Abstract
There is increasing evidence that vascular endothelial growth factor (VEGF) has autocrine as well as paracrine functions in tumour biology. Vascular endothelial growth factor-mediated cell survival signalling occurs via the classical tyrosine kinase receptors Flt-1, KDR/Flk-1 and the more novel neuropilin (NP) receptors, NP-1 and NP-2. A 24-mer peptide, which binds to neuropilin-1, induced apoptosis of murine and human breast carcinoma cells, whereas a peptide directed against KDR had no effect. Both anti-NP1 and anti-KDR peptides induced endothelial cell apoptosis. Confocal microscopy using 5-(6)-carboxyfluorescein-labelled peptides showed that anti-NP1 bound to both tumour and endothelial cells, whereas anti-KDR bound endothelial cells only. This study demonstrates that NP-1 plays an essential role in autocrine antiapoptotic signalling by VEGF in tumour cells and that NP1-blockade induces tumour cell and endothelial cell apoptosis. Specific peptides can therefore be used to target both autocrine (tumour cells) and paracrine (endothelial cells) signalling by VEGF.
Similar content being viewed by others
Main
Angiogenesis, the growth of new capillaries, is a critical process in tumour growth and metastasis (Folkman, 1995). Numerous angiogenic factors have been identified to date, the most potent of these being vascular endothelial growth factor (VEGF), otherwise known as vascular permeability factor (VPF). Anti-VEGF strategies have been shown to potently inhibit tumour growth and metastasis (Kim et al, 1993; Millauer et al, 1996; Yuan et al, 1996; Xu et al, 2000).
In endothelial cells, the biological activities of VEGF are mediated through its binding to two high-affinity tyrosine kinase receptors, Flt-1 (VEGFR-1), KDR (VEGFR-2) and its murine homologue, Flk-1 (Waltenberger et al, 1994). At least five isoforms of VEGF are produced as a result of alternative mRNA splicing, where these isoforms differ with regard to the expression of exons 6 and 7 of the VEGF gene. More recently, neuropilin-1 (NP-1) has been identified as an isoform-specific receptor for VEGF (Soker et al, 1996; Gagnon et al, 2000). Originally identified as a receptor for the semaphorin/collapsin family of neuronal guidance mediators (Fujisawa and Kitsukawa, 1998), NP-1 is expressed on endothelial cells and is also found on the cell surface of several tumour cells such as breast, prostate and melanoma cells. Despite a similar domain structure, NP-1 shares approximately 44% amino-acid homology with neuropilin-2 (NP-2). The NP-1-binding site in VEGF165 is encoded by VEGF exon 7, which is absent in the VEGF isoforms VEGF121 and VEGF145 (Soker et al, 1997). The related NP-2 receptor also behaves as a splice form-specific VEGF receptor that also binds VEGF165. Unike NP-1, NP-2 binds VEGF145 (Gluzman-Poltorak et al, 2000). Neither receptor binds VEGF121, which lacks the exon 7 domain.
As neuropilins lack an intracellular tyrosine kinase domain, they may act in conjunction with other cell surface receptors in order to mediate cell signalling. It has been reported that neuropilin receptors can form complexes with members of the plexin family, where the plexin is the signal transducing element of this neuropilin/plexin complex (Tamagnone et al, 1999; Rohm et al, 2000). Plexin-A1 and plexin-A2 form complexes with the neuropilins. This complex formation does not depend on the presence of semaphorin (Takahashi and Strittmatter, 2001).
Until recently, VEGF produced by tumour cells and stromal cells such as macrophages was thought to act in a paracrine manner stimulating endothelial cell growth and differentiation. However, VEGF was recently shown to be an autocrine factor for breast carcinoma invasion in vitro (Bachelder et al, 2002). We and others have also demonstrated that VEGF acts in an autocrine fashion (Bachelder et al, 2001; Pidgeon et al, 2001). Furthermore, we demonstrated that VEGF-neutralising antibodies induced apoptosis of murine 4T1 and human MDA-MB-231 mammary adenocarcinoma cells. In the present study, we examined VEGF receptor expression and the effect of peptides targeting Flk-1/KDR and NP-1 on apoptosis of 4T1 and MDA-MB-231 breast carcinoma cells.
Materials and methods
Cell culture
The murine mammary adenocarcinoma 4T1 cell line was generously provided by Dr Fred Miller (Duke University, USA). The MDA-MB-231 human mammary adenocarcinoma cell line was purchased from the American Tissue Culture Collection (ATCC). 4T1 tumour cells were maintained in RPMI 1640 medium in a humidified atmosphere of 5% CO2 in air at 37°C. MDA-MB-231 cells were maintained in sealed flasks with L-15 medium at 37°C. The RENCA cell line (murine renal cell carcinoma), a gift from IJ Fidler, MD Anderson Cancer Centre, USA, were grown in MEM medium supplemented with nonessential amino acids (1 ×), L-glutamine (200 mM) and sodium pyruvate (100 mM). All media were supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1) (Gibco-BRL, Paisley, UK). Primary human umbilical vein endothelial cells (HUVEC), a gift from Ambrose Clarke, Department of Clinical Pharmacology, RCSI, were cultured in M-199 culture medium supplemented with 20% fetal calf serum, endothelial cell growth mitogen (Sigma, Ireland), penicillin (100 U ml−1) and streptomycin (100 μg ml−1). All cells were maintained as monolayer cultures, and exponentially growing cultures were used for experiments.
Western immunoblotting
Cells were lysed for 1 h on ice in 1 ml of lysis buffer (5 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% Triton –X-100, 0.5% SDS, 0.5% deoxycholic acid, 1 mM phenylmethylsulphonyl fluoride). Total protein concentration was determined using the Bicinchoninic Acid assay according to the manufacturer's instructions (Pierce, IL, USA).
In total, 30 μg of total protein was separated on an 8% denaturing polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (25 mM Tris-HCl, pH 7.6, 150 mM NaCl) containing 0.1% Tween-20 (TBST) for 1 h at room temperature and incubated for 60 min with NP-1 antibody (Santa Cruz Biotech, CA, USA), diluted 1 : 100 in 5% nonfat dry milk in TBST. Blots were stripped and reprobed with NP-2 antibody (Upstate Biotechnology, NY, USA), diluted 1 : 500 under similar conditions as that used for NP-1. Following six 5 min washes in TBST, membranes were incubated for 90 min with horseradish peroxidase-conjugated goat anti-rabbit antibody (DAKO, Glostrup, Denmark), diluted 1 : 2000 in TBST. Blots were probed using anti-Flt-1 and anti-Flk-1/KDR antibodies (R&D Systems, UK) at a concentration of 0.20 μg ml−1 and using a HRP-conjugated mouse anti-goat antibody, 1 : 2000. Bound antibody was detected using enhanced chemiluminescence (Pierce, IL, USA).
Peptide synthesis and purification
A 24-mer anti-NP1 VEGF peptide corresponding to exon 7 of VEGF165, CSCKNTDSRCKARQLELNERTCRC-NH2 (Soker et al, 1997), a corresponding ‘scrambled’ control peptide, NCTESKARCRLCSRCNDELTRKCQ-NH2, and a 7-mer anti-KDR/Flk-1 peptide, ATWLPPR-OH (Binetruy-Tournaire et al, 2000) were synthesised using an automated 433A peptide synthesizer (Applied Biosystems Inc., USA). Chromatographic analysis and purification were performed using a BioCAD SPRINT Perfusion Chromatography workstation (PerSeptive Biosystems) using POROS 20R2 Reversed Phase Perfusion Chromatography packing for analysis or a Jupiter Column (Phenomenex) for purification. For characterisation of peptides, Matrix Assisted Laser Desorption Ionisation-Time of Flight (MALDI-TOF) mass spectrometry was used.
Confocal microscopy
MDA-MB-231, 4T1 and HUVEC cells were grown on glass chamber slides (Becton Dickinson, UK) and treated with 5(6)-carboxyfluorescein labelled anti-NP1 and anti-KDR peptides for 18 h. Slides were washed four times in TBS and mounted in fluorescent mounting medium (DAKO, Glostrup, Denmark). Peptide binding was examined using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss International, Germany). The objective used was a PLAN-NEOFLUAR 40 × /1.3 oil DIC objective.
Flow cytometry analysis
Cells (5 × 104) were seeded in six-well culture plates and treated for 24 h with VEGF control (125 μg ml−1), anti-NP1 (125 μg ml−1) and anti-KDR (210 μg ml−1) peptides in medium containing 1% FCS. Cells were washed in 1 × PBS containing 2% BSA and apoptosis was assessed using the TACS™ Annexin V-FITC/PI apoptosis detection kit (R&D Systems, UK) according to the manufacturer's instructions. Flow cytometric analysis was performed using the FACSCalibur analysis system (Becton Dickinson, CA, USA). Apoptosis was measured as a percentage of the total number of cells and expressed as a percentage of controls in three independent experiments.
Hoechst staining of apoptotic cells
Nuclear staining of apoptotic tumour cells was examined by Hoechst staining (Singhal et al, 1999). Cells were treated with blocking peptides, as before, for 24 h. At the end of the incubation period, cells were washed in PBS and pelleted by centrifugation at 1500 r.p.m. for 5 min. Cells (1 × 105) were resuspended in 100% methanol containing Hoechst 33342 (Sigma-Aldrich, Ireland) at a final concentration of 1 μg ml−1 and incubated at 37°C for 10 min. Cytospins were prepared, mounted in fluorescent mounting medium (DAKO, Glostrup, Denmark) and viewed by UV light microscopy (Nikon, Melville, USA).
Statistical analysis
Statistical comparison between groups was carried out using analysis of variance (ANOVA) with LSD post hoc correction, using the SPSS™ statistical software package (SPSS Inc., IL, USA). Data were expressed as mean±standard error of the mean (s.e.m) and taken as significant where P<0.05.
Results
Tumour cell expression of VEGF receptors
Vascular endothelial growth factor is constitutively expressed by murine 4T1 and human MDA-MB-231 tumour cells (4T1, 59.10±1.87 pg VEGF perμg protein; MDA-MB-231, 20.56±1.51 pg VEGF per μg protein, Pidgeon et al, 2001). In the present study, we examined the effect of blocking the binding of VEGF to the NP-1 and KDR receptors using a peptide-based approach. Western blot analysis identified NP-1 and NP-2 receptors as a 130 and 135 kDa protein, respectively, in 4T1 (NP-1), MDA-MB-231 (NP-1 and NP-2) and HUVEC (NP-1 and NP-2) cells (Figure 1A), but both were absent in the RENCA cell line (Figure 1B). Of the tumour cell lines examined, we identified the expression of Flt-1 in 4T1, RENCA and HUVEC cells only. To date, we have been unable to detect Flk-1/KDR in 4T1 and MDA-MB-231 tumour cells using immunocytochemistry and Western blotting.
Vascular endothelial growth factor receptor expression. Tumour cells (4T1, MDA-MB-231, RENCA) and endothelial cells (HUVEC) were cultured for 24 h, and VEGF receptor expression was anlaysed by Western blot. 4T1, MDA-MB-231 and HUVEC cells expressed a 130 kDa protein corresponding to the NP-1 receptor, while MDA-MB-231 and HUVEC cells also expressed NP-2 as a 135 kDa protein (A). Flt-1 was identified as a 175 kDa protein in murine 4T1 and RENCA cells and as a 128 kDa protein in endothelial cells (B).
Anti-NP1 peptides bind to tumour and endothelial cells, while anti-KDR peptides bind to endothelial cells only
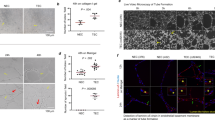
We examined the binding of anti-NP1 and anti-KDR peptides labelled with 5(6)-carboxyfluorescein to tumour and endothelial cells by confocal microscopy. The anti-NP1 peptide bound to 4T1 (Figure 2A), MDA-MB-231 (Figure 2B) and HUVEC (Figure 2C) cells. In contrast, the anti-KDR peptide did not bind to murine 4T1 and human MDA-MB-231 tumour cells but did bind to HUVEC cells, where these are known to express functional KDR.
Anti-NP1 and anti-KDR peptide binding. Tumour and endothelial cells were incubated with 5-(6)-carboxyfluorescein-labelled anti-NP1 and anti-KDR peptides on chamber slides for 18 h. Peptide binding to the VEGF receptors, NP-1 and KDR, was examined on 4T1 (A), MDA-MB-231 (B) and HUVEC (C) cells by confocal microscopy (× 400 magnification). Images are representative of a scan zoom of between 1- and –4.2-fold.
Vascular endothelial growth factor receptor blockade induces apoptosis of tumour and endothelial cells
The anti-KDR peptide, ATWLPPR, was previously identified from a phage-display library and shown to block the KDR receptor, inducing apoptosis of endothelial cells (Binetruy-Tournaire et al, 2000). We also synthesised a 24-mer anti-NP1 peptide corresponding to exon 7 of VEGF165 (Soker et al, 1997) as the NP-1-binding motif has been shown to reside within exon 7 of this VEGF isoform. Having demonstrated that murine 4T1 and human MDA-MB-231 tumour cells express NP-1 and endothelial cells (HUVEC's) express both NP-1 and KDR, we studied the effect of anti-NP1 and anti-KDR peptides on apoptosis. HUVECs were used as a positive control as these are known to express Flt-1, KDR and NP-1 (Peters et al, 1993). Treatment with NP-1-blocking peptide resulted in a significant increase in apoptosis of both tumour cells and endothelial cells (4T1, 226.72±38.80%; MDA-MB-231, 126.99±14.49%; HUVECs, 196.77±29.51%, P<0.05) relative to cells treated with a scrambled control peptide (4T1, 88.66±22.77%; MDA-MB-231, 71.45±7.32%; HUVECs, 83.40±8.78%, p<0.05, Figure 3A, B and C, respectively). Anti-KDR peptide induced significant apoptosis of HUVECs relative to controls (141±7% vs control, P<0.05) but had no effect on tumour cell apoptosis in murine and human tumour cells (4T1, 77.12±20.82%; MDA-MB-231, 86.24±5.61% vs control), consistent with the lack of expression of Flk-1/KDR in these cells. Representative dot plots showing induction of tumour cell (4T1) and endothelial cell (HUVEC) apoptosis by anti-NP1-blocking peptide relative to a VEGF control peptide are shown (Figure 3D), following analysis by flow cytometry. RENCA cells were also treated with anti-NP1 and anti-KDR-blocking peptides as previously described. These cells do not express the VEGF receptors NP-1 or Flk-1. As expected, neither peptide induced apoptosis of these tumour cells (data not shown), demonstrating that the anti-NP1 and anti-KDR peptides only induce apoptosis in NP-1-positive or KDR-positive cells, respectively.
Effect of anti-NP1 and anti-KDR peptides on tumour cell and endothelial cell apoptosis. Murine 4T1 (A), human MDA-MB-231 (B) tumour cells and HUVEC endothelial cells (C) were treated with anti-NP1 and anti-KDR peptides or a ‘scrambled’ control peptide for 24 h. Annexin V binding to phosphatidylserine residues on the suface of apoptotic cells was assessed by flow cytometry and the percentage apoptotic cells was expressed relative to controls (mean±s.e.m) from three independent experiments (*P<0.05 vs untreated cells, $P<0.05 vs control peptide). Representative dot-plots demonstrating Annexin V-positive apoptotic cells are shown in the lower right-hand quadrant following treatment of murine 4T1 tumour cells (top panel) and HUVECs (lower panel) with a scrambled control peptide and anti-NP1 peptide (D).
To demonstrate that the induction of apoptosis in NP1-expressing 4T1 and MDA cells by anti-NP1 peptide was not a consequence of nonspecific cytotoxicity, RENCA cells, which do not express the NP1 receptor, were treated with a scrambled control peptide and the anti-NP1 peptide. As expected, neither peptide induced apoptosis in NP1-negative RENCA cells.
Morphological evaluation of tumour cell apoptosis using anti-NP1 and anti-KDR blocking peptides
To confirm the induction of 4T1 and MDA-MB-231 tumour cell apoptosis using anti-NP1 blocking peptides, nuclear morphology was examined by Hoechst 33342 staining. Tumour cells treated for 24 h with anti-NP1 blocking peptide (Figure 4C) demonstrated typical apoptotic nuclei relative to untreated cells (Figure 4A) or cells treated with a scrambled control peptide (Figure 4B). Anti-KDR peptides however did not induce apoptosis of 4T1 and MDA-MB-231 tumour cells (Figure 4D). These findings at the morphological level are consistent with our findings using Annexin V-FITC/PI by flow cytometry.
Hoechst staining of apoptotic tumour cells. 4T1 and MDA-MB-231 tumour cells were incubated for 24 h with control peptide (B), anti-NP1 (C) and anti-KDR (D) peptides. Following incubation, cells were stained with Hoechst 33342 (1 μg ml−1) in methanol and examined under UV light (× 400 magnification). Treatment of 4T1 and MDA-MB-231 tumour cells with anti-NP1 peptides induced apoptosis, as seen by bright fluorescing cells relative to control peptide and cells treated with anti-KDR peptide.
Discussion
In contrast to the classical VEGF receptors, Flt-1 (VEGFR-1) and Flk-1/KDR (VEGFR-2), and the mechanisms of VEGF signalling via these tyrosine kinase receptors, relatively little is known regarding the mechanisms governing VEGF signalling via the neuropilins.
A review of the literature of VEGF receptors and MDA-MB-231 tumour cells appears controversial, with a number of groups reporting different VEGF receptor profiles. Soker et al (1998) report that NP-1 is the only VEGF receptor associated with these cells. This is consistent with the findings from Bachelder et al (2001) who also failed to identify KDR expression by Western blot. However, Tae-Hee et al (2003) found detectable levels of Flk-1/KDR mRNA in MDA-MB-231 cells by Northern blot analysis. Such findings are consistent with our observations using RT–PCR. Despite a lack of evidence for Flt-1 expression at the protein level, we detected Flt-1 mRNA (data not shown). This is in contrast to that reported by Soker et al (1996) where they report undetectable levels of Flt-1 mRNA. Similarly, there have been a number of contrasting reports regarding NP-2 expression by MDA-MB-231 cells. Using 125I-VEGF145- and 125I-VEGF165-binding studies, Gluzman-Poltorak et al (2000) deduced that MDA-MB-231 tumour cells do not express detectable levels of functional NP-2 receptors. Mamluk et al (2002) on the other hand report NP-2 expression on these cells. Our findings that MDA-MB-231 cells and endothelial cells express NP-2 at the protein level are in agreement with those reported by the latter. We found that 4T1 tumour cells express only NP-1 and Flt-1 receptors.
It has recently been reported that MDA-MB-231 breast carcinoma cells express sema-3A and plexin-A1, which can both bind to NP-1. Indeed Sema-3A and VEGF compete for binding to NP-1. These act as antagonistic autocrine NP-1 ligands that regulate breast carcinoma cell migration (Bachelder et al, 2003). Plexin-A1 and plexin-A2 can form complexes with NP-1 and NP-2. Having found NP-1 and NP-2 as the only VEGF receptors expressed by these cells, we propose that VEGF signalling in MDA-MB-231 cells may be mediated by plexin/NP complexes. Since Bachelder et al demonstrated a role for such receptors in tumour cell chemotaxis, these receptors may also play a functional role in VEGF-mediated tumour cell survival. Since 4T1 tumour cells only express NP-1 and Flt-1, both receptors may interact with each other in order to transduce signalling via the classical tyrosine kinase receptor, Flt-1. Alternatively, such signalling may be mediated via plexin-A1. In endothelial cells (HUVEC), there exists a number of possibilities whereby VEGF mediates signalling through a number of its receptors. Evidence to date suggests that VEGF signalling in endothelial cells occurs via the formation of complexes involving KDR and NP-1. This in turn enhances the binding of VEGF165 to KDR. Both KDR and NP-1 play an important role in VEGF-mediated survival signalling as blockade of either receptor induces apoptosis of endothelial cells.
In conclusion, we have demonstrated that a 24-mer peptide targeting the VEGF165-binding site on the novel VEGF receptor, NP-1, antagonises the autocrine antiapoptotic effects of VEGF on breast carcinoma cells. The use of peptides over monoclonal antibodies offer a number of advantages including low manufacturing costs, lower risk of an immune response, improved organ and tumour penetration and greater stability. Owing to their reduced size, peptides offer an attractive therapeutic option over antibodies as inducers of apoptosis and angiogenesis inhibitors. As chemo/radiotherapy increases VEGF expression (Gorski et al, 1999), an autocrine role for VEGF in protecting tumour cells from apoptosis may explain the synergistic effect of VEGF blockade when combined with chemo/radiotherapy (Harmey and Bouchier-Hayes, 2002). Thus, it is likely that anti-NP1 peptides will be most effective when used in combination with other antiangiogenic strategies and traditional apoptosis-inducing chemotherapy/radiotherapy regimens.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM (2001) Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res 61: 5736–5740
Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM (2003) Competing autocrine pathways involving alternative neuropilin-1 ligands regulates chemotaxis of carcinoma cells. Cancer Res 63: 5230–5233
Bachelder RE, Wendt MA, Mercurio AM (2002) Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res 62: 7203–7206
Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, Plouet J, Derbin C, Perret G, Mazie JC (2000) Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J 7: 1525–1533
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med 1: 27–30
Fujisawa H, Kitsukawa T (1998) Receptors for collapsin/semaphorins. Curr Opin Neurobiol 8: 587–592
Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M (2000) Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and anti-tumor activity. Proc Natl Acad Sci USA 97: 2573–2578
Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G (2000) Neuropilin-2 and neuropilin-1 are receptors for the 165-amino acid form of vascular endothelial growth factor (VEGF) and of placenta growth factor-2, but only neuropilin-2 functions as a receptor for the 145-amino acid form of VEGF. J Biol Chem 275: 18040–18045
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Hari DM, Kufe DW, Weichselbaum RR (1999) Blockade of the vascular endothelial growth factor stress response increases the anti-tumor effects of ionizing radiation. Cancer Res 59: 3374–3378
Harmey J, Bouchier-Hayes D (2002) Vascular endothelial growth factor (VEGF), a survival factor for tumor cells: implications for anti-angiogenic therapy. BioEssays 24: 280–283
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 362: 841–844
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M (2002) Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277: 24818–24825
Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, Strawn LM (1996) Dominant negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res 56: 1615–1620
Peters KG, De Vries C, Williams LT (1993) Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc Natl Acad Sci USA 90: 8915–8919
Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ (2001) Vascular endothelial growth factor (VEGF) upregulates Bcl-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer 85: 273–278
Rohm B, Ottemeyer A, Lohrum M, Pueschel AW (2000) Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev 93: 95–104
Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G (1999) Morphine promotes apoptosis in Jurkat cells. J Leuk Biol 66: 650–658
Soker S, Fidder H, Neufeld G, Klagsbrun M (1996) Characterization of a novel vascular endothelial growth factor (VEGF) receptor on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem 271: 5761–5767
Soker S, Gollamundi-Payne S, Fidder H, Charmahelli H, Klagsbrun M (1997) Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165 . J Biol Chem 272: 31582–31588
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92: 735–745
Tae-Hee L, Avraham HK, Jiang S, Avraham S (2003) Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278: 5277–5284
Takahashi T, Strittmatter SM (2001) Plexin-A1: autoinhibition by the plexin sema domain. Neuron 29: 429–439
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99: 71–80
Waltenberger J, Claesson-Welsh L, Siegbahn M, Heldin CH (1994) Different signal transduction properties of KDR and Flt-1, two receptors for vascular endothelial growth factor. J Biol Chem 269: 26988–26995
Xu L, Yoneda J, Herrera C, Wood J, Killion JJ, Fidler IJ (2000) Inhibition of malignant ascites and growth of human ovarian carcinoma by oral administration of a potent inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Int J Oncol 16: 445–454
Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK (1996) Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 93: 14765–14770
Acknowledgements
This study was supported by Health Research Board Grant RP182/2000, Higher Education Authority, Cycle II and RCSI Research Committee Grant 014/02. We gratefully acknowledge Dr Vivienne Foley, Centre for Synthesis and Chemical Biology, Department of Pharmaceutical and Medicinal Chemistry, RCSI, for her assistance in the preparation and purification of peptides used in this study. Also, to Ms Pamela Connolly, Department of Clinical Pharmacology, RCSI, for her assistance with confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Barr, M., Byrne, A., Duffy, A. et al. A peptide corresponding to the neuropilin-1-binding site on VEGF165 induces apoptosis of neuropilin-1-expressing breast tumour cells. Br J Cancer 92, 328–333 (2005). https://doi.org/10.1038/sj.bjc.6602308
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602308
Keywords
This article is cited by
Prediction and therapeutic targeting of the tumor microenvironment-associated gene CTSK in gastric cancer
Discover Oncology (2023)
Silencing Neuropilin 1 gene reverses TGF-β1-induced epithelial mesenchymal transition in HGC-27 gastric cancer cell line*
Oncology and Translational Medicine (2020)
A comprehensive review of tumor proliferative and suppressive role of semaphorins and therapeutic approaches
Biophysical Reviews (2020)
Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer
npj Precision Oncology (2019)
Genetic status of KRAS modulates the role of Neuropilin-1 in tumorigenesis
Scientific Reports (2017)