-
PDF
- Split View
-
Views
-
Cite
Cite
Mario Lorenz, Nicoline Jochmann, Amélie von Krosigk, Peter Martus, Gert Baumann, Karl Stangl, Verena Stangl, Addition of milk prevents vascular protective effects of tea, European Heart Journal, Volume 28, Issue 2, January 2007, Pages 219–223, https://doi.org/10.1093/eurheartj/ehl442
Close - Share Icon Share
Abstract
Aims Experimental and clinical studies indicate that tea exerts protection against cardiovascular diseases. However, a question of much debate is whether addition of milk modifies the biological activities of tea. We studied the vascular effects of tea, with or without milk, in humans and elucidated the impact of individual milk proteins in cell culture experiments, with isolated rat aortic rings and by HPLC analysis.
Methods and results A total of 16 healthy female volunteers consumed either 500 mL of freshly brewed black tea, black tea with 10% skimmed milk, or boiled water as control. Flow-mediated dilation (FMD) was measured by high-resolution vascular ultrasound before and 2 h after consumption. Black tea significantly improved FMD in humans compared with water, whereas addition of milk completely blunted the effects of tea. To support these findings, similar experiments were performed in isolated rat aortic rings and endothelial cells. Tea induced vasorelaxation in rat aortic rings and increased the activity of endothelial nitric oxide synthase by phosphorylation of the enzyme in endothelial cells. All effects were completely inhibited by the addition of milk to tea. Of the various kinds of milk proteins, the caseins accounted for these inhibiting effects of milk, probably by formation of complexes with tea catechins.
Conclusion Milk counteracts the favourable health effects of tea on vascular function. This finding indicates the need for particular awareness in the interpretation and design of studies comprising nutritional flavonoids.
Introduction
Consumption of tea has been inversely associated with cardiovascular morbidity and mortality.1 A broad body of evidence from experimental and clinical studies indicates that tea exerts antioxidative, anti-inflammatory, and vasodilating effects, thereby rendering protection against cardiovascular diseases.2,3 As the worldwide consumption of tea is second only to water, the beneficial effects of tea represent important public health issues. However, up to now, it is not known whether addition of milk to tea, as widely practiced in the UK, influences these vasoprotective properties. We therefore investigated the effects of tea with and without milk on endothelial function as a sensitive parameter of vascular wall homeostasis.4 Many pathophysiological conditions in the cardiovascular system are characterized by attenuated production of protective vasoactive substances in endothelial cells, resulting in a condition known as endothelial dysfunction.5 Undisturbed flow-mediated dilation (FMD) of human blood vessels is a hallmark of a functional endothelium, with impairment of FMD representing an early marker of vascular dysfunction.4,6,7
We address the question whether milk affects the beneficial effects of tea on endothelial function. We show here that black tea significantly improves endothelial function in humans, whereas the addition of milk completely blunts this amelioration. In our in vitro experiments, we demonstrate that milk caseins account for these inhibiting effects of milk.
Methods
Study design
Postmenopausal women were recruited by press advertisements. A total of 229 women responded, and interviews were conducted with these women. Subjects with chronic diseases and known cardiovascular risk factors such as high blood cholesterol, diabetes, arterial hypertension, and obesity as well as with certain food patterns (to exclude high regular tea consumption and vegetarian lifestyle) were excluded. A total of 42 women were invited for clinical examination. All measures of clinical parameters were required to be within the normal range to allow study inclusion: lipid profile, blood pressure, body mass index, as well as routine internal parameters such as thyroidea-stimulating hormone and sexual steroids. A total of 16 healthy women (mean age 59.5 ± 5) completed the study. None of the participants had taken medication for at least 3 months before entering the study. The participants were asked to refrain from consuming tea 4 weeks before and during the study. Study subjects were required to make three clinical visits, at least 3 days apart and at the same time of day (i.e. 8 a.m. after fasting overnight). Each subject consumed 500 mL of boiled water, freshly brewed black tea (with 10% water to achieve the same dilution as tea with milk), or black tea with 10% skimmed milk in a crossover study design. Five grams of tea leaves (Darjeeling black tea, provided by King's Teagarden, Berlin, Germany) were brewed for 3 min with 500 mL of boiled water. All participants were required to complete all three visits. FMD and nitro-mediated dilation (NMD) were measured before and 2 h after consumption of the beverages. The participants had a standardized breakfast (one croissant) while they drank the beverage. The study was approved by the Charité University Hospital Ethics Committee, and participants provided their written informed consent.
Endothelial function in humans
Endothelial function was measured by high-resolution vascular ultrasound (Siemens Sonoline Antares), with use of a 13 MHz linear array transducer. Endothelium-dependent FMD was assessed by measuring the change in the brachial artery diameter during reactive hyperaemia after cuff occlusion of the forearm for 5 min, according to established guidelines.8 The nature of the stimulus and conditions for the measurement of FMD were chosen to obtain a nitric oxide-dependent response after a brief period of shear stress in the brachial artery.9,10 Changes in the arterial diameter were measured every 15 s for up to 2 min. FMD was defined as the maximum percentage change in diameter compared with baseline measurement. Endothelium-independent vasodilation (NMD) was measured after sublingual application of nitroglycerin spray (0.4 mg). The increase in diameter of the brachial artery was measured from 1–6 min, and NMD was defined as the maximum percentage change in diameter compared with baseline measurement. Ultrasound images were digitized online and saved. Analyses of diameter changes were conducted offline (Tom Tec Imaging Systems) by two different investigators blinded to subject treatment.
Endothelial nitric oxide synthase activity and phosphorylation in endothelial cells
Bovine aortic endothelial cells (BAEC) were maintained in EGM-MV (endothelial cell growth medium), supplemented with 5% fetal bovine serum, 10 pg/mL endothelial growth factor, 1 µg/mL hydrocortisone, 12 µg/mL bovine brain extract, and 0.1% gentamicin. For experiments, cells in 6 cm dishes were incubated for 30 min in 1.5 mL HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffer, pH 7.4, containing, in mmol/L, NaCl 145, KCl 5, MgSO4 1, HEPES-Na 10, glucose 10, and CaCl2 1, followed by treatment with 100 µL of black tea for 15 min. Tea and tea with milk were prepared as described earlier. Individual milk proteins were added to tea at 900 µg/mL. Endothelial nitric oxide synthase (eNOS) activity was assessed by formation of l-[3H]citrulline from l-[3H]arginine after separation of the amino acids by cation-exchange chromatography, as described.11 Briefly, stimulation was initiated by addition of tea beverages, 10 µmol/L cold l-arginine, and 3 µCi/mL l-[3H]arginine. After 15 min, the reaction was terminated with ice-cold stop solution containing 5 mmol/L l-arginine and 4 mmol/L EDTA. Cells were denatured with 96% ethanol, and after evaporation, the soluble cellular components were extracted with 20 mmol/L HEPES-Na, pH 5.5. l-[3H]citrulline was separated from l-[3H]arginine by Dowex chromatography, and l-[3H]citrulline formation was quantified by liquid scintillation counting.
For western blots, cells were treated as described earlier, washed twice with phosphate buffered saline, and lysed in buffer containing, in mmol/L, HEPES 20 (pH 7.9), NaCl 100, Na3VO4 1, sodium pyrophosphate 4, EDTA 10, phenylmethylsulfonyl fluoride 1, NaF 10, okadaic acid 0.1, and 1% Triton X-100. Total protein (15 µg per lane) was subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis, and membranes were probed with anti-phospho-eNOS (Ser1179) antibody (1:1000) from Cell Signaling. Bands were visualized by using BCIP (5-bromo-4-chloro-3-indolyl phosphate, p-toluidine salt) and Nitro Blue Tetrazolium (Sigma).
Vasorelaxation studies in isolated rat aortic rings
Thoracic aortas from male Wistar rats were rapidly excised, cleaned of connective tissue, and cut into rings 2–3 mm in length for organ-chamber experiments. The rings were then mounted on platinum hooks in 10 mL jacketed organ baths containing modified Krebs–Henseleit solution (composition, in mmol/L, NaCl 144, KCl 5.9, CaCl2 1.6, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, and d-glucose 11.1) and 1 µmol/L diclofenac. Tension was gradually adjusted to 2 g over 1 h. The solution in the bath was maintained at 37°C with a gas mixture of 5% CO2 and 95% O2. Following equilibration and submaximal precontraction with phenylephrine (0.05 µmol/L), relaxation to cumulative doses (5–50 µL) of black tea was performed in a volume of 10 mL Krebs–Henseleit solution. Preparation of the various tea beverages was as described earlier, with the exception that 300 µg/mL of individual milk proteins were used. Selected studies were conducted in rings treated with the NOS inhibitor N-nitro-l-arginine methyl ester (L-NAME, 1 mmol/L) before phenylephrine exposure. Vasorelaxation is expressed as percentage of precontraction with phenylephrine.
Concentrations of individual tea compounds
Tea was prepared as described in study protocols. The concentrations of individual tea substances in brewed tea, in the supernatant of tea with or without addition of 10% milk after centrifugation of the beverage at 13 000 g for 20 min, were determined as described with slight modifications.12 In brief, tea samples were diluted with 10% acetonitrile containing 500 µg/mL EDTA and ascorbic acid. The samples were analysed by HPLC. The HPLC detection system consisted of an Agilent 1100 (Agilent Technology, San Diego) with a binary pump, thermostatted autosampler, column oven, photodiode array detector, and a data system with Agilent 1100 ChemStation software. The column was eluted at 35°C with a binary gradient of 100% solution A (9% acetonitrile, 2% acetic acid, containing 20 µg/mL EDTA) for 10 min, 68% solution A and 32% of solution B (80% acetonitrile, 2% acetic acid, containing 20 µg/mL EDTA) for 10 min at a flow rate of 1.0 mL/min. The eluent was monitored at 278 nm. The signals were verified using UV spectra (dioden array detector) and comparisons of the retention times with reference compounds. Quantification was carried out using the relative response factor (RRF) concept of ISO 14505:2.
Statistical analysis
A two-step testing procedure was performed for the comparison of the three beverages: water, black tea, and tea with milk. In cases of overall significance, pairwise tests were performed for the three possible comparisons between beverages. For three conditions and overall significance, no further adjustment for multiple testing was necessary. Since time points and beverages were not balanced, all comparisons between beverages were adjusted for a time effect by use of a linear model. The dependent structure of the data was taken into account by use of generalized estimating equations. No time effects or time vs. treatment interactions were found. All statistical tests were two-sided (level of significance 0.05). The statistical analysis was performed using SPSS, Release 12.0.1, and SAS, Release 9.1. All values are expressed as mean ± SD in the table and mean ± SEM in the figures.
Results
Endothelial function in human volunteers
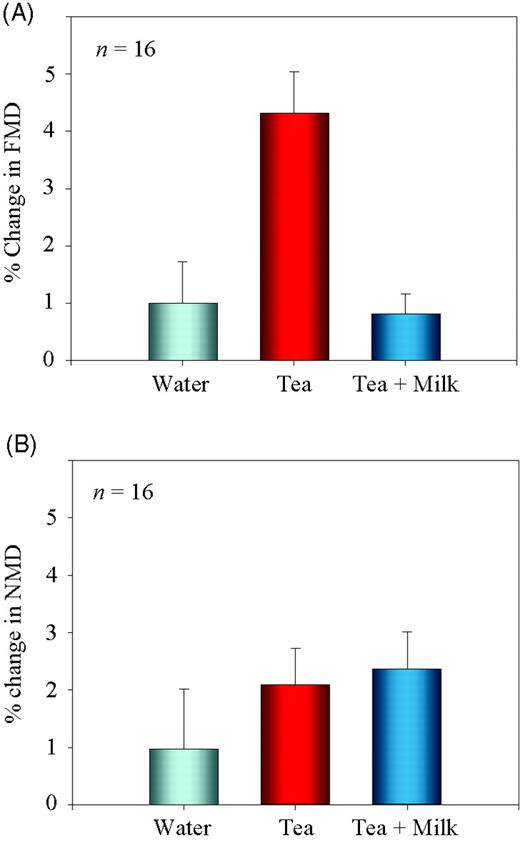
Baseline characteristics of the study group are summarized in Table 1. Endothelial function was assessed by measuring FMD of the forearm brachial artery in 16 healthy females, before and 2 h after ingestion of 500 mL water (control), black tea alone, or black tea with 10% milk in a crossover study. Whereas tea significantly increased FMD compared with control, this effect was completely prevented by ingestion of tea with milk (Figure 1A). In contrast, endothelial-independent vasodilation (NMD) was not affected by addition of milk to tea (Figure 1B).
Changes in FMD and NMD after consumption of tea with and without milk in humans. (A) A total of 16 volunteers consumed water, black tea, or black tea with 10% milk, and changes in FMD were measured. FMD significantly (P < 0.01 compared with water) increased after consumption of black tea. Addition of milk to tea suppressed the vasodilatory effects of tea (P < 0.01 compared with tea alone). (B) Beverages were consumed as in (A), and NMD was measured after sublingual application of nitroglycerin spray (0.4 mg). FMD and NMD were defined as the maximum percentage change in diameter compared with baseline measurement.
Baseline characteristics of the study population
| . | Mean ± SD (n = 16) . |
|---|---|
| Age (years) | 59.5 ± 5 |
| Height (cm) | 162 ± 6 |
| Weight (kg) | 61 ± 6 |
| Body mass index (kg/m2) | 23 ± 2 |
| Diastolic blood pressure (mmHg) | 73 ± 8 |
| Systolic blood pressure (mmHg) | 117 ± 14 |
| . | Mean ± SD (n = 16) . |
|---|---|
| Age (years) | 59.5 ± 5 |
| Height (cm) | 162 ± 6 |
| Weight (kg) | 61 ± 6 |
| Body mass index (kg/m2) | 23 ± 2 |
| Diastolic blood pressure (mmHg) | 73 ± 8 |
| Systolic blood pressure (mmHg) | 117 ± 14 |
Baseline characteristics of the study population
| . | Mean ± SD (n = 16) . |
|---|---|
| Age (years) | 59.5 ± 5 |
| Height (cm) | 162 ± 6 |
| Weight (kg) | 61 ± 6 |
| Body mass index (kg/m2) | 23 ± 2 |
| Diastolic blood pressure (mmHg) | 73 ± 8 |
| Systolic blood pressure (mmHg) | 117 ± 14 |
| . | Mean ± SD (n = 16) . |
|---|---|
| Age (years) | 59.5 ± 5 |
| Height (cm) | 162 ± 6 |
| Weight (kg) | 61 ± 6 |
| Body mass index (kg/m2) | 23 ± 2 |
| Diastolic blood pressure (mmHg) | 73 ± 8 |
| Systolic blood pressure (mmHg) | 117 ± 14 |
Measuring of eNOS activity in endothelial cells
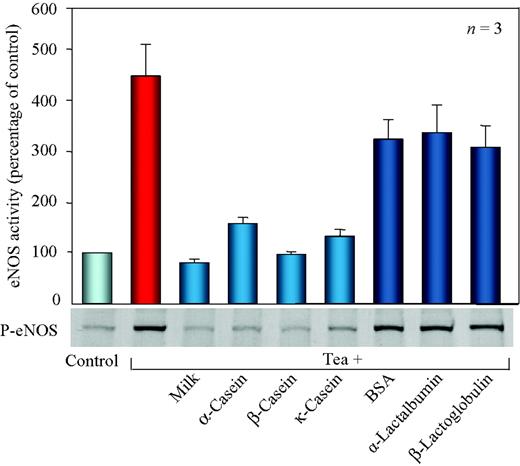
FMD is evoked by nitric oxide generated by eNOS during a short period of shear stress.13,14 We therefore measured generation of nitric oxide in response to tea with or without milk in cell culture experiments. Tea increased eNOS activity to more than 400% in BAEC. Addition of 10% milk to tea completely eliminated the rise in eNOS activity (Figure 2, upper panel). To identify the milk compounds responsible for the inhibiting effects, we performed experiments with single milk proteins. The major proteins in milk are α-casein (13 mg/mL), β-casein (9.3 mg/mL), κ-casein (3.3 mg/mL), α-lactalbumin (1.2 mg/mL), β-lactoglobulin (3.2 mg/mL), and serum albumin (0.4 mg/mL).15 Individual milk proteins were added to tea at 900 µg/mL to avoid the influence of different amounts of protein per se. This amount corresponds to 9 mg/mL of protein concentration in the milk, after 10% of milk is added to tea. All three caseins blunted eNOS activity to an extent similar to milk, whereas equal amounts of bovine serum albumin (BSA), α-lactalbumin, and β-lactoglobulin had little effects (Figure 2, upper panel). The activity of eNOS is regulated by phosphorylation of the enzyme.16 Correspondingly, milk and single milk caseins blocked tea-induced eNOS phosphorylation at Ser1179, whereas the other three milk proteins were without effect (Figure 2, lower panel).
Effect of milk and individual milk proteins on tea-induced nitric oxide production in endothelial cells. Cells were treated for 15 min with the indicated beverages, and eNOS activity was measured (upper panel). Western blot showing the phosphorylation of eNOS (lower panel) was performed with an antibody specific to the eNOS Ser1179 phosphorylation site. The western blot is representative from three independent experiments.
Vasorelaxation in rat aortic rings
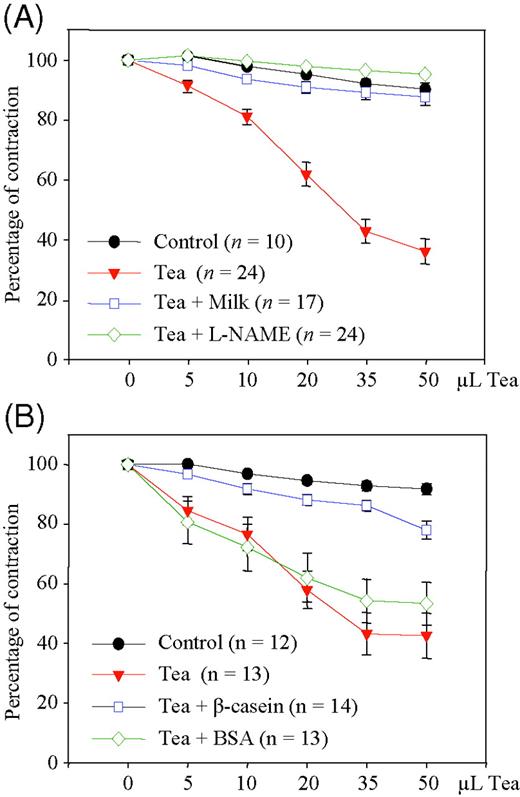
To extend these findings to a functional model, we determined vasodilation in isolated rat aortic rings. Addition of tea cumulatively relaxed precontracted rat aortic rings, which was prevented by the NOS-inhibitor L-NAME. Similarly, addition of 10% milk completely inhibited tea-induced relaxation (Figure 3A). In corroboration of our data in endothelial cells, addition of β-casein to tea inhibited relaxation of aortic rings, whereas BSA had little effect (Figure 3B).
Tea-induced nitric oxide-dependent vasorelaxation in rat aortic rings after addition of milk and individual milk proteins to tea. (A) Precontracted rat aortic rings were treated with water (control), tea alone, or tea with 10% milk, and vasorelaxation was determined. L-NAME indicates NOS-inhibitor. Vasorelaxation is expressed as percent of contraction. (B) Rat aortic rings were treated with water (control), tea alone, tea with β-casein, or tea with BSA, and vasorelaxation was determined as in (A). Data are mean ± SEM from the indicated numbers of experiments.
Interaction of milk with tea compounds
Tea polyphenols have been shown to interact with proteins.17 In an attempt to elucidate the underlying mechanism for the diminished biological activities of tea after addition of milk, we measured the concentrations of various tea compounds, including the catechins, in our tea preparations. The concentrations of single tea compounds were determined before, and in the supernatants, after centrifugation of the tea beverages at 13 000 g for 20 min. Contents of individual tea compounds without addition of milk did not significantly change upon centrifugation. However, addition of 10% milk to tea selectively and markedly decreased the concentrations of various catechins in tea, whereas the contents, for example, of caffeine, gallic acid, and others were not affected (Table 2).
Concentrations of tea ingredients (µmol/L)
| . | TB . | TG . | Gallic acid . | Caffeine . | EC . | EGC . | ECG . | EGCG . |
|---|---|---|---|---|---|---|---|---|
| Tea | 40 | 136 | 91 | 1034 | 50 | 70 | 116 | 324 |
| Tea centrifugationa | 39 | 139 | 93 | 1035 | 40 | 52 | 117 | 322 |
| Tea + milk centrifugationa | 40 | 138 | 93 | 995 | 8 | 47 | 27 | 62 |
| . | TB . | TG . | Gallic acid . | Caffeine . | EC . | EGC . | ECG . | EGCG . |
|---|---|---|---|---|---|---|---|---|
| Tea | 40 | 136 | 91 | 1034 | 50 | 70 | 116 | 324 |
| Tea centrifugationa | 39 | 139 | 93 | 1035 | 40 | 52 | 117 | 322 |
| Tea + milk centrifugationa | 40 | 138 | 93 | 995 | 8 | 47 | 27 | 62 |
TB, theobromine; TG, theogallin; EC, epicatechin; EGC, epigallocatechin; ECG, epicatechin gallate; EGCG, epigallocatechin gallate.
aTea was centrifuged at 13 000 g for 20 min.
Concentrations of tea ingredients (µmol/L)
| . | TB . | TG . | Gallic acid . | Caffeine . | EC . | EGC . | ECG . | EGCG . |
|---|---|---|---|---|---|---|---|---|
| Tea | 40 | 136 | 91 | 1034 | 50 | 70 | 116 | 324 |
| Tea centrifugationa | 39 | 139 | 93 | 1035 | 40 | 52 | 117 | 322 |
| Tea + milk centrifugationa | 40 | 138 | 93 | 995 | 8 | 47 | 27 | 62 |
| . | TB . | TG . | Gallic acid . | Caffeine . | EC . | EGC . | ECG . | EGCG . |
|---|---|---|---|---|---|---|---|---|
| Tea | 40 | 136 | 91 | 1034 | 50 | 70 | 116 | 324 |
| Tea centrifugationa | 39 | 139 | 93 | 1035 | 40 | 52 | 117 | 322 |
| Tea + milk centrifugationa | 40 | 138 | 93 | 995 | 8 | 47 | 27 | 62 |
TB, theobromine; TG, theogallin; EC, epicatechin; EGC, epigallocatechin; ECG, epicatechin gallate; EGCG, epigallocatechin gallate.
aTea was centrifuged at 13 000 g for 20 min.
Discussion
The most striking finding of our study is that addition of milk to black tea completely prevents the biological activity of tea in terms of improvement of endothelial function. Our results thus provide a possible explanation for the lack of beneficial effects of tea on the risk of heart disease in the UK, where milk is usually added to tea.18 To follow the common practice of drinking tea in the UK, we added 10% milk to our black tea preparations. The well-established health benefits of tea, as described by numerous studies,1–3 are mainly attributed to various flavonoids, especially catechins.19 Previously, we have shown that the major catechin in tea, epigallocatechin-3-gallate (EGCG), induces eNOS activation in endothelial cells and leads to vasorelaxation in rat aortic rings.11 In the present study, we found a particular, selective decrease in the concentrations of a number of catechins after centrifugation of freshly brewed tea with milk compared with tea without milk, which suggests complex formation between catechins and milk proteins. Concentrations of other tea compounds were not affected by addition of milk. It was previously reported that polyphenols can bind to proteins,20,21 and interaction between flavanoids and proteins affects their antioxidant capacity in vitro.22 There is some evidence that polyphenols possess a high binding affinity for proline-rich proteins such as caseins.23 A very recent study demonstrated the non-covalent cross-linking of EGCG by caseins, emphasizing the interaction of tea catechins with milk caseins.24
Interactions of food-derived flavonoids with milk proteins may impede their physiological effects. An in vitro gastrointestinal model to simulate the conditions in the human digestive tract demonstrated that addition of milk to tea inhibited its antimutagenic activity.25 Tea possesses strong antioxidant properties, in vitro and in vivo, that are affected by addition of milk.26,27 In analogy, consumption of dark chocolate that contains epicatechin, but not milk chocolate or dark chocolate with extra milk, increased the antioxidant capacity of human blood plasma.28 However, it should be noted that other studies failed to establish an effect of milk on antioxidant properties of tea.29,30 The reasons for these discrepant findings are largely unknown but may be attributed to different physiological/experimental endpoints (e.g. in vitro and in vivo, and assays for measurements of antioxidant activities).
In conclusion, milk may counteract the favourable health effects of tea on vascular function. The finding that the tea-induced improvement of vascular function in humans is completely attenuated after addition of milk may have broad implications on the mode of tea preparation and consumption. In addition, it indicates that caution is warranted in the design of studies involving nutritional flavonoids.
Acknowledgements
There was no funding or support for this study. We are grateful to Thomas Düsterhöft, Angelika Vietzke and Wanda Michaelis for their excellent technical assistance.
Conflict of interest: none declared.