-wide f3 -statistics. R.N. and M.D. developed and implemented the ancestry test. M.R., S.L.A., M.R.W., P.S., M.D., R.N. and E.W. wrote most of the manuscript with input from T.W.S., M.E.A., A.-S.M., S.R., I.M., A.A., G.D.P., K.T., M.M., R.G., V.G., R.Y., P.D.H., O.E.C., M.C., F.B., A.M., L.S. and the remaining authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 DNA fragmentation, damage and type-specific error.
a, Left, fragment length distribution of the Anzick-1 DNA sequences mapping to a human reference genome. The maximum read length with the applied chemistry on the HiSeq Illumina platform is 94 base pairs (100 − 6 base pair index read), hence the large peak at this length simply represents the entire tail of the distribution. Right, the declining part of the distribution for the nuclear DNA, and the fit to an exponential model. The decay constant (λ) is estimated to 0.018. b, Damage patterns for the Anzick-1 individual in a random 0.5% subset of all mapped reads. Mismatch frequency relative to the reference as function of read position, C to T in red and G to A in blue. c, Type-specific error rates for the Anzick-1 sample and the individual libraries. Estimates of overall error rates are given on the right.
Extended Data Figure 2 mtDNA and Y-chromosome subtrees.
a, Schematic phylogenetic tree of mtDNA haplogroup D4h3 and its sub-branch D4h3a. Mutations from the root of haplogroup D4h are specified only for haplogroup D4h3a lineage; diagnostic mutations are shown only for defined sub-branches on solid lines. The haplotypes of Anzick-1, identical with the root haplotype of D4h3a, and an ancient full sequence from the northwestern coast of North America (Ancient939), are indicated in red. Insertions are indicated with ‘.’ followed by a number of inserted nucleotides (X if not specified), deletions are indicated with ‘d’ and back mutations to ancestral state with ‘!’. The geographical spread of sub-branches of haplogroup D4h is shown with different colours specified in figure legend. b, Placement of Anzick-1 within the Y-chromosome phylogeny. Anzick-1 (circled) represents Y-chromosome haplogroup Q-L54*(xM3) (blue), which is offset by haplogroup Q-M3 (dark blue). The lineage carried by the ancient Saqqaq Palaeo-Eskimo (light blue) constitutes an outgroup to Q-L54. Each branch is labelled by an index and the number of transversion SNPs assigned to the branch (in brackets). Terminal taxa (individuals) are also labelled by population, ID and haplogroup. Branches 21 and 25 represent the most recent shared ancestry between Anzick-1 and other members of the sample. Branch 19 is considerably shorter than neighbouring branches, which have had an additional ∼12,600 years to accumulate mutations.
Extended Data Figure 3 Ancestry proportions of Anzick-1 as determined by Admixture assuming the number of hypothetical ‘ancestral’ populations or genetic components, K, is 3 to 5 and 9 to 11 for a set of 135 extant Eurasian, Oceanian and New World populations.
Shown are results from one of the converged runs at each K. We note that the model at K = 11 was found to have the best predictive accuracy as determined by the lowest cross validation index values (see Supplementary Information). At each K each sample is represented by a stacked vertical bar whereas these of Anzick-1 are magnified and presented horizontally at the top. Note that irrespective of the number of genetic components, K, assumed, the Anzick-1 sample shares all the components present in different contemporary Native American populations.
Extended Data Figure 4 Anzick-1 is closer to Central/Southern Native Americans than Northern Native Americans.
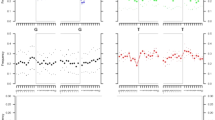
a–d, The closer relationship between Anzick-1 and Southern Native Americans compared to Algonquin, Cree, Ojibwa and a Yaqui individual is consistent for different 44 Southern and Central Native American populations to Anzick-1. We used the test D(Han, Anzick-1; Algonquin/Cree/Ojibwa/Yaqui, Central/Southern Native Americans). Thick and thin whiskers represent 1 and 3 standard errors, respectively.
Extended Data Figure 5 Outgroup f3 -statistics contrasted for different combinations of populations.
a, Shared genetic history with Anzick-1 compared to shared genetic history with the three Northern Amerind-speaking populations. b, c, Shared genetic history with the Anzick-1 individual compared to the ∼4,000-year-old Saqqaq from Greenland. d, e, Anzick-1 compared to shared genetic history with the 24,000-year-old MA-1 individual from Central Siberia. f, g, Shared genetic history with Anzick-1 compared to shared genetic history with the 40,000-year-old Tianyuan individual from China.
Extended Data Figure 6 Pairwise outgroup f3 statistics computed using Saqqaq, Han, French or ancient MA-1 (Mal’ta) on the x axis and Anzick-1, Karitiana or Mayan on the y axis.
The black line indicates the y = x line.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Supplementary References – see contents page for details. (PDF 770 kb)
Rights and permissions
About this article
Cite this article
Rasmussen, M., Anzick, S., Waters, M. et al. The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature 506, 225–229 (2014). https://doi.org/10.1038/nature13025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13025
This article is cited by
The Allen Ancient DNA Resource (AADR) a curated compendium of ancient human genomes
Scientific Data (2024)
Genetic continuity and change among the Indigenous peoples of California
Nature (2023)
Genomic history of coastal societies from eastern South America
Nature Ecology & Evolution (2023)
South-to-north migration preceded the advent of intensive farming in the Maya region
Nature Communications (2022)
Genomic selection signatures in autism spectrum disorder identifies cognitive genomic tradeoff and its relevance in paradoxical phenotypes of deficits versus potentialities
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.