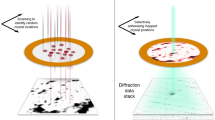
Abstract
Traditionally, crystallographic analysis of macromolecules has depended on large, well-ordered crystals, which often require significant effort to obtain. Even sizable crystals sometimes suffer from pathologies that render them inappropriate for high-resolution structure determination. Here we show that fragmentation of large, imperfect crystals into microcrystals or nanocrystals can provide a simple path for high-resolution structure determination by the cryoEM method MicroED and potentially by serial femtosecond crystallography.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Darwin, C.G. Philisophical Magazine Series 6 43, 800–829 (1922).
Nave, C. Acta Crystallogr. D Biol. Crystallogr. 54, 848–853 (1998).
Cusack, S. et al. Nat. Struct. Biol. 5 (Suppl.), 634–637 (1998).
Schlichting, I. IUCrJ 2, 246–255 (2015).
Shi, D., Nannenga, B.L., Iadanza, M.G. & Gonen, T. eLife 2, e01345 (2013).
Weierstall, U. Phil. Trans. R. Soc. Lond. B 369, 20130337 (2014).
Sierra, R.G. et al. Acta Crystallogr. D Biol. Crystallogr. 68, 1584–1587 (2012).
Henderson, R. Q. Rev. Biophys. 28, 171–193 (1995).
Nannenga, B.L., Shi, D., Leslie, A.G.W. & Gonen, T. Nat. Methods 11, 927–930 (2014).
Nannenga, B.L., Shi, D., Hattne, J., Reyes, F.E. & Gonen, T. eLife 3, e03600 (2014).
Colliex, C. et al. in International Tables for Crystallography, Vol. C (eds. Prince, E. & Wellberry, T.R.) Ch. 4.3 (International Union of Crystallography, 2006).
Yonekura, K., Kato, K., Ogasawara, M., Tomita, M. & Toyoshima, C. Proc. Natl. Acad. Sci. USA 112, 3368–3373 (2015).
Hattne, J., Shi, D., de la Cruz, M.J., Reyes, F.E. & Gonen, T. J. Appl. Crystallogr. 49, 1029–1034 (2016).
Rodriguez, J.A. et al. Nature 525, 486–490 (2015).
Sawaya, M.R. et al. Proc. Natl. Acad. Sci. USA 113, 11232–11236 (2016).
Von Laue, M. in Nobel Lectures Physics 1901–1921, 347–355 (Elsevier, 1967).
Stevenson, H.P. et al. Acta. Crystallogr. D Struct. Biol. 72, 603–615 (2016).
Bublitz, M. et al. IUCrJ 2, 409–420 (2015).
The PyMOL Molecular Graphics System v. 1.8 (Schrödinger, LLC, 2015).
Zúñiga, J.E. et al. J. Mol. Biol. 354, 1052–1068 (2005).
Watanabe, N., Akiba, T., Kanai, R. & Harata, K. Acta Crystallogr. D Biol. Crystallogr. 62, 784–792 (2006).
English, A.C., Done, S.H., Caves, L.S.D., Groom, C.R. & Hubbard, R.E. Proteins 37, 628–640 (1999).
de la Cruz, M.J. et al. Micro- and nanocrystal preparation for MicroED and XFEL serial crystallography by fragmentation of imperfect crystals. Protocol Exchange http://dx.doi.org/10.1038/protex.2017.010 (2017).
Hattne, J. et al. Acta Crystallogr. A Found. Adv. 71, 353–360 (2015).
Shi, D. et al. Nat. Protoc. 11, 895–904 (2016).
Leslie, A.G.W. & Powell, H.R. (eds.) Evolving Methods for Macromolecular Crystallography (Springer, 2007).
Battye, T.G.G., Kontogiannis, L., Johnson, O., Powell, H.R. & Leslie, A.G.W. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011).
Kabsch, W. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P.R. & Murshudov, G.N. Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Vagin, A. & Teplyakov, A. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Sheldrick, G.M. Acta Crystallogr. A Found. Adv. 71, 3–8 (2015).
Winn, M.D. et al. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Afonine, P.V. et al. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Brunger, A.T. Nat. Protoc. 2, 2728–2733 (2007).
Gonen, T. et al. Nature 438, 633–638 (2005).
Meyer, P.A. et al. Nat. Commun. 7, 10882 (2016).
Acknowledgements
The Gonen laboratory is supported by funds from the Howard Hughes Medical Institute. The Eisenberg laboratory is supported by HHMI, NIH grant 1R01-AG029430 (D.E.), and DOE grant DE-FC02-02ER63421 (D.E.). The Calero laboratory is supported by NIH grants RO1GM120292 and RO1DK102495 (G.C.) and BioXFEL-STC1231306 (G.C.). The Hinck laboratory is supported by NIH grants R01-GM58670 and R01-CA172886 (A.P.H.).
Author information
Authors and Affiliations
Contributions
D.S. and T.G. designed the experiment; M.J.d.l.C. and F.E.R. prepared lysozyme, xylanase, thaumatin, trypsin, proteinase K, and thermolysin samples; J.R. prepared tau peptide samples; S.C.W., S.K.K., C.S.H., A.P.H., and C.G. prepared TGF-βm–TßRII samples; M.J.d.l.C., D.S., J.R., and S.C.W. collected data; M.J.d.l.C., J.H., P.S., M.R.S., D.C., S.C.W., and G.C. analyzed data and refined and determined models; J.H., J.R., and M.R.S. prepared figures; M.J.d.l.C., J.H., and T.G. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 X-ray and corresponding MicroED diffraction pattern from protein tau.
(Left) When extracted from hanging drops, a cluster of microneedle crystals of the amyloid-forming protein tau diffracts as powder to no better than 4.2 Å using a rotating anode X-ray source. Physically breaking these needle clusters and selecting individual sub-micron thick crystal fragments yields diffraction to atomic resolution by MicroED (right) and a structure solvable by direct methods. The X-ray diffraction pattern was collected over a 6° oscillation range; the MicroED pattern spans a 0.6° wedge. See main text for data collection details.
Supplementary Figure 2 X-ray and corresponding MicroED diffraction pattern from Zn2+-NNQQNY.
The structure has previously been solved by X-ray diffraction, albeit at lower resolution [Nelson et al. (2005) Nature 435, 773–778]. It was readily redetermined by direct methods from the MicroED data [Sawaya et al. (2016) Proc Natl Acad Sci 113, 11232–11236]. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 3 X-ray and corresponding MicroED diffraction pattern from Cd2+-NNQQNY.
This structure was not previously solved by X-ray diffraction, but was readily determined by direct methods from the MicroED data [Sawaya et al. (2016) Proc Natl Acad Sci 113, 11232–11236]. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 4 X-ray and corresponding MicroED diffraction pattern from GNNQQNY.
The structure has previously been solved by X-ray diffraction, albeit at lower resolution [Nelson et al. (2005) Nature 435, 773–778]. It was readily redetermined by direct methods from the MicroED data [Sawaya et al. (2016) Proc Natl Acad Sci 113, 11232–11236]. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 5 X-ray and corresponding MicroED diffraction pattern from lysozyme.
The X-ray diffraction pattern displays multiple lattices. No optimization of crystal growth was done; instead crystals were sonicated and probed by MicroED. The obtained resolution was as good as what was obtained from the parent crystal. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 6 X-ray and corresponding MicroED diffraction pattern from TGF-βm–TβRII.
The X-ray diffraction pattern was collected at an X-ray free-electron laser, but the crystals diffracted better under MicroED. No optimization of crystal growth was done; instead crystals were vortexed with glass beads and then probed by MicroED. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 7 X-ray and corresponding MicroED diffraction pattern from xylanase.
The X-ray diffraction pattern displays several multiple lattices. No optimization of crystal growth was done; instead crystals were sonicated and probed by MicroED. The obtained resolution was as good as what was obtained from the parent crystal. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 8 X-ray and corresponding MicroED diffraction pattern from proteinase K.
The X-ray diffraction pattern displays multiple lattices. No optimization of crystal growth was done; instead crystals were sonicated and probed by MicroED. The obtained resolution was better than what was obtained from the parent crystal. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 9 X-ray and corresponding diffraction pattern from thermolysin.
The X-ray diffraction pattern is powder-like. No optimization of crystal growth was done; instead crystals were sonicated and probed by MicroED. The obtained resolution was better than what was obtained from the parent crystal. For the MicroED pattern the inset shows a close-up of the spot indicated by the blue circle.
Supplementary Figure 10 Rfree – Rwork as a function of refinement cycle.
The R-factor gap is always less than 6% and the variation is generally within ±1% after the first few cycles. The variation is greater for TGF-βm–TβRII, thaumatin, and thermolysin, which are the lowest-resolution structures in this study. (Left) tau peptide (dashed black curve), lysozyme (solid orange curve), TGF-βm–TβRII (solid blue curve), and xylanase (dashed green curve). (Right) thaumatin (solid black curve), trypsin (dashed orange curve), proteinase K (solid blue curve), and thermolysin (dashed green curve). All refinements were performed using phenix.refine [Afonine et al. (2012) Acta Crystallogr D Biol Crystallogr 68, 352–367].
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1055 kb)
Supplementary Text
Supplementary Protocol (PDF 134 kb)
Source data
Rights and permissions
About this article
Cite this article
de la Cruz, M., Hattne, J., Shi, D. et al. Atomic-resolution structures from fragmented protein crystals with the cryoEM method MicroED. Nat Methods 14, 399–402 (2017). https://doi.org/10.1038/nmeth.4178
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4178
This article is cited by
Growing and making nano- and microcrystals
Nature Protocols (2023)
Fortilin interacts with TGF-β1 and prevents TGF-β receptor activation
Communications Biology (2022)
Rational design and synthesis of a novel BODIPY-based probe for selective imaging of tau tangles in human iPSC-derived cortical neurons
Scientific Reports (2022)
Nucleation of protein mesocrystals via oriented attachment
Nature Communications (2021)
Establishing electron diffraction in chemical crystallography
Nature Reviews Chemistry (2021)