-
PDF
- Split View
-
Views
-
Cite
Cite
Adrian Puren, Jay L. Gerlach, Bernhard H. Weigl, David M. Kelso, Gonzalo J. Domingo, Laboratory operations, specimen processing, and handling for viral load Testing and surveillance, The Journal of Infectious Diseases, Volume 201, Issue Supplement_1, April 2010, Pages S27–S36, https://doi.org/10.1086/650390
Close - Share Icon Share
Abstract
RNA remains the most informative and accurate biomarker for human immunodeficiency virus type 1 load diagnostics and for surveillance of drug resistance markers. Viral load testing by nucleic acid amplification currently is a complex and expensive test that is restricted to centralized laboratory testing. Successful extension of centralized viral load testing to rural or remote settings is a major challenge. Emerging nucleic acid-based technologies are progressing rapidly toward platforms appropriate for field use in low-resource settings, leaving a growing gap for sample processing technologies that complement them. One area in which new technologies could be applied to improve access is clinical specimen preservation and processing. Novel technologies that extract nucleic acid from clinical specimens and stabilize it at the point of specimen collection could fill this gap. In addition, these technologies may provide alternative viral load detection and surveillance solutions to the current centralized laboratory testing paradigm.
The human immunodeficiency virus (HIV) epidemic has galvanized several countries and organizations, either in collaboration or individually, to increase access to therapy in developing countries. This has included setting targets, such as theWorld Health Organization's (WHO's) 3 by 5 Initiative and “All by 2010,” or funding programs, such as the Global Fund for AIDS, Tuberculosis, and Malaria; the US President's Emergency Plan for AIDS Relief; Botswana's Masa program; and the South African Comprehensive Plan on Prevention Treatment and Care. Because it is closely linked to access to therapy, there has been significant recognition and effort to introduce laboratory-based testing as part of monitoring the efficacy of treatment and subsequent treatment failure, as reflected specifically by CD4 cell count and HIV-1 load testing and HIV-1 drug resistance testing [1–3].
Technologies for viral load testing have recently been reviewed [2, 4]. These can be categorized into 3 types of assays: (1) commercially available US Food and Drug Administration-approved or CE-marked nucleic acid- based tests (NATs), such as the Roche Amplicor HIV-1 Monitor test, the bioMe'rieux NucliSENS EasyQ System HIV-1 QT test, and the Bayer Versant HIV-1 RNA bDNA test; (2) in-house or “home-brew” NATs [5, 6]; and (3) nonnucleic acid-based tests, such as the Cavidi ExaVir Load HIV-specific reverse-transcriptase activity assay, shown to correlate well to viral load over all HIV- 1 clades [7, 8], and the Perkin Elmer Ultrasensitive p24 antigen detection assay, which still requires further validation [2, 4]
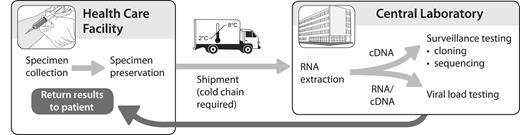
Currently, all NAT-based HIV-1 load testing in the developing world is performed at centralized facilities (Figure 1). This makes good sense, because central laboratories allow tests to be performed in optimal batch sizes in a carefully monitored, quality-controlled environment (discussed below). However, central laboratories are few in number, compared with the projected population that they must serve, and their reach is limited to patients whose samples can be effectively collected and transported to the laboratory and have the results transmitted correctly to their care provider.
Current paradigm for viral load testing. Specimens are preserved at the point of care and are typically shipped under cold chain to a central laboratory that has the facilities for high-throughput sample processing and nucleic acid amplification testing.
Attempts to streamline the operations of centralized laboratories must be complemented by new technologies and systems that extend the reach of laboratory testing facilities by enabling specimens to be collected far from the laboratory, enabling viral load testing at lower-level laboratories, and/or moving testing to the point of care (POC). Different settings will require different solutions.
Here, we present the benefits and challenges of extending the reach of centralized testing and focus on the particular challenges to ensuring safe specimen handling and processing for HIV-1 load NAT. We discuss a technology gap in sample processing that should be addressed as part of a solution to add versatility to the current options for viral load testing.
Centralized HIV-1 load testing
The current laboratory capacity and specific requirements that are unique to HIV-1 load testing in part determine the choice among centralized testing, decentralized testing, or combinations of centralized and regional or tiered testing in a country. Consideration can also be given to policy decisions on antiretroviral treatment adherence, HIV-1 drug resistance, and the type of viral load tests to perform. How to assess the scientific rationale for such decisions requires investigation. For example, clinical assessment of responses or use of CD4 cell count monitoring has not proven to be entirely beneficial, and thus, use of viral load testing to monitor responses is strongly favored [1]. How frequently viral load should be measured and which cutoff levels to use are part of a broader debate and are linked to discussions about tiers of testing [3, 4].
Current state of laboratory support in Africa. The documented approaches to quality assurance systems reflect the unevenness of laboratory systems in Africa. The differences can be seen in the same country, where state-of-the-art reference laboratories coexist with minimally resourced laboratories at lower levels of the health system. There is an urgent need to improve laboratory services in and among countries in Africa [9]. The reality is that resources through various efforts are required to establish the necessary infrastructure (eg, laboratory and transportation systems; recruitment, training, and retention of staff; and development and maintenance of quality assurance systems [9]). Various efforts by governments and/or consortia are currently under way at different levels to ensure that laboratory capacity needs are being met. These include the National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group, the National Health Laboratory Services (NHLS) in South Africa, the Joint Clinical Research Centre in Uganda, and the US President's Emergency Plan for AIDS Relief, through the US Centers for Disease Control and Prevention and partners.
Centralized testing: laboratory and throughput requirements. Since the introduction of commercial viral load testing in 1996, there have been technological advancements, particularly in highlevel automation, which involves walk-away systems that do not require high-level training. More recently, there have been steps toward providing in-house alternatives or less technically advanced approaches, such as the Cavidi assay [10, 11]. The decision to introduce a specific technology can be a fairly complex process. One of the advantages of introducing, for example, Food and Drug Administration-approved (United States) or CEmarked (European) technologies is that there is some form of traceable standardization, compared with in-house approaches. Selection of a particular HIV-1 load test should also be based on regional performance evaluations [12, 13].
The cost of the viral load assay is an important consideration.
A very detailed approach to costing was recently presented in the case of 2 assays: the Bayer Versant bDNA test and the Roche Amplicor PCR COBAS test performed with automated sample preparation on the COBAS AmpliPrep instrument at a central facility in the United States [14]. Such a detailed approach is important to avoid the various pitfalls when considering the introduction of high-end technology in centralized laboratories. The cost models developed by Elbeik et al [14] incorporate kit tests, disposables, equipment, equipment service plans and maintenance, equipment footprint, waste disposal, and labor. On the basis of these models, the throughput of samples tested by a facility has the greatest impact on cost per reportable test. The minimum number of controls required for each run and the configuration of the kit and platform dictate the optimum number of samples per run. The cost per reportable test for the bDNA test and the PCR test decreased from ∼$131 to ∼$60 and from ∼$140 to ∼$68, respectively, with the lowest costs for both tests resulting from using full kits per run [14]. A central facility with a high throughput of reportable tests is more likely to test the optimum number of specimens per run and have fewer suboptimal runs and, thus, sustain the best cost per reportable test. Additional costs that should be considered include external quality assurance and proficiency testing and internal quality control costs. Costs are not necessarily absolute and include the differential negotiated pricing by existing procurement processes. Service level agreements, access to technical assistance and support, and supply chain management are other factors that play a role in the final cost of testing.
Quality assurance as a key to laboratory support. A key aspect for ensuring quality results is the development of laboratory quality assurance systems. The important role of quality assurance and quality control in the context of introducing new technologies, such as HIV-1 load testing and CD4 cell count testing for HIV treatment and monitoring, is reviewed elsewhere [15]. The approach outlined for HIV testing is similar to that adopted in the development of HIV vaccine trial monitoring, as described elsewhere [16]. The emphasis in vaccine trial monitoring, as for diagnostic and therapeutic monitoring, is placed on the development of systems approaches to ensure high-quality data.
For centralized and/or regional testing, a limited number of laboratories can be monitored. Provided there is a good information infrastructure, it is possible to monitor external quality assurance and proficiency testing and internal quality control performance. For example, the National Institute for Communicable Diseases (the reference laboratory for the NHLS), in collaboration with Quality Control for Molecular Diagnostics, provides an NHLS-specific report that provides information on all participating NHLS laboratories' performance. For reporting, the current practice at the NHLS is that laboratories open resolution forms and perform analyses of the root cause if scores are lower than expected. The National Institute for Communicable Diseases and Quality Control for Molecular Diagnostics recently embarked on an internal quality control program that is supported using an informatics platform.
Staff requirements. Centralized testing using the abovementioned automated platforms requires staff with a high level of training and expertise, although this requirement is partly reduced by the ease of use of tests. Nevertheless, training on routine maintenance and repairs is a useful adjunct when instruments are placed and technical support is limited. In South Africa, training duration is usually 3–4 years at a technical university (technikon), followed by experiential training for up to 18 months. In certain instances, there is a requirement to register to practice, and the scope of practice is relatively well defined.
Data and specimen tracking. Centralized testing requires a well-developed information system to ensure that the incoming and outgoing flow of information (eg, patient information and reporting results) is captured consistently and that there is a capacity to store and integrate the information. The approach is both human resource and infrastructure intensive and requires back-up and maintenance systems, as do laboratories that perform the testing, in the form of back-up power and other requirements, such as barcode printers and barcode readers.
Data can reach sites from centralized testing locations electronically, provided that the informatics system is accessible to all sites. This requires human capacity and infrastructure. The alternative approach would be delivery of hard copies using the courier services that collect specimens. However, there must be systems in place to ensure that results are delivered as part of the quality assurance process. The interaction of geographically distant laboratories with clinical support needs to be in place in terms of access to results, interpretation of results, and support for clinical problems, such as requirements for drug resistance testing. The development of accessible communication systems in hard-to-reach areas can be achieved by using such tools as mobile telephones to provide both results and technical support via short message systems. Such systems need to be secure to ensure patient confidentiality. Recent advancements in the field include the TherapyEdge system (Advanced Biological Laboratories), which is currently under evaluation in South Africa. It is designed to provide information to clinic sites through servers using 3G mobile phone technology. The aim is to integrate laboratory data as part of clinical management at remote sites.
Specimen handling and shipment. Centralized and/or regional testing requires that specimens, such as whole blood, reach the laboratories in a timely manner, so that the integrity of the specimens is maintained and results are provided in a reasonable time. Approaches include the use of nucleic acid stabilizers, placement of specimens in cooler boxes, use of dried blood spots (DBSs), and assessment of patterns of collection (eg, tiered collection systems or direct collections from clinics or hospitals; discussed below). Laboratories with limited operating times may change to "shift work" to ensure that the specimens are processed to allow for a reasonable turn-around time. Such considerations have obvious implications in terms of human capacity costs, infrastructural overheads, and maintenance. The type of transportation that has to be used includes in-house transportation or courier services or a combination of both, as is the case for the NHLS in South Africa. Development of protocols, training of drivers, and provision of the necessary and sufficient collection materials (eg, cooler boxes and requisition slips) are essential. Correct management of couriers also requires attention. Additional considerations may be the type of vehicles used, such as standard delivery or pickup vehicles or motorcycles for hard-to-reach areas, and the overhead costs for these services.
Specimen preservation and processing technologies and impact on HIV load testing
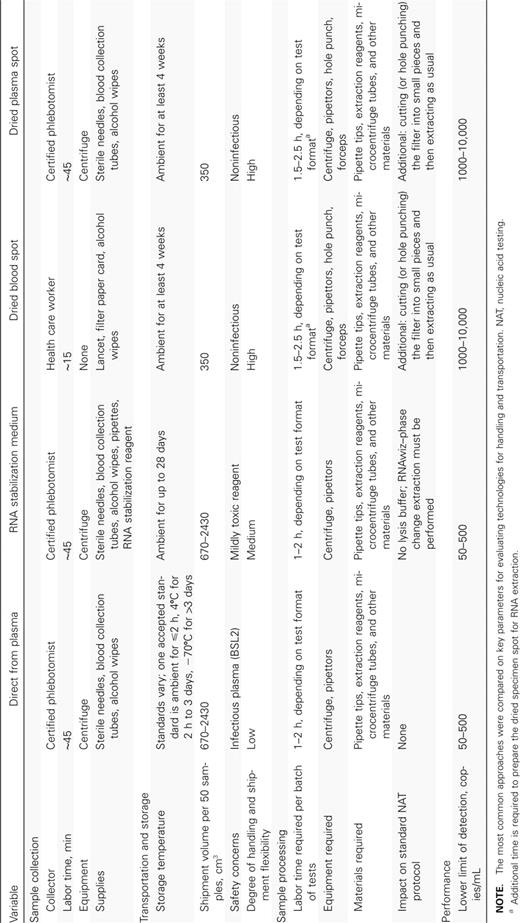
Standard protocols for RNA-based viral load testing call for RNA to be extracted directly from plasma. Performance of viral load testing in this fashion demands a great deal of resources to collect and store specimens properly and sound practices to ensure biosafety when handling specimens with live virus. Simpler technologies, such as filter spot preservation of specimens, have a large impact on the specimen handling costs (Table 1). A major determinant of the complexity and cost of specimen processing is the intended target limit of detection. Achieving a low limit of detection (50 copies/mL) requires processing of large specimen volumes (⩾1 mL of plasma). This introduces complexity and resource and/or reagent cost implications in the whole process, from specimen collection (venipuncture) and specimen shipment to RNA extraction. A higher target limit of detection (>1000 copies/mL) significantly reduces the complexity and resource and reagent costs for specimen collection (a fingerstick), specimen shipment, and RNA extraction.
Summary of Current Methods of Viral RNA Specimen Handling and Preservation for Viral Load Testing
Specimen collection and preservation. There is a need for the development of standard protocols and guidelines for evaluating new sample collection and preservation technologies specifically for HIV-1 load testing. There is, however, a consensus that EDTA plasma, compared with other anticoagulant plasmas, serum, or whole blood, is the better source for cellfree viral RNA determination [17–20]. PPT vacutainers (plasma preparation tubes; Beckton Dickinson) provide a good solution to plasma specimen preservation during shipment [17, 21]. Whole blood with EDTA and cell-free EDTA plasma can be stored at room temperature for up to 30 h, at 4°C for up to 14 days, and at −70°C for extended periods of time without significant decreases in viral load signal [17, 22]. In one study, chaotropic lysis buffer did not perform well against plasma EDTA [23], whereas RNAlater (Qiagen) has been shown to stabilize HIV RNA at room temperature for up to 28 days and to ensure a greater degree of integrity of the RNA sample [24].
The use of smaller blood volumes, such as from a finger prick or heelstick, if sufficient, would preclude the need for a phlebotomist for sample collection. Dried plasma spots (DPSs) or DBSs have been used extensively for DNA-based tests and, more recently, for viral RNA-based tests [25, 26]. This method has a number of benefits that make it useful in developing countries. After the blood or plasma spot has dried, the RNA is stable at ambient temperature for long periods (reported times have varied from 4 weeks to 1 year) and the virus is inactivated [27, 28]. DBSs have been demonstrated to be a very effective specimen preservation technology for diagnosis of HIV infection during infancy, either through viral RNA or DNA testing, with little impact on the sensitivity of the test [29, 30]. DBSs and DPSs were successfully evaluated for viral load testing, with good correlation between the 2 methods [25, 27, 31, 32]. Filter spots allow for greater flexibility in storage and transportation of specimens, and DBSs also eliminate the need for venipuncture and separation of plasma from cells and require inexpensive materials (a lancet and filter paper). The usefulness of DBSs and DPSs in therapy monitoring is still not clear [23, 33]. Efficiency of RNA extraction and reproducibility of viral load NATs from filter spots is dependent on both RNA extraction protocol and the downstream NAT. Further work is required to evaluate and standardize the different combinations of these 2 steps. Although contamination during excision of the filter spots can be averted [34], this step is still significantly cumbersome and labor intensive or requires expensive automated instrumentation. In addition, because the current limit of detection for viral load testing using DBSs or DPSs is an HIV-1 RNA load of 3 log10 or 4 log10 copies/mL, this technology may need further improvement to meet the requirements for therapy monitoring.
New technologies to stabilize specimens are emerging. Two such technologies are the SampleTanker (Technology Think Tank) [52] and RNAStable (Biomatrica). The latter claims to preserve purified RNA at 50°C for up to 4 months. As mentioned above, development of standard protocols for evaluating these and other new technologies for specimen preservation is required.
RNA extraction. A number of methods have been developed to extract viral RNA from plasma and to separate it from inhibitors that can impair amplification [35]. These generally involve (1) lysing virons and denaturing proteins, (2) adsorbing RNA on solid phases, (3) removing interferents, and (4) desorbing RNA from the solid phase. With plasma specimens, lysing and denaturing is done with chaotropic agents, such as guanidine thiocyanate; detergents, such as Triton X-100 or Tween 20; and the proteolytic enzyme Proteinase K. Alternatively, when plasma or whole blood is dried on 903 filter paper, RNA is released and protected from RNAases [31].
Particulate silica surfaces are widely used to adsorb RNA in the presence of alcohol, which reduces its solubility. Separations are accomplished either by flow-through columns packed with solid silica particles [36, 37] or magnetically by coating paramagnetic particles with silica surfaces [38, 39]. Alternatively, charged surfaces have been shown to efficiently adsorb RNA without the use of alcohol [40, 41]. After RNA is adsorbed, particles are washed with a series of buffers to remove inter fering substances. If alcohol is used in the initial step, it also must be eliminated by evaporation because it is a very potent inhibitor of polymerases. For silica surfaces, RNA is released by lowering the ionic strength of the buffer, and charged surfaces are switched off by changing the pH of the buffer.
Extraction of RNA from plasma is resource intensive, requiring specialized equipment, disposable reagents, and training. Analysis of the costs for performing viral load testing with commercial kits and in-house tests showed that sample preparation accounts for 65%–81% of labor required to perform a test and 60%–75% of the cost of disposables (above kit costs) [6, 14, 42]. In addition, commercially available kits require a substantial amount of disposables and instrumentation beyond those provided with the kit. Managing the labor requirements and the procurement logistics for RNA extraction in settings with medium to poor laboratory infrastructures becomes a significant barrier to introduction of HIV-1 load testing.
POC sample processing and stabilization
The requirement for cold chain during the shipment of viral specimens and the technical and resource demands for nucleic acid extraction are among several barriers to extending the reach of NAT to remote and low-resource settings. Easier, lowcost sample processing technologies that can “plug and play” into current and emerging NATs appropriate for low-resource settings and that simultaneously stabilize the viral nucleic acid may address some of these barriers.
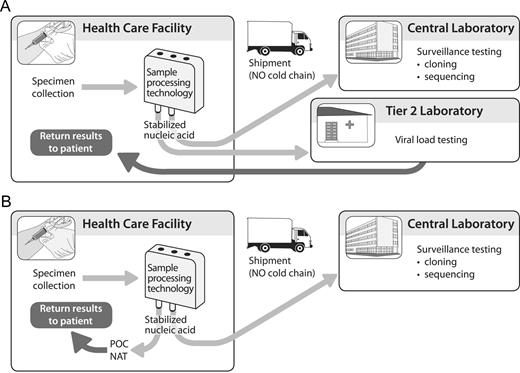
An affordable, easy-to-use, POC sample processing technology could distribute the labor required for sample processing from highly skilled laboratory technicians to health care providers, who are generally compensated at lower rates. In addition, this distribution of the work burden for middle-tier laboratories in combination with emerging low resource-requiring NATs, such as loop-mediated isothermal amplification platforms [43], may facilitate their ability to determine HIV-1 load (Figure 2A).
Added versatility to the current viral load testing systems through a low-cost, simple-to-use RNA extraction and stabilization technology. Such a technology could enhance the capacity of second-tier laboratories to perform viral load tests (A) or to feed into emerging point-of-care nucleic acid-based diagnostics (B).
Several research groups and private enterprises are developing POC nucleic acid technologies, some of which are highlighted in this supplement. Some platforms are complete in that they provide a solution for sample processing at the POC, whereas others offer only nucleic acid amplification and detection. A POC sample processing technology could be integrated with a diversity of POC platforms to provide a complete solution (Figure 2B). A POC sample processing technology could also extend the reach of surveillance efforts by providing stabilized nucleic acid (figure 2A and 2B). Of importance, a sample processing technology should contribute to providing a broad offering of versatile technology solutions to help test users adopt the solutions that best meet their needs.
Desired technology specifications
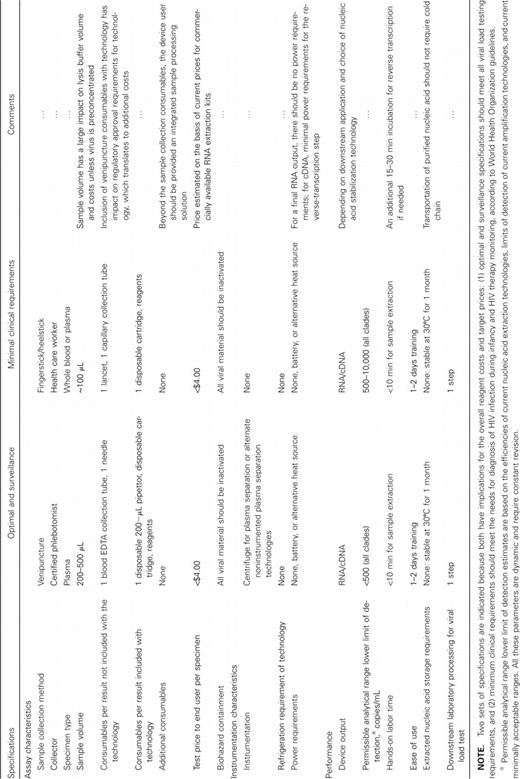
Product specifications for a low-cost viral load test are described elsewhere [1].We describe the product characteristics for a POC sample processing technology that extracts RNA from a clinical specimen at the point of specimen collection and stabilizes the RNA for HIV-1 load testing and surveillance purposes (Table 2). Key product specifications are defined by acceptability of the product to end users and to NAT developers and of the cost to manufacture. An integrated sample processing device holding all reagents may be more acceptable to users, but the manufacturing costs may be prohibitive.
Specifications for an Integrated Disposable Sample Processing Technology
Table 1 outlines product features for 2 potential technologies. Option A (optimal and surveillance) should meet the product specifications for clinical viral load applications, both for monitoring of therapy response and for diagnosis during infancy, and for surveillance applications and vaccine trial monitoring. Option B (minimal clinical requirements) would have a limit of detection inferior to standard viral load testing protocols but would offer a dramatically simplified sample collection. Option B is more limited in downstream applications but potentially significantly less costly, compared with option A. A true target price is not trivial to determine and should be based on detailed operational research and cost analysis of current practices, as suggested in “Future Research.” In the absence of detailed price and cost analyses, we suggest a price target in Table 2 that is based on the current prices for commercially available RNA extraction kits. We would expect a product offered at this price to generate savings, because additional disposables and reagents would not be required to perform extraction; they would be for commercially available RNA extraction kits. We would also expect cost savings to be realized by introducing logistical flexibility to testing systems (allowing sample batching) and by reducing the labor required to extract RNA and shifting this labor from the laboratory to the point of sample collection. It is more difficult to predict the cost implications of improvements in logistics and labor costs.
Rouet and Rouzioux [3] have suggested a 3-tiered approach to address the viral load testing needs. Each tier addresses a different testing need and has a panel of test types that are suitable to fulfill the need. Offering a variety of solutions to address the specific needs for different settings will be important in creating optimal testing systems.
Other uses
A versatile and modular sample processing technology may facilitate extension of surveillance and molecular diagnostics to remote settings for several key pathogens. RNA viruses are the culprits for several infectious diseases with a major global health burden (eg, influenza, hepatitis C, and HIV infection). For most of these pathogens, in addition to the diagnostic need, there is a strong public health need for surveillance through further analysis of the specimens in terms of virus typing and drug resistance marker monitoring [44–46]. Specifically for HIV-1, both viral RNA and DNA are required to identify circulating antiretroviral resistance markers [47, 48].
Future research
The acceptability of sample preparation methods that compromise sensitivity for cost and ease of use is heavily influenced by the clinically relevant limits of detection. Access to testing should perhaps not be sacrificed at the expense of maximizing performance beyond the clinical needs of a particular setting. Clear guidelines about clinically relevant limits of detection for each specific application will aid in developing new technologies that meet the criteria at the right price.
Elbeik et al [14, 42] provide a thorough cost analysis of testing in high- and middle-income areas. Similar analyses are needed for established viral load testing systems in low-income countries. Cost evaluations of viral load testing systems should take into account significant attrition in successfully reported test results because of sample loss, test result loss, and loss to follow-up [49–51]. Expanding the analysis to investigate sample collection, transportation, and storage and the efficiency with which results are correctly transmitted back to the patient will help identify opportunities for improvement of the testing system as a whole and not just the laboratory component. It would also be valuable to model the impact of potential solutions, such as enabling viral load testing at lower-level laboratories, moving sample processing to the point of specimen collection, or using a fully integrated POC viral load test. These models should include the cost of implementing appropriate quality assurance processes at this level. Determination of whether these improvements are likely to have favorable impacts and at what price point will help guide efforts to develop technologies to facilitate them. An alternative approach that is currently being studied in South Africa is the use of a high-end laboratory set-up for HIV-1 load and linked testing in converted mobile containers that can be taken into peripheral regions. The laboratories are linked to a central laboratory in terms of communications and monitoring. The feasibility and sustainability of such an approach is worth reviewing.
References
Author notes
Potential conflicts of interest: J.L.G., B.H.W., and G.J.D. are employees at PATH, a nonprofit organization. A.P. reports no conflicts.
Financial support: The Bill & Melinda Gates Foundation (48591 and 37774), United States Agency for International Development (GPH-A-00-01-00005-00), and the National Institute of Biomedical Imaging and Bioengineering (1U54EB007949- 01).
Supplement sponsorship: This article is part of a supplement entitled “Need for Point-of-Care HIV Molecular Diagnostic Technologies in Resource-Limited Settings,” which is based on the workshop “Novel Technologies in Rapid HIV-1 Viral Detection” and was sponsored by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services.