Abstract
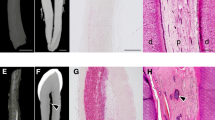
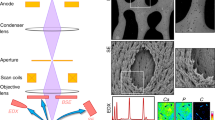
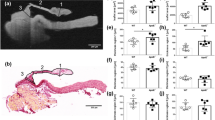
The accumulation of calcified material in cardiovascular tissue is thought to involve cytochemical, extracellular matrix and systemic signals; however, its precise composition and nanoscale architecture remain largely unexplored. Using nano-analytical electron microscopy techniques, we examined valves, aortae and coronary arteries from patients with and without calcific cardiovascular disease and detected spherical calcium phosphate particles, regardless of the presence of calcific lesions. We also examined lesions after sectioning with a focused ion beam and found that the spherical particles are composed of highly crystalline hydroxyapatite that crystallographically and structurally differs from bone mineral. Taken together, these data suggest that mineralized spherical particles may play a fundamental role in calcific lesion formation. Their ubiquitous presence in varied cardiovascular tissues and from patients with a spectrum of diseases further suggests that lesion formation may follow a common process. Indeed, applying materials science techniques to ectopic and orthotopic calcification has great potential to lend critical insights into pathophysiological processes underlying calcific cardiovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
24 April 2013
In the version of this Article originally published online, the first name of the fourth author was incorrect: it should have read 'Adrian H. Chester'. This error has been corrected in all versions of the Article.
References
Butler, D. UN targets top killers. Nature 477, 260–261 (2011).
Rajamannan, N. M. et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107, 2181–2184 (2003).
Lindroos, M., Kupari, M., Heikkila, J. & Tilvis, R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J. Am. Coll. Cardiol. 21, 1220–1225 (1993).
Nkomo, V. T. et al. Burden of valvular heart diseases: A population-based study. Lancet 368, 1005–1011 (2006).
Roger, V. L. et al. Heart disease and stroke statistics—2011 update. Circulation 123, e18–e209 (2011).
Iung, B. & Vahanian, A. Epidemiology of valvular heart disease in the adult. Nature Rev. Cardiol. 8, 162–172 (2011).
Sekikawa, A. et al. Aortic stiffness and calcification in men in a population-based international study. Atherosclerosis 222, 473–477 (2012).
Sage, A. P., Tintut, Y. & Demer, L. L. Regulatory mechanisms in vascular calcification. Nature Rev. Cardiol. 7, 528–536 (2010).
Langanay, T. et al. Aortic valve replacement in the elderly: The real life. Ann. Thorac. Surg. 93, 70–78 (2012).
Thomas, M. et al. Thirty-day results of the sapien aortic bioprosthesis european outcome (source) registry. Circulation 122, 62–69 (2010).
Duer, M. J. et al. Mineral surface in calcified plaque is like that of bone further evidence for regulated mineralization. Arterioscler. Thromb. Vasc. Biol. 28, 2030–2034 (2008).
Mody, N., Tintut, Y., Radcliff, K. & Demer, L. Vascular calcification and its relation to bone calcification: Possible underlying mechanisms. J. Nucl. Cardiol. 10, 177–183 (2003).
Delogne, C., Lawford, P. V., Habesch, S. M. & Carolan, V. A. Characterization of the calcification of cardiac valve bioprostheses by environmental scanning electron microscopy and vibrational spectroscopy. J. Microsc. 228, 62–77 (2007).
Weska, R. F. et al. Natural and prosthetic heart valve calcification: Morphology and chemical composition characterization. Artif. Organs 34, 311–318 (2010).
Demer, L. L. & Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 117, 2938–2948 (2008).
Fratzl, P. & Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 52, 1263–1334 (2007).
Gentleman, E. et al. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nature Mater. 8, 763–770 (2009).
Mahamid, J. et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl Acad. Sci. USA 107, 6316–6321 (2010).
Biltz, R. M. & Pellegri, E. D. Chemical anatomy of bone I. A comparative study of bone composition in 16 vertebrates. J. Bone Joint Surg.-Am. A 51, 456–466 (1969).
Mohler, E. R. III et al. Bone formation and inflammation in cardiac valves. Circulation 103, 1522–1528 (2001).
Rajamannan, N. M. et al. Calcific aortic valve disease: Not simply a degenerative process. Circulation 124, 1783–1791 (2011).
Su, X., Sun, K., Cui, F. Z. & Landis, W. J. Organization of apatite crystals in human woven bone. Bone 32, 150–162 (2003).
Mohr, W. & Görz, E. Verkalkungen der temporalarterien - ihre morphogenese im vergleich zur physiologischen osteogenese. Z. Kardiol. 92, 60–72 (2003).
Mohr, W. & Gorz, E. Does calcification of arteriosclerotic vessels imitate osteogenesis? Pathomorphological study of arteriosclerotic plaques. Z. Kardiol. 91, 212–232 (2002).
Kockx, M. M., Muhring, J., Bortier, H., DeMeyer, G. R. Y. & Jacob, W. Biotin- or digoxigenin-conjugated nucleotides bind to matrix vesicles in atherosclerotic plaques. Am. J. Pathol. 148, 1771–1777 (1996).
Johnston, K. W. et al. Determining the quantity and character of carotid artery embolic debris by electron microscopy and energy dispersive spectroscopy—discussion. J. Vasc. Surg. 45, 724–725 (2007).
Miller, V. M. et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am. J. Physiol. Heart Circ. Physiol. 287, H1115–H1124 (2004).
Koutsopoulos, S., Kontogeorgou, A., Dalas, E. & Petroheilos, J. Calcification of porcine and human cardiac valves: testing of various inhibitors for antimineralization. J. Mater. Sci.-Mater. Med. 9, 421–424 (1998).
Anderson, H. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 5, 222–226 (2003).
Boonrungsiman, S. et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl Acad. Sci. USA 109, 14170–14175 (2012).
Tanimura, A., McGregor, D. H. & Anderson, H. C. Matrix vesicles in artherosclerotic calcification. Proc. Soc. Exp. Biol. Med. 172, 173–177 (1983).
Komori, T. et al. Targeted disruption of cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Satokata, I. et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nature Genet. 24, 391–395 (2000).
Nakashima, K. et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29 (2002).
Jantou, V., Turmaine, M., West, G. D., Horton, M. A. & McComb, D. W. Focused ion beam milling and ultramicrotomy of mineralised ivory dentine for analytical transmission electron microscopy. Micron 40, 495–501 (2009).
McNally, E. A., Schwarcz, H. P., Botton, G. A. & Arsenault, A. L. A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS ONE 7, e29258 (2012).
Landis, W. J., Hodgens, K. J., Arena, J., Song, M. J. & McEwen, B. F. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc. Res. Tech. 33, 192–202 (1996).
Nudelman, F. et al. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature Mater. 9, 1004–1009 (2010).
New, S. E. P. & Aikawa, E. Cardiovascular calcification: An inflammatory disease. Circ. J. 75, 1305–1313 (2011).
Yuan, H. et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl Acad. Sci. USA 107, 13614–13619 (2010).
Yamasaki, H. & Sakai, H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials 13, 308–312 (1992).
Yang, Z. J. et al. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: Variability among different kinds of animals. Biomaterials 17, 2131–2137 (1996).
Kurashina, K., Kurita, H., Wu, Q., Ohtsuka, A. & Kobayashi, H. Ectopic osteogenesis with biphasic ceramics of hydroxyapatite and tricalcium phosphate in rabbits. Biomaterials 23, 407–412 (2002).
Le Nihouannen, D. et al. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 36, 1086–1093 (2005).
Ducheyne, P. & Qiu, Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials 20, 2287–2303 (1999).
LeGeros, R. Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Rel. Res. 395, 81–98 (2002).
LeGeros, R. Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 108, 4742–4753 (2008).
Miller, J. D., Weiss, R. M. & Heistad, D. D. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ. Res. 108, 1392–1412 (2011).
Chen, J. H. & Simmons, C. A. Cell-matrix interactions in the pathobiology of calcific aortic valve disease critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 108, 1510–1524 (2011).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Acknowledgements
S.B. was supported by the Rosetrees Trust and the Junior Research Fellowship scheme at Imperial College London. E.G. was supported by a fellowship from the Wellcome Trust. K.L.C. was supported by a studentship from the British Heart Foundation Centre of Research Excellence at Imperial College London. M.M.S. gratefully acknowledges financial support from the Rosetrees Trust. We would like to thank K. Nitiputri for providing the murine osteoblast cells line MC3T3-E1. We would like to acknowledge the provision of preliminary animal tissues by W. Jahnen-Dechent and A. Kinkeldey.
Author information
Authors and Affiliations
Contributions
S.B. designed the study, performed sample preparation, conducted all the experimental work, carried out data interpretation and aided with manuscript preparation and writing. E.G. aided with study design and data interpretation and wrote most of the manuscript. K.L.C. helped with sample preparation. A.H.C. and M.H.Y helped with sample procurement and aided in data interpretation. M.M.S. supervised the study, aided with study design and data interpretation and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1716 kb)
Rights and permissions
About this article
Cite this article
Bertazzo, S., Gentleman, E., Cloyd, K. et al. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nature Mater 12, 576–583 (2013). https://doi.org/10.1038/nmat3627
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3627
This article is cited by
68Ga-bisphosphonates for the imaging of extraosseous calcification by positron emission tomography
Scientific Reports (2023)
Calcific aortic valve disease: mechanisms, prevention and treatment
Nature Reviews Cardiology (2023)
Inflammatory macrophages interrupt osteocyte maturation and mineralization via regulating the Notch signaling pathway
Molecular Medicine (2022)
Biomineralization of bone tissue: calcium phosphate-based inorganics in collagen fibrillar organic matrices
Biomaterials Research (2022)
Epigenetics: a new warrior against cardiovascular calcification, a forerunner in modern lifestyle diseases
Environmental Science and Pollution Research (2022)