-
PDF
- Split View
-
Views
-
Cite
Cite
Jeremy Sobel, Botulism, Clinical Infectious Diseases, Volume 41, Issue 8, 15 October 2005, Pages 1167–1173, https://doi.org/10.1086/444507
Close - Share Icon Share
Abstract
Botulism is a rare disease with 4 naturally occurring syndromes: foodborne botulism is caused by ingestion of foods contaminated with botulinum toxin, wound botulism is caused by Clostridium botulinum colonization of a wound and in situ toxin production, infant botulism is caused by intestinal colonization and toxin production, and adult intestinal toxemia botulism is an even rarer form of intestinal colonization and toxin production in adults. Inhalational botulism could result from aerosolization of botulinum toxin, and iatrogenic botulism can result from injection of toxin. All forms of botulism produce the same distinct clinical syndrome of symmetrical cranial nerve palsies followed by descending, symmetric flaccid paralysis of voluntary muscles, which may progress to respiratory compromise and death. The mainstays of therapy are meticulous intensive care (including mechanical ventilation, when necessary) and timely treatment with antitoxin.
Botulism is a rare, naturally occurring disease that can also be caused by accidental or intentional exposure to botulinum toxins. All forms of botulism manifest essentially the same distinct clinical syndrome of symmetrical cranial nerve palsies that may be followed by descending, symmetric flaccid paralysis of voluntary muscles, which may progress to respiratory compromise and death.
Rapid diagnosis, provision of intensive care, and administration of botulinum antitoxin are the cornerstones of treatment. Antitoxin is available exclusively from public health authorities, who immediately investigate potential sources to prevent additional illness [1]. Botulinum toxin is a category A biological agent, and it has been extensively weaponized by governmental military programs [2–6] and was deployed by a terrorist group [7, 8].
The Organism and Its Toxins
Clostridium botulinum is ubiquitously found in soil and aquatic sediments. C. botulinum produces 7 immunologically distinct toxins, which are designated by the letters A–G. Several related clostridial species (e.g., Clostridium baratii and Clostridium butyricum) can produce some botulinum toxins as well. All produce a clinically similar, highly recognizable syndrome. Human cases are caused mostly by toxin types A, B, E, and (rarely) F. All of the toxins are large, single polypeptides of similar structure that exert their action on the cholinergic system at the presynaptic motor-neuron terminal by blocking acetylcholine transmission across the neuromuscular junction [9–12], causing neuromuscular blockade, resulting in flaccid paralysis. The toxins also affect the adrenergic system, but this apparently happens without significant consequences.
Although the exact lethal dose has not been quantified, botulinum toxins are the most potent toxins known; extrapolations can be made from primate studies. Commonly cited estimated lethal doses for purified crystalline botulinum toxin type A for a 70-kg man are 0.09–0.15 µg when introduced intravenously, 0.80–0.90 µg when introduced inhalationally, and 70 µg when introduced orally [2]. However, considerably lower figures have been generated by other studies [13, 14] and from estimates based on cases in humans [15]. Because mouse bioassay is the standard method for detection and quantification of toxin, toxin is usually quantified in terms of biological activity in terms of mouse intraperitoneal lethal dose (MIPLD50).
Under stress, C. botulinum forms a spore that survives standard cooking and food-processing measures. However, the confluence of conditions permitting spore germination—anaerobic milieu, nonacidic pH, and low salt and sugar content—is rarely achieved in food, which explains the small number of foodborne botulism cases. The technique of modern industrial canning (i.e., retort canning) was developed expressly for killing C. botulinum spores [16, 17]. In contrast with the spore, botulinum toxins are temperature sensitive, and all toxins are inactivated by heating to 85°C for 5 min [17].
Conditions in the normal human intestine are not conducive to germination and vegetation of C. botulinum. C. botulinum spores are routinely ingested and excreted by humans without germination, toxin production, or any harm to the person through whom they pass. The exceptions are the small number of infants who develop infant botulism and the handful of adults who develop adult toxemic infectious botulism.
Botulism Syndromes and Their Epidemiology
Foodborne botulism.Foodborne botulism is caused by consumption of foods contaminated with botulinum toxin. C. botulinum grows and elaborates toxin only when the food presents conditions that include an anaerobic milieu, a pH of <4.5, low salt and sugar content, and a temperature of 4°C–121°C [18]. Home-canned foods have long constituted a major source of intoxication in the continental United States [19, 20]. Traditional Alaska Native dishes, which are fermented and consumed without cooking, also pose a substantial risk [20].
In the United States, during 1990–2000, the median number of foodborne botulism cases per year was 23 (range, 17–43 cases) Most cases are sporadic (i.e., they are not part of outbreaks); outbreaks are typically small, involving 2 or 3 persons. Nevertheless, outbreaks caused by commercial or restaurant-prepared foods do occur [22].
Additional evidence for the diagnosis of foodborne botulism can be provided by obtaining a 3–5-day food history from the patient; reported consumption of home-canned food substantially enhances the probability of foodborne botulism. Whether close contacts may have shared foods should also be noted. It is important that the clinician solicit this information early, because if the patient's case progresses to respiratory failure and mechanical ventilation despite administration of supportive and specific therapy, the patient's ability to communicate further information will be compromised.
Wound botulism.Wound botulism is caused by contamination of a wound with C. botulinum spores from the environment and subsequent germination of these spores and production of toxin in the anaerobic milieu of an abscess. The condition was exceedingly rare until the early 1990s; since that time, the western United States has experienced a dramatic and continuing increase in its incidence, almost exclusively among injection drug users [23]. Almost all persons with injection drug–associated cases are users of so-called "black tar heroin," a specific preparation of heroin, and persons who partake in "skin-popping" (i.e., injection of the black tar heroin into tissues, as opposed to veins) [24]. The typical patient is an adult in the fourth or fifth decade of life, who has a long history of use of injected black tar heroin, and who resides in the western United States. The incubation period is hard to establish, because most patients inject drugs several times daily. The clinical syndrome is indistinguishable from that of foodborne botulism, except for the absence of gastrointestinal symptoms. Often, the abscess is a minor lesion, at times no more than a small furuncle or an abscess that resembles mild cellulitis. Whenever possible, abscesses should be cleaned and debrided, tissue material should be collected in anaerobic culture tubes for testing, and appropriate antimicrobial therapy should be provided.
For the clinician, ascertainment of a history of injection drug use—particularly use of black tar heroin—is crucial. When combined with a compatible presentation, a history of such drug use is highly predictive of wound botulism.
Infant botulism.Infant botulism results from absorption of toxin produced in situ by C. botulinum colonization of the intestines of certain infants aged <1 year [25]. This is the most common form of botulism, with ∼80–100 cases reported annually in the United States [19]. Colonization is believed to occur because normal bowel florae that could compete with C. botulinum have not been fully established. Studies have implicated honey consumption as a risk factor for illness, but honey consumption probably accounts for only up to 20% of cases [26]. For unknown reasons, the highest incidence is found in the vicinity of Philadelphia, Pennsylvania [27]. The clinical presentation resembles that of adult forms of disease, with common symptoms that include inability to suck and swallow, weakened voice, ptosis, and floppy neck and that may progress to generalized flaccidity and respiratory compromise [28]. Specific therapy for infant botulism is a newly licensed human-source antitoxin, which halves the median hospitalization period from 6 to 3 weeks. With appropriate intensive care, the survival rate is nearly 100% with or without antitoxin therapy [25].
Adult intestinal toxemia botulism.Adult intestinal toxemia botulism has resulted from absorption of toxin produced in situ by rarely occurring intestinal colonization in a few adults in the United States by botulinum-toxin producing Clostridia. Typically, patients have some anatomical or functional bowel abnormality or are using antimicrobials, which may permit protection of normally fastidious Clostridia species from competition with normal bowel flora [29–33]. Protracted symptoms and relapse in the face of antitoxin treatment due to ongoing intraluminal production of toxin may be observed. Diagnosis in a patient with sporadic botulism and no known food or wound source rests on demonstration of prolonged excretion of organisms and toxin in the stool.
Inhalational botulism.Inhalational botulism is not a naturally occurring disease. The syndrome was described once among German laboratory workers in 1962, with symptoms resembling those of foodborne botulism [3]. Deliberate dissemination of botulinum toxin by aerosol could produce an outbreak of inhalational botulism [2].
Iatrogenic botulism.Iatrogenic botulism is caused by injection of botulinum toxin for cosmetic or therapeutic purposes. Doses recommended for cosmetic treatment are too low to cause systemic disease. Higher doses injected for treatment of muscle movement disorders have caused anecdotal cases of systemic botulism-like symptoms [34]. Injection of unlicensed, highly concentrated botulinum toxin caused severe botulism in 4 patients who received it for cosmetic purposes (unpublished data).
Clinical Presentation
The clinical syndrome of botulism is highly distinctive, consisting of symmetrical cranial nerve palsies, followed by symmetrical descending flaccid paralysis that may progress to respiratory arrest [19, 35]. For a sporadic (isolated) case, the differential diagnosis is not extensive, and the combination of neurological findings and specific laboratory tests provide highly sensitive clinical diagnosis pending laboratory confirmation [17]. A cluster of ⩾2 cases with compatible symptoms is essentially pathognomonic, because the illnesses that most resemble botulism do not produce outbreaks. The diagnosis in sporadic cases and even in small outbreaks is frequently missed, however, because botulism is a rare disease with which most clinicians are unfamiliar [36]. Every case of botulism is a public health emergency, and immediately upon suspecting the diagnosis, the clinician should report the suspected case to the 24-h emergency telephone number of the state health department [1]. The state health department will initiate an epidemiologic investigation to determine the source of infection and to identify exposed persons, and it will also put the physician in contact with the Centers for Disease Control and Prevention's (CDC's) 24-h botulism consultancy service (table 1). The on-call CDC consultant will review the case with the clinician over the telephone and, if indicated, will help arrange for laboratory confirmation by testing appropriate specimens at a public health laboratory; the CDC will also arrange for shipment of antitoxin, which, in the United States, is available exclusively from the CDC [17]. The state health departments of California and Alaska maintain their own botulism clinical consultation services.
Protocols for clinicians evaluating suspected cases of botulism.
Infant botulism causes sporadic illness only and has no potential to cause an epidemic [25]. Clinical consultation and human-source antitoxin licensed for treatment of infant botulism are available on a 24-h basis from the California Department of Health Services' Infant Botulism Treatment and Prevention Program (telephone, 510-540-2646; table 1).
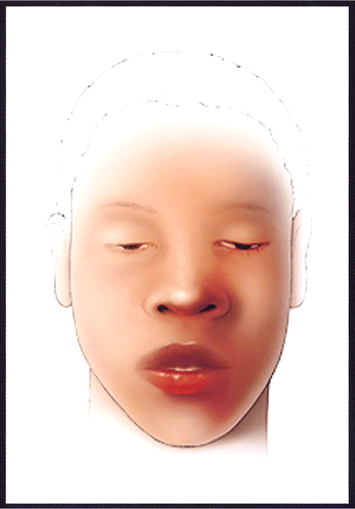
Cranial nerve palsies are invariably presenting symptoms of botulism (figure 1). The absence cranial nerve palsies or their onset after other true neurological symptoms have made their appearance rules out the disease. Extraoccular muscle paralysis is due to paralysis of cranial nerves III, IV, and VI and manifests as blurry vision or frank diplopia and as an inability to accommodate near vision. Ptosis is prominent. Paralysis of cranial nerve VII produces expressionless facies, and dysphagia is caused by cranial nerve IX paralysis, which may present as regurgitation (at times nasal) of masticated food or beverages. Dysarthria is prominent. Prominent autonomic symptoms include anhydrosis with severe dry mouth and throat (which may produce pronounced mucosal erythema and pain and which has been mistaken for pharyngitis) and postural hypotension. In some cases, pharyngeal collapse secondary to cranial nerve paralysis may compromise the airway and require intubation in the absence of respiratory muscle compromise. In foodborne botulism, particularly that involving toxin types B and E, the gastrointestinal symptoms nausea and vomiting may precede neurological symptoms. It is unknown whether these symptoms are caused by direct action of botulinum toxin, other products of C. botulinum, or some other contaminant of spoiled food. These symptoms have never been reported in cases of wound botulism [32], nor have corresponding signs been observed in primate experiments in which pure toxin was administered intragastrically or intravenously [13, 37–41]. Therefore, these symptoms may be absent in patients with illness resulting from exposure to pure toxin.
Artist's rendition of an adult patient with mild botulism. Note ptosis and facial paralysis, manifesting as youthful, unlined, and seemingly inexpressive facies. The patient is fully alert. (Illustrator, James K. Archer).
Cranial nerve palsies may be followed by flaccid, descending, completely symmetric paralysis of voluntary muscles, affecting (in order) the muscles of the neck, shoulders, the proximal and then distal upper extremities, and the proximal followed by distal lower extremities. Paralysis of the diaphragm and accessory breathing muscles may result in respiratory compromise or arrest. Eventual constipation is a nearly universal symptom; vital signs are usually normal, with preservation of normal range blood pressure possibly representing equilibrium between vagal blockade and extensive peripheral vasodilatation, both caused by the toxin. In some cases, hypotension occurs. Deep tendon reflexes progressively disappear. The ultimate extent of paralysis in untreated patients and the rapidity of progression are variable. Symptoms may be limited to a few cranial nerves or may progress to complete paralysis of all voluntary muscles, and symptoms may progress over hours to days, with the rate apparently proportional to the dose. Toxin binding is noncompetitive and irreversible. Nerve terminals do regenerate slowly, allowing for eventual full recovery in 95% of cases in the United States. Paralysis resolves in weeks to months and often requires extended outpatient rehabilitation therapy.
The sensory system is unaffected. Intellectual function is preserved throughout. Patients are able to respond appropriately to questions. Once intubated, they can continue communicating by signal using fingers or toes, so long as paralysis has not affected the digits. Tragically, in some instances, the patient's ptosis, expressionless facies, and altered voice have been interpreted as signs of mental status changes due to alcohol intoxication, drug overdose, encephalitis, or meningitis, and critical components of the history, including potential sources of toxin, were not sought by questioning. Because of skeletal muscle paralysis, patients who experience respiratory distress do not present with signs of agitation, such as restlessness, tossing, gasping, thrashing, or flailing, and they may appear to be placid and detached, even as they near respiratory arrest. Death in patients with untreated botulism results from airway obstruction from pharyngeal muscle paralysis and inadequate tidal volume, resulting from paralysis of diaphragmatic and accessory respiratory muscles.
Routine laboratory tests and radiological studies are not useful for diagnosis of botulism. Lumbar puncture reveals normal CSF values—in particular, the protein level is normal, in contrast to Guillain-Barré syndrome (GBS). Brain imaging studies may help rule out rare stroke syndromes that produce nonlateralizing symptoms. The Tensilon test helps for diagnosis of myasthenia gravis. In experienced hands, electromyography can be an exceedingly helpful adjunct to diagnosis. In affected muscles, findings consistent with neuromuscular junction blockage, normal axonal conduction, and potentiation with rapid repetitive stimulation are indicative of botulism [42].
Differential diagnosis.In the setting of an outbreak, in which several persons would present with signs and symptoms of botulism, the diagnosis readily suggests itself. The situation for the lone or sporadic patient with botulism (who may, in fact, be the herald case in a larger outbreak) is more precarious, because there is general unfamiliarity with the syndrome. However, if the diagnosis is considered, the clinician should immediately call the state health department emergency number, and expert consultation at no charge will be provided within minutes over the phone [1]. The differential diagnosis includes GBS, myasthenia gravis, stroke syndromes, Eaton-Lambert syndrome, and tick paralysis. Less likely conditions include tetrodotoxin and shellfish poisoning, antimicrobial-associated paralysis, and a host of conditions due to even rarer poisons. A thorough history and meticulous physical examination can effectively eliminate most competing diagnoses. GBS, a rare autoimmune, demyelinating polyneuropathy that follows an acute infection (with Campylobacter jejuni in one-third of cases), presents in 95% of cases as an ascending paralysis and never occurs in outbreaks [43]. Five percent of GBS cases present with the Miller Fisher variant; this is characterized by the triad of ophthalmoplegia, ataxia, and areflexia, which are easily mistaken for descending paralysis [44, 45]. A history of recent diarrheal or respiratory infection may be elicited, and in the case of the former, stool culture may yield an enteric pathogen, especially C. jejuni, although this is of no use in the acute setting. CSF protein levels are elevated in persons with all forms of GBS. However, these elevations may be delayed until several days after symptom onset, so a negative finding must be interpreted in light of the duration of symptoms, and lumbar puncture may need to be repeated. In experienced hands, electromyography may demonstrate findings consistent with GBS but not botulism in adult patients; electromyography should not be performed in infants. A strongly positive Tensilon test result, with or without presence of autoantibodies, confirms the diagnosis of myasthenia gravis. Borderline positive Tensilon test results have been reported for patients with botulism. In most cerebrovascular accidents, a careful physical examination will usually uncover asymmetry of paralysis and upper motor neuron signs; brain imaging studies can help reveal the rare basilar stroke that produces symmetric bulbar palsies. Eaton-Lambert syndrome usually manifests as proximal limb weakness in a patient already debilitated by cancer. Tick paralysis is a rare condition of flaccid paralysis that closely resembles botulism and that is caused by neurotoxins present in certain ticks. The ticks should be sought, especially on the scalp (but also on other parts of the body), and removal of the ticks reportedly results in rapid reversal of paralysis [46].
Laboratory diagnosis and confirmation.Confirmation of botulism rests on demonstration of the toxin in specimens of patient serum, gastric secretions, or stool or in a food sample [17]. Demonstration of C. botulinum in a patient's stool sample or in cultures of wound material is generally satisfactory for diagnosis of adult botulism syndromes and is considered definitive for diagnosis of infant botulism. The standard test is a bioassay involving intraperitoneal injection of toxin into mice and observation of the development of botulism-specific symptoms. This test can detect concentrations on the order of 1 MIPLD50/mL. Toxin type is determined by injecting a panel of mice with mixtures of test sample and a monoclonal type-specific antitoxin (e.g., anti-A or anti-B) and by observing which antitoxin confers protection on the mice. The mouse bioassay is performed in a limited number of public health laboratories. From the time that mice are injected, final results may not be available for 24 h or even 48 h. Accordingly, all clinical management decisions and initial public health interventions are determined solely on the basis of clinical diagnosis.
Clinical samples for suspected cases of foodborne botulism include serum (10 mL), vomitus or gastric secretions, stool (ideally ⩾25 mg), and suspect foods in original containers. For suspected wound botulism cases, samples include 10 mL of serum and anaerobic wound material; for infant botulism, stool is the preferred material [17]. The overall sensitivity of laboratory tests of clinical specimens has been reported to be as low as 33%–44% [47, 48] but varies inversely with the time elapsed between symptom onset and sample collection. Accordingly, when syndromes other than infant botulism are suspected, serum (at least one 10-mL red-top serum tube, spun and separated) should be obtained immediately, and it should always be obtained before administration of antitoxin, because antitoxin will neutralize all circulating toxin and render the test meaningless. If possible, the earliest available serum sample (such as the sample obtained for hospital admission bloodwork) should be salvaged and preserved for testing. Vomit samples should be collected, and if a nasogastric tube is placed, gastric secretions should be collected immediately. Because constipation almost always occurs, stool samples should be collected by means of a sterile water enema. These samples should be refrigerated but not frozen pending shipping directions from public health officials. In general, ingested toxin is not demonstrable in serum >1 week after exposure. Toxin can be isolated from stool samples farther in the course of illness, and the toxin is stable in many food matrices for a considerably longer period.
Therapeutics
Supportive intensive care.During the first decades of the 20th century in the United States, the mortality rate among patients with botulism was 60%–70%, even when equine antitoxin was administered in heroic doses. During the late 1940s and 1950s, the mortality rate decreased precipitously, until it reached the current rate of 3%–5% [20]. The difference resulted largely from the development of modern intensive care techniques—principally, mechanical ventilation. Persons with suspected botulism should be placed immediately in an intensive care setting, with frequent monitoring of vital capacity and institution of mechanical ventilation if required. Paralysis due to botulism is protracted, lasting weeks to months, and meticulous intensive care is required during this period of debilitation.
Antitoxin therapy.The only specific treatment for botulism is administration of botulinum antitoxin. Antitoxin can arrest the progression of paralysis and decrease the duration of paralysis and dependence on mechanical ventilation. Antitoxin should be given early in the course of illness, ideally <24 h after onset of symptoms [49, 50], because antitoxin neutralizes only toxin molecules that are yet unbound to nerve endings. Animal experiments confirm this relationship [13, 14, 38, 41]. Use of antitoxin is associated with adverse effects, including anaphylaxis, other hypersensitivity reactions, and serum sickness. Approximately 9% of persons treated in previous decades, when the recommended antitoxin dose was 2–4-fold higher than at present, experienced hypersensitivity reactions [51]. Of patients who were treated with 1 vial of antitoxin in the past few years, <1% experienced serious reactions. Before administration of antitoxin, skin testing should be performed to test for sensitivity to serum or antitoxin. Administration of 1 vial of botulism antitoxin produces serum levels of toxin type–specific antibodies capable of neutralizing serum toxin concentrations many-fold in excess of those reported for patients with botulism [52].
Given the high predictive value of objectively noted symmetric cranial nerve palsies in the setting of an outbreak, all previously healthy patients who have this symptom during an outbreak should received a diagnosis of probable botulism. During outbreaks, for reasons that remain uncertain, not all exposed persons manifest symptoms of botulism [53–55]. Toxin may be distributed unevenly in contaminated foods. However, host factors may play a role, because at least 1 person has had demonstrable levels of toxin in circulation without manifesting any clinical symptoms [53]. Persons who may have been exposed by consumption of implicated foods should be observed closely, and if they develop symptoms compatible with botulism, they should be treated with antitoxin immediately.
Isolation and infection control.Standard precautions should be exercised when evaluating and treating patients. Botulinum toxin cannot be absorbed through intact skin. Toxin can be absorbed through mucosal surfaces, the eye, and nonintact skin. No case of person-to-person transmission of botulinum has ever been described, including in patient care settings. Nevertheless, persons exposed to bodily fluids or stool from patients with botulism should be advised of the early signs of botulism and should report for evaluation if these are noted. At present, there is no licensed vaccine for botulinum toxins.
Conclusions
Regardless of the mode of exposure, botulinum toxins produce a distinctive syndrome of cranial nerve palsies that may be followed by descending flaccid paralysis. Effective treatment depends on provision of intensive care and rapid administration of botulinum antitoxin based on clinical presentation, because laboratory diagnosis is time-consuming. Free expert clinical consultation and antitoxin can be rapidly obtained from the CDC by contacting public health authorities (table 1). Rapid diagnostics and more-advanced specific therapy may accelerate diagnosis and facilitate treatment.
Acknowledgments
Potential conflicts of interest.J.S.: no conflicts.






Comments