-
PDF
- Split View
-
Views
-
Cite
Cite
Kinya G. Ota, Shigeru Kuratani, Cyclostome embryology and early evolutionary history of vertebrates, Integrative and Comparative Biology, Volume 47, Issue 3, September 2007, Pages 329–337, https://doi.org/10.1093/icb/icm022
Close - Share Icon Share
Abstract
Modern agnathans include only two groups, the lampreys and the hagfish, that collectively comprise the group Cyclostomata. Although accumulating molecular data support the cyclostomes as a monophyletic group, there remain some unsettled questions regarding the evolutionary relationships of these animals in that they differ greatly in anatomical and developmental patterns and in their life histories. In this review, we summarize recent developmental data on the lamprey and discuss some questions related to vertebrate evolutionary development raised by the limited information available on hagfish embryos. Comparison of the lamprey and gnathostome developmental patterns suggests some plesiomorphic traits of vertebrates that would have already been established in the most recent common ancestor of the vertebrates. Understanding hagfish development will further clarify the, as yet, unrecognized ancestral characters that either the lampreys or hagfishes may have lost. We stress the immediate importance of hagfish embryology in the determination of the most plausible scenario for the early history of vertebrate evolution, by addressing questions about the origins of the neural crest, thyroid, and adenohypophysis as examples.
Introduction—phylogeny and evolution
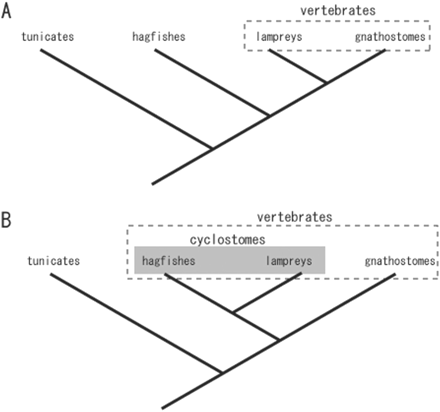
In their basal position on the phylogenetic tree of the vertebrates, the extant agnathans (the lampreys and the hagfish) are considered important for any understanding of the history of the vertebrates (reviewed by Kuratani et al. 2002). Vertebrates, in this context, can be defined as having a clearly differentiated head (as the taxonomic term “craniate” implies), peripheral nerve ganglia, a neural crest and its derivatives, placodes and their derivatives, a thyroid gland, and a well-developed skeletal system, all of which the cephalochordates and urochordates lack. The origins of the neural crest and placodes are especially central issues because these embryonic structures contribute to the development of the characters specific to the head (Gans and Northcutt 1983). In this context, the phylogenetic relationships between the lampreys, the hagfish, and the gnathostomes are a fundamental problem.
Until recently, the hagfish have tended to be placed as a sister group of the vertebrates based mainly on their lack of vertebrae (Fig. 1). In the strict sense, the hagfish is an invertebrate, and the taxon “craniates” can be applied to the group consisting of vertebrates and the hagfish (Janvier 1996). Other than their lack of vertebrae, the hagfish exhibit apparently primitive patterns in their anatomy (Janvier 1996; see subsequently), which may, of course, simply reflect a secondary degenerative condition in this animal. We should also remember that the lampreys also lack a cartilaginous skeleton in the trunk in the larval stages, and even after metamorphosis, only the neural-arch-like nodules of cartilage arise in the trunk, and no ventral vertebral elements differentiate in the lamprey, unlike the condition in all the gnathostome species. In terms of vertebral development, the hagfish and the lampreys may represent a similar state of skeletal evolution.
Phylogenetic relationships among the gnathostomes, the lampreys, hagfish, and tunicates. Morphological data tend to cluster the gnathostomes and the lampreys in the same group, “Vertebrata,” because of the presence of vertebrae (A). Molecular phylogenetic analysis supports “Cyclostomata,” consisting of the lampreys and hagfish, as the monophyletic group (B).
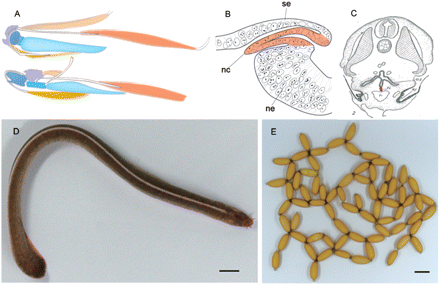
Both the lampreys and the hagfish may have undergone extensive modifications to their anatomy as a result of their accommodatation to their specialized modes of life (burrowing, parasitic), which tend to obscure any phylogenetic relationships based only on anatomical features that are absent or apparently primitive. Therefore, it is important to focus on the more organized and derived features shared by the lampreys and the hagfish, as Yalden (1985) showed explicitly for the oral apparatus (Fig. 2A). According to that author, the muscular and skeletal elements can be strictly homologized between these two animals, although the functions of these organs differ greatly in the two groups, implying that these animals share a fundamental pattern of mesenchymal differentiation, at a very basic level of their development. Functional and morphological diversity based on homologous embryological elements is reminiscent of the mammalian middle ear that evolved from a the basic developmental pattern of the shared gnathostome mandibular and hyoid-arch skeletons (Goodrich 1930; Allin 1975; also see Tucker et al. 2004 for the shared dorsoventral patterning mechanism of the mandibular arch in amniotes). Mammals are still regarded as gnathostomes since the equivalent jaw joint can be identified in the articulation between the malleus and incus. Considering the morphological and organizational complexity of the shared morphological plan, the cyclostome oral region may represent a true synapomorphy that supports their monophyly, but not the pre-gnathostome state of agnathans.
Morphological and histological features of hagfishes. (A) Comparison of the feeding apparatus between lampreys (upper) and hagfish (lower). Diagrammatic representations of the muscles and skeletal elements comprising the mandibular-arch-derived feeding organs seen from left lateral view. Homologous elements are indicated by the same color. Redrawn from Yalden (1985). (B) A diagrammatic illustration of the putative neural crest (red) in an early embryo of Eptatretus stouti. The neural crest (nc) of this animal is believed to grow as an epithelial pocket arising between the surface ectoderm (se) and the neural tube (ne). Modified from Conel (1942). (C) An Illustration of a section of an E. stouti embryo at the level of the fifth gill, with the thyroid Anlage colored red. Modified from Stockard (1906b). (D) Inshore hagfish species, E. burgeri, kept in our aquarium. It was collected from the shallow-water seas off Shimane Prefecture, Japan (60 m deep). (E) Deposited eggs of E. burgeri. All the eggs were obtained in November 2005 from a female maintained in the aquarium. Scale bars: 1 cm.
It is, of course, possible that such a highly organized oral pattern in the cyclostomes (Janvier 1996) represents a plesiomorphic state of the vertebrates, which leads to the conclusion that the gnathostomes are secondarily simplified animals in terms of their oral patterning. The evaluation of comparative anatomical data such as these will require more information about the internal structures of fossil agnathans. Importantly, if the hagfish and the lampreys really constitute a monophyletic cyclostome group that diverged from the basal position on the vertebrate tree, most fossil agnathans will have to be regarded as stem gnathostomes. Therefore, the split between the cyclostomes and the gnathostomes will not be equivalent only to the possession of the jaw (Janvier 1996).
Molecular data also seem to favor the monophyly of the cyclostomes (Fig. 1). Stock and Whitt (1992) first pointed out this possibility from the nucleotide sequences of ribosomal RNA. Similar conclusions were then drawn by Mallatt and Sullivan (1998), Furlong and Holland (2002), and Takezaki et al. (2003) using multiple genes (for the molecular phylogeny of cyclostomes, also see Kuratani et al., 2002 and Kuraku and Kuratani, 2006). Notably, it has recently been found that hagfish and lampreys share a similar mechanism of generating the variable lymphocyte receptors composed of highly diverse leucine-rich repeats, in contrast to the Ig-based system for adaptive immune responses shared by gnathostomes (Pancer et al. 2004, 2005). The latter findings also strengthen the monophyly of cyclostomes. In the present article, we will use the taxonomic name “cyclostome” to represent a monophyletic clade that includes the modern lampreys and the hagfish, and therefore the taxon “vertebrates” will also include the hagfish.
Lamprey and evolutionary developmental studies
A character that is shared by the lampreys and the gnathostomes is likely to represent a feature established in the most recent common ancestor of all vertebrates. If this feature is not shared by the nonvertebrate chordates, like the tunicates and amphioxus, it is highly likely that it represents a key to understanding the evolutionary origin of the vertebrates, and a change in their developmental program (Kuratani et al. 2001; Murakami et al. 2004).
The embryology of the lamprey has a long history, and it has often been used to demonstrate that this animal is either an intermediate form linking amphioxus and the shark (Alcock 1898; Neal 1897, 1914; Damas 1944), or a typical vertebrate, sharing the same embryonic structures observed in elasmobranch embryos (Koltzoff 1901; for “elasmobranch worship” in comparative vertebrate embryology, see Gee 1996). These studies commonly emphasize the presence of mesodermal head segments, which are primarily hypothetical in vertebrates (see subseqently).
So far, the embryonic developmental patterns of the lamprey have been shown to be surprisingly similar to gnathostome patterns. The morphology of the embryo at stages ranging from neurula to late pharyngula stage is characterized by several basic features already recognized in model vertebrates. These include the configuration of the mesoderm, endoderm, and neural-crest-derived ectomesenchyme, the early morphology of the peripheral and central nervous systems, and the muscular system (Horigome et al. 1999; Kuratani et al. 1999, 2001; Kusakabe and Kuratani 2005; Kusakabe et al. 2004; Ogasawara et al. 2000; Uchida et al. 2003; Murakami et al. 2001, 2002; Boorman and Shimeld 2002; Takio et al. 2004; McCauley and Bronner-Fraser 2006). The mesoderm consists of the unsegmented head mesoderm and the mesoderm of the trunk. The latter is further subdivided into the somites and lateral mesoderm. If there are any segments in the head mesoderm, they would be the premandibular mesoderm that arises secondarily, rostral to the mandibular mesoderm, in the late pharyngula stage. The more caudal head mesoderm, however, is only secondarily regionalized into several domains by epigenetic divisions caused by the surrounding structures, such as the pharyngeal pouches and otocyst (Jacobson and Meier 1984; Kuratani et al. 1999, 2004). These mesodermal domains are not associated with crest-derived ectomesenchyme, as is the case between the crest cells and somites in the trunk.
The distribution of the crest cells is also similar in lamprey and gnathostome embryos (Horigome et al. 1999; McCauley and Bronner-Fraser 2006). In the head, the crest cells form three major cell populations associated with the pharyngeal arches, as well as with the rhombomeres, the segmental bulges of the hindbrain. The crest cells adhere proximally to the even-numbered rhombomeres, prefiguring the future cranial nerve roots in a configuration that is generally observed in the early pharyngula stage in the gnathostomes (Kuratani 1997; Kuratani et al. 2001). This conserved segmental organization of the embryonic structures must be linked to some specific patern of gene expression. As expected, the expression patterns of some Hox genes [paralogue group (PG) 2 genes expressed in the ectomesenchyme of the second and more-posterior pharyngeal arches, and the PG3 gene expressed in arch 3 and posterior to it] are shared by the lampreys and the gnathostomes, including the absence of any Hox transcripts in the mandibular arch (Hox code default state; see Takio et al. 2004, but also see Cohn 2002). Although the evolution of the jaw seems to have involved the heterotopic shift of tissue interactions to exclude the premandibular domain from the induction of the oral apparatus, and not simply the transformation of the mandibular arch (Shigetani et al. 2002; Kuratani 2004), this novelty also seems to have been dependent upon the shared basic developmental program already established in the common ancestor of all vertebrates (Shigetani et al. 2005).
The lampreys also lack some of the characteristic gene expression patterns observed in the gnathostomes, which may be associated with the absence of the apomorphic characters that define the gnathostomes. For example, the Hox code in the head region of the lamprey does not perfectly match that of the gnathostomes. Generally speaking, the segmental organization of Hox gene regulation is clearer in the gnathostomes, as are the developmental patterns of some interneurons (Murakami et al. 2004). Thus, the simple and organized segmental pattern in the gnathostome hindbrain does not represent a primitive developmental program, but it rather seems to have been established secondarily from a less-organized pattern similar to that found in the lamprey. By comparing the effect of all-trans retinoic acid on the hindbrain patterning between lamprey and gnathostomes, it has been suggested that regulation of the Hox genes and hindbrain segmentation were secondarily coupled in the agnathan to gnathostome transition, gaining the rhombomeric boundary-associated expression of the Hox genes (Murakami et al. 2004).
A more striking feature is the apparent absence of the Dlx code, which functions in the dorsoventral specification of the pharyngeal arches (see Neidert et al. 2001 for Dlx gene expression; for the Dlx code, see Depew et al. 2002). Therefore, the Dlx code may represent a typical example of a gnathostome-specific apomorphic character in the developmental program.
Molecular developmental studies of the lampreys should continue to provide more information to help us understand the part of the developmental program that is shared by the agnathans and the gnathostomes, thus representing the ancestral part, and the part that apparently reflects the features newly added or modified to establish the gnathostome body plan. To refine the scenario of early vertebrate evolution, we must study the development of another cyclostome species, the hagfish.
Hagfish and evolutionary development
Because it is difficult to access their deep-sea habitat, our knowledge of hagfish embryos is very limited (Wicht and Tusch 1998; Ota and Kuratani 2006). To define more precisely the plesiomorphic features in the developmental program of the vertebrates, hagfish embryos must be examined. Although the embryonic morphology of Eptatretus stouti has been reported (Dean 1899), knowledge of the histology of this species is still very limited (reviewed by Ota and Kuratani 2006). The problems to be solved include:
Development of the neural crest;
Development of the thyroid gland;
Patterning of the somites and their derivatives;
Morphology of the pharyngeal pouches;
Development of the pharyngeal arches (and their skeletons);
Patterning of the oral apparatus; and
Origin of the adenohypophysis.
A few of these evolutionary traits will be discussed subsequently from comparative and developmental perspectives.
Neural crest
As has been emphasized earlier, the neural crest and placodes represent two major embryonic synapomorphies in the vertebrates because the development of the vertebrate head depends on these two types of ectodermal primordia (Gans and Northcutt 1983). The placode and one of its derivatives, the lateral line system, have been described in early hagfish embryos (Wicht and Northcutt 1994). Another derivative of the placode, the cranial nerve ganglion, is also apparent in the hagfish, as implied by the morphology of the peripheral nervous system in this animal (Cole 1905, 1906).
As for the development of the neural crest, Conel (1942) reported that the hagfish crest arises as an epithelial pocket between the surface ectoderm and the neural tube (Fig. 2B). According to this author, this ectodermal sheet grows between the somites, and does not migrate as delaminated cells as is normally observed in the crest cells of vertebrate embryos, to form an epithelial nodule that later differentiates directly into the dorsal root ganglion. This observation raises the possibility that the vertebrate neural crest originally evolved as an epithelial structure and secondarily acquired its migratory capability through an epithelium–mesenchyme transition.
Needless to say, the problem of crest evolution is again tightly linked to the phylogenetic relationships between the hagfish, the lampreys, and the gnathostomes, as discussed earlier. Because the lamprey neural crest develops as a delaminating structure, and the crest cells migrate (von Kupffer 1899; Horigome et al. 1999; McCauley and Bronner-Fraser 2006), the epithelial state of the hagfish crest may represent a hagfish-specific trait, if the hagfish and the lampreys form a monophyletic clade. This also appears to hold true for the recent finding of crest-like cells in tunicate larvae (Jeffery et al. 2004), which implies that the neural crest in chordates arose as migrating cells from the beginning. Alternatively, if the epithelial crest in hagfish really represents a plesiomorphic trait in the vertebrates, the phylogenetic position of the hagfish must be re-examined, as must the nature of the crest-like cells in the tunicates. In this context, we should bear in mind that Conel's study was entirely based on the embryos collected by Dean (1899), which had been preserved in toto in alcohol, encapsulated in the egg shells. Under these conditions, the embryos seem to have undergone enormous distortion, especially in the neurepithelial structures, which could have blurred the histology of the neural crest. There is no doubt that the morphology of the early hagfish neural tube represents an artifact, because older embryos show brain primordia that appear typical of vertebrates (Conel 1942). Thus, it is very important to examine properly fixed embryos of the hagfish, especially at early stages when neurulation is still in progress.
Thyroid gland and endostyle
Because the developmental processes of the thyroid gland are different in the hagfish and lamprey, the evolutionary origin of this gland has also been a matter of debate. Stockard (1906b) described the thyroid gland in the hagfish embryo as a trough-like structure that develops on the floor of the pharynx (Fig. 2C). There is no evidence of a paired origin of this organ, as is observed in gnathostome embryos. In the lamprey, the thyroid gland develops from the larval endostyle, which undergoes metamorphosis (for the expression of regulatory genes in the endostyle and thyroid primordia of the chordates, see Ogasawara et al. 2001).
From the thyroid developmental processes of the embryos and larvae of the two cyclostomes, we can draw two different evolutionary scenarios: (1) the direct development of the thyroid gland in hagfish and the gnathostomes represents parallel evolution, and was independently acquired in each lineage, and the endostyle represents the ancestral precursor of the thyroid; or (2) the origination of the thyroid gland from the endostyle-evolved secondarily in the lamprey lineage, and these two organs are not primarily associated with one another. The latter scenario leads to the further hypothesis that the ammocoete endostyle represents a larval organ specific to the lampreys and is not necessarily homologous with the organ of the same name in the amphioxus.
The first hypothesis has been supported by some authors, because of the morphological similarity of the endostyles of the lampreys and some protochordate animals (Leach 1944; Etkin and Gona 1974; Ogasawara et al. 2001). In this case, hagfish are assumed to have undergone the secondary loss of endostyle differentiation from their developmental program. However, because there are no fossil agnathans to confirm the possession of the endostyle (including the recently discovered Devonian lamprey, which does not seem to have included the larval stage), the second scenario seems equally plausible (Hardisty 1982; Gess et al. 2006). This question could be answered, at least in part, by examining endostyle-specific gene expression in the early hagfish embryo prior to differentiation of the thyroid.
Development of the pharyngeal arches (and their skeletons)
The evolution and development of the pharyngeal arches are also central and problematic issues in the understanding of the vertebrate body plan. In a broad sense, this problem also includes the origin of the jaw, because the gnathostome jaw is a derivative of the rostralmost pharyngeal arch (Shigetani et al. 2005). To determine the histogenetic evolution of the pharyngeal arch cartilage, the origins and functions of some developmental regulatory genes have recently been compared between the lampreys and gnathostome model animals (McCauley and Bronner-Fraser 2006; Zhang and Cohn 2006). According to these studies, the functions of Sox9 cognates, a master control gene for chondrification, and a gene encoding a component of the cartilage matrix, type II collagen, which could be a downstream target of Sox9, are shared between these animals, possibly as part of a plesiomorphic developmental program (for different types of cartilage matrices in cyclostomes and the gnathostomes, see Hall 1999, 2005).
It has long been known that the lamprey branchial arch cartilages are also derived from the neural crest, as in the gnathostomes (Newth 1951; Langille and Hall 1988; McCauley and Bronner-Fraser 2006; reviewed by Kimmel et al. 2001). Morphologically, however, the branchial arch cartilages of lampreys and gnathostomes show a fundamental difference, insofar as that of the lamprey occupies the lateral part of the arch (predominantly populated by crest cells), whereas in the gnathostomes, the cartilage develops in the medial portion. The gnathostome condition may be explained as a secondary shift of the ectomesenchyme of the arch prior to chondrification (Cerny et al. 2004; Ericsson et al. 2004). It is also possible, however, as assumed by Holmgren (1940) and by Mallatt (1984), that the arch of the ancestral animal had two cartilaginous elements, the medial and lateral ones, and different elements remain functional in each lineage (the lateral element in the lamprey, the medial one in the gnathostomes). The situation in the hagfish seems to complicate this scenario. The hagfish may possess anteroposteriorly separated skeletal elements, as discussed subsequently.
The anatomy of the branchial skeleton is obviously different in the lampreys and the hagfish. The lampreys have a large branchial skeleton, which is a complicated framework that forms their characteristic branchial basket, whereas the branchial skeleton of the hagfish is represented by only small cartilaginous nodules, each associated with the distal part of the pharyngeal pouch. At the same time, hagfish also have remnant wire-like cartilages, located more rostrally, where the visceral skeleton is normally expected. Although several authors have described the skeletal anatomy in the branchial region of the adult hagfish and lamprey (Ayers and Jackson 1900; Cole 1905), the lack of detail in these description preclude morphological evaluation. The identities of the wire-like cartilages are also curious; Janvier (1996) saw no clear homology with any other skeletal elements in lampreys or in gnathostomes. Based on the more precise descriptions by Marinelli and Strenger (1954), it seems that these cartilaginous elements are associated with muscles innervated by the facial and glossopharyngeal verves, respectively, apparently supporting the branchiomeric nature of these cartilages. There have been no further reports of the branchial skeletons of hagfish embryos since that of Stockard (1906a). According to the latter author, however, initially, the pharyngeal pouches arise in a metamerical pattern as seen in other vertebrates. Soon afterwards the first pouch and posterior ones shift posteriorly, to a huge gap between the mandibular arch and the hyoid. This is apparently due to the enormous expansion of the mandibular-arch derivative, the lingual apparatus. The wire-like skeletons noted earlier apparently stay in the rostral position during this shift, and this leaves open the question as to the developmental sequence of pharyngeal slits. If the latter cartilage really represents the visceral skeleton, the pharynx of the hagfish seems to have undergone enormous modification, which no other vertebratess have experienced. Detailed embryological study of the hagfish is again required to clarify the early cellular components of the branchial arches and to construct the evolutionary scenario of the vertebrate branchial arches.
Origin and evolution of the adenohypophysis
The origin of the adenohypophysis may be one of the most popular issues among vertebrate comparative embryologists. This organ has been reported to arise from the oral endoderm in the hagfish (reviewed by Gorbman 1997 and Jefferies 1986), whereas in all other vertebrate species, including the lamprey, the organ undoubtedly arises from the rostral ectoderm (if not from the oral ectoderm in the lamprey; see subsequently).
The problem is multifold. First, the adenohypophysis in the gnathostomes develops from an epidermal structure called “Rathke's pouch,” which arises as part of the oral ectoderm. This structure is induced by the ventral diencephalon, or the hypothalamic primordium, and this topographical relationship between the two tissues plays a crucial role in adenohypophysial development (Dasen and Rosenfeld 2001; reviewed by Uchida et al. 2003). This relationship is not conserved in the lamprey, however, because the adenohypophysis of this animal originates in a single median placode called the “nasohypophysial plate,” located rostral to the oral ectoderm. Consistent with this difference, the patterns of regulatory gene expression differ greatly between the lampreys and the gnathostomes, especially those of genes encoding signaling molecules (Fgfs, Bmps, and Hh) (Uchida et al. 2003). Thus, the morphological homology of the adenohypophysis and/or oral ectoderm cannot be strictly established between the lampreys and the gnathostomes (reviewed by Kuratani et al. 2001; Uchida et al. 2003 and references therein). Since this part of the ectoderm overlaps with the domain that induces the proximodistal patterning of the oral apparatus (Shigetani et al. 2002), this phenomenon is coupled with the origin of the jaw [as well as with the monorhiny-to-diplorhiny transition (Janvier 1996)]: the extent of the oral ectoderm differs between the lampreys and the gnathostomes, as implied by the heterotopy theory of the origin of the jaw (Shigetani et al. 2002; Kuratani 2004).
As its name implies, the nasohypophysial plate is a common Anlage for the nasal epithelium and the adenohypophysis in the lamprey. In the hagfish, a similar median placode has been described as arising in the rostralmost part of the head in the early embryo. Its differentiation into the adenohypophysis, however, has not been demonstrated. In later development, the adenohypophysial primordium is certainly drawn as a part of the endodermal epithelium (Gorbman 1997). Thus, the homology of this placode is also questionable in the cyclostomes, again implying heterotopic evolution by means of a topographical shift in tissue interactions.
The epithelial reorganization between the ectoderm and endoderm has often been described in vertebrate embryos, as seen in the pharyngeal pouches of amniotes (Kastschenko 1887). In the latter case, the original pharyngeal pouch ruptures to form a pharyngeal slit of the pharyngula, and then the slit closes again. This secondarily formed pharyngeal pouch thus contains a piece of ectoderm in its distal part. A similar transformation may also be involved in hagfish development. Stockard (1906a) first dealt with this problem and, in this context, he assumed that the nasopharyngeal duct of the hagfish may represent the original mouth of the vertebrate ancestor. Unlike that of the lamprey, in which the nasal cavity ends in a blind sac, the external nostril of the hagfish leads caudally to the pharynx via this duct. The rostral epithelial configuration is thus utterly enigmatic in the hagfish embryo with the information available today. We should remember that, in the same context, pharyngeal pouch “0” has been assumed to occur in the hagfish rostral to the pharyngeal pouch equivalent to the first pouch in the gnathostomes, namely the mandibulo-hyoid pouch (Dean 1899; Stockard. 1906a; Wicht and Tusch 1998).
Conclusions and perspectives
To obtain properly prepared hagfish embryos, we launched the Hagfish Embryology Project in 2004. As part of this project, we have collected the Japanese inshore hagfish (Eptatretus burgeri; Fig. 2D), because of the wide range of its temperature tolerance and its easy accessibility. In contrast to most other hagfish, this species tends to live in shallow-water areas. For collecting samples of this species, we had the assistance of a local fisherman familiar with the seasonal behavior of this animal in the area around Shimane Prefecture, Japan. Adult male and female individuals were collected during the putative spawning season (August–October) and were maintained in aquarium tanks designed for their spawning (Ota and Kuratani 2006; Fig. 2E). This aquarium system still requires improvement to supply constant hagfish embryos.
In conclusion, the confusion and uncertainty associated with the questions discussed earlier and all other related questions stem primarily and clearly from the paucity of information about hagfish embryos and the regulatory gene expression patterns in these embryos. With this incomplete set of developmental data, comparison of these organisms with nonvertebrate chordates, such as the amphioxus and the tunicates, cannot be meaningful. It is possible that some of these questions will be answered merely by observing several different stages of properly prepared specimens of hagfish embryos. Although experimental accessibility appears to be rather low, as is the case with elasmobranch eggs, the anticipated information is relevant to the more basal events of vertebrate evolution. In this sense, hagfish embryology truly represents the final frontier of vertebrate comparative zoology.
Acknowledgments
The authors are grateful to Capt. Osamu Kakitani and the members of the Fishery Association in Gotsu City for their assistance in collecting hagfish. This work was supported by Grants-in-Aid from the Ministry of Education, Science, and Culture of Japan.
References
Author notes
From the symposium “Linking Genes and Morphology in Vertebrates” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2007, at Phoenix, Arizona.