A Floral Diet Increases the Longevity of the Coccinellid Adalia bipunctata but Does Not Allow Molting or Reproduction
- Department of Plant and Environmental Sciences, University of Copenhagen, Frederiksberg, Denmark
Adalia bipunctata L. (Coleoptera: Coccinellidae) is a generalist aphidophagous coccinellid and an important natural enemy in many agroecosystems including orchards. Coccinellid species have been observed to consume non-prey food like nectar and pollen, but the value of these foods for A. bipunctata is poorly known. The objective of this study was to determine the effect of different prey and non-prey diets on A. bipunctata larval development and adult longevity and fecundity. Larval development was studied on three prey diets: The aphids Dysaphis plantaginea and Myzus persicae and Lepidopteran eggs of Ephestia kuehniella; five flower diets: Matricaria chamomilla, Daucus carota, Fagopyrum esculentum, Anethum graveolens, and Sinapis alba; four pollen diets from three plant species: Typha angustifolia, Malus pumila (two varieties) and A. graveolens; and 1 M solutions of three sugars: glucose, fructose, and sucrose. Adult longevity and fecundity were tested on one prey diet (E. kuhniella eggs), three flower diets (F. esculentum, A. graveolens, and S. alba); the same four pollen diets and three sugar diets with larvae; and finally a mixed diet of sucrose and A. graveolens pollen. A water-only (starvation) control was used for both larval development and adult longevity and fecundity. Adult lipid content was assessed as a measure of how non-prey food affects the ladybeetles' nutritional status. Larvae did not develop beyond the first instar on any of the non-prey diets, but they lived more than twice as long as on F. esculentum and sugar diets than on water. Sugar and flower diets improved A. bipunctata adult longevity (71–92 days and 10–66 days, respectively) over a pure pollen diet (6–7 days). Fecundity was nil on all non-prey diets, and within a normal range on E. kuhniella eggs. The results suggest that pure floral diets do not support A. bipunctata molting or reproduction, but flowering plants can prolong A. bipunctata larval survival and adults longevity considerably when prey are absent. Adults on sugar diets had high lipid content, indicating that sugar feeding can improve overwintering survival. The findings could be used in agroecosystem design, such as the composition of flower strips for optimal functional diversity.
Introduction
The two-spotted ladybird Adalia bipunctata L. (Coleoptera, Coccinellidae) is native to Europe, Central Asia and North America (Majerus, 1994), and one of the most common coccinellids in orchards (Doumbia et al., 1998; Mehrnejad et al., 2011), preferring arboreal habitats, but also found on herbaceous plants in nature (Leather et al., 1999). It is a commercially available species, widely used for aphid control in many countries (Majerus, 1994; Jalali et al., 2010) and for psyllid control (Khan et al., 2016).
Adalia bipunctata is a polyphagous predator with a wide range of prey (Omkar, 2005). Adalia bipunctata was observed visiting flowering plants (Free et al., 1975), and pollinivory by A. bipunctata in early spring has been detected by gut dissection, especially within the pollen of Rosaceae (Hemptinne and Desprets, 1986). Feeding experiments indicate that pollen represents an alternative food source, enabling females to promptly oviposit at the time of aphid population increase (Hemptinne and Desprets, 1986). Furthermore, Hemptinne and Desprets (1986) reported that A. bipunctata larvae completed the development on pollen of Rosaceae alone. In the laboratory, Harmonia axyridis (Pallas) can complete development and reproduce on bee pollen alone (Berkvens et al., 2008), whereas Coleomegilla maculata (DeGeer) can develop and reproduce on a diet consisting solely of maize pollen (Lundgren and Wiedenmann, 2004). Ladybeetles oviposit in aphid patches (Dixon, 1959; Mills, 1979), but the number of aphids in each patch changes over time, often dramatically, even in the absence of natural enemies (Dixon, 1985). Polyphagy may have served as an evolutionary stepping stone for primarily predaceous groups to adopt new feeding habits (Giorgi et al., 2009), and non-prey foods are probably used by coccinellids to increase survival when prey is scarce (Lundgren, 2009). Thus, the exploitation of non-prey food may expand biological control services by coccinellids.
Plants are sources of pollen and nectar and can provide a habitat for alternative prey and natural enemies too. Flowering plants have been widely used in conservation biological control (Fiedler et al., 2008; Haaland et al., 2011), and can increase natural enemies' longevity, fecundity, and predation or parasitism rates, which in turn can enhance the effectiveness of natural enemies as biocontrol agents (Lee and Heimpel, 2008; Russell, 2015; van Rijn and Wäckers, 2016). The role of pollen and nectar is well studied for hymenopteran parasitoids (e.g., Winkler et al., 2009; Russell, 2015), but increasing attention is being given to the role of non-prey food for predator fitness components such as survival or reproduction, involving studies on coccinellids (Bertolaccini et al., 2008), neuropterans (Resende et al., 2017), predatory mites (Khodayari et al., 2013; Khanamani et al., 2016; Riahi et al., 2017), spiders (Pollard et al., 1995; Nyffeler et al., 2016), and syrphids (van Rijn and Wäckers, 2016). Sugar feeding can improve fitness and performance, as well as nutritional status in coccinellids (Lundgren and Seagraves, 2011; Seagraves et al., 2011).
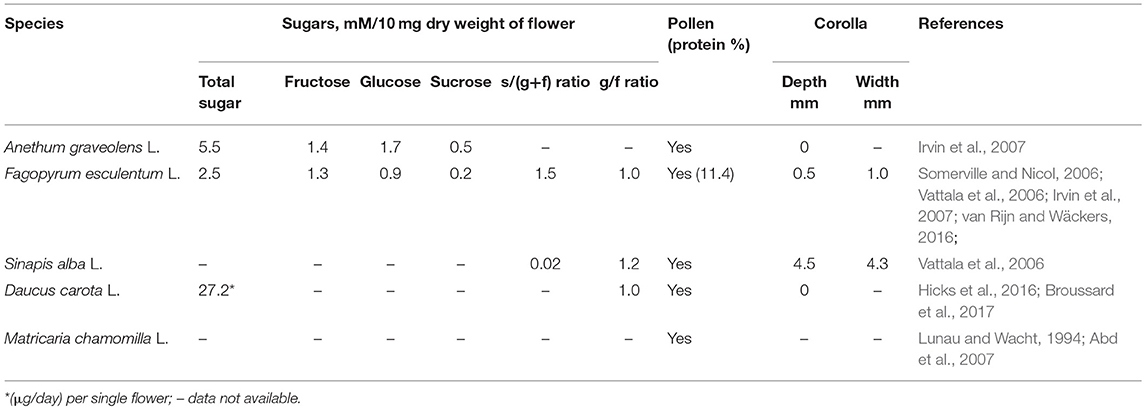
To contribute to the design of agricultural systems to support A. bipunctata, it is important to determine the value of different floral diets and their main sugar constituents for larvae and adults. Five annual flowering plants often used in flower strip mixtures, which represented four different plant families, were tested. Since A. bipunctata is an important predator in apple orchard and has a preference for pollen of Rosaceae (Hemptinne and Desprets, 1986), two varieties of apple pollen were included in the study, as well as cattail pollen (Typha angustifolia) and A. graveolens pollen. The major components of plant nectars are sucrose, fructose and glucose, occurring in different proportions in different plant species (Baker and Baker, 1983), and their value to a given insect species may differ. Therefore, these three sugars were also included in the study. Prey species used were the rosy apple aphid (Dysaphis plantaginea L.), a principal apple orchard pest of which A. bipunctata is a known major enemy (Wyss et al., 1999), and the peach aphid (Myzus persicae L.), occurring in many crops including orchards. Finally, Ephestia kuhniella Zeller eggs were tested, as this is a high-value prey often used in mass-rearing. The objective of this study was to determine the effect of whole floral diets, as well as selected pollens and main sugars, on life history parameters related to A. bipunctata immature development and adults' survival, reproduction and overwintering. Lipid content was analyzed since it is important for survival and would serve as a measure of how non-prey food affects the ladybeetles' nutritional status.
Materials and Methods
Adalia bipunctata Rearing
Insects were taken from a laboratory stock colony at the University of Copenhagen, which started in March 2017 with eggs purchased from EWH BioProduction ApS (Tappernøje, Denmark). In the stock colony, A. bipunctata larvae and adults were fed on D. plantaginea [leaves of Plantago lanceolata L. (Lamiales; Plantaginaceae) infested with D. plantaginea were offered]. The colony was maintained in plastic containers, with ventilation holes in the lid screened with fine nylon mesh. A soaked cotton plug fitted into an Eppendorf tube served as a source of water. The stock colony was maintained in a growth chamber at 23 ± 1°C and a 16: 8 h (L: D) photoperiod. For experiments on juvenile development, A. bipunctata eggs were collected from the colony and placed in 14-cm Petri dishes in the same growth chamber until hatched, and larvae were isolated after hatching. Newly hatched larvae (<24 h) were used to assess larval development. Larvae were collected from 14-cm Petri dishes and given E. kuhniella eggs to feed on. The newly-emerged adults (<24 h) were used for the longevity and fecundity experiment.
Aphid Rearing
Dysaphis plantaginea was reared on its summer host, P. lanceolata (Plantain), at 20°C and 16:8 L: D photoperiod. Aphids originated from collections made on Zealand in 2015–2016 in the University of Copenhagen's Pometum and an organic orchard near Køge (Ventegodtgaard, Lille Skensved). The M. persicae culture was maintained on pepper plant Capsicum annuum var. grossum L. (Solanales; Solanaceae). Both P. lanceolata and C. annuum were grown in the greenhouse under L: D 16:8 conditions at a minimum of 20°C.
Non-prey Food
Plants
Five plant species were selected from four different plant families: Polygonaceae (Fagopyrum esculentum L.), Cruciferae (Sinapis alba L.), Apiaceae (Daucus carota L. and Anethum graveolens L.), and Asteraceae (Matricaria chamomilla L.) (Table 1). These plants are often found in flower strip mixtures. Plants were grown from seed in 13 cm pots in a greenhouse (L: D = 16:8 h, min. 20°C), and were used when flowering. Cohorts of plants were sown once a week from March to July to ensure a steady supply of flowering plants for the duration of the experiment. D. carota, A. graveolens, and M. chamomilla started flowering in May; while F. esculentum and S. alba started flowering in June.
Pollen
Four types of pollen were tested. Two varieties of apple pollen (Malus pumila L. Rome and Malus pumila L. Red Delicious; Firman Pollen Company, WA, United States); cattail pollen (Typha angustifolia L.; Biobest NV, Westerlo, Westerlo, Belgium) and A. graveolens pollen collected from flowers grown in the greenhouse. Apple pollen is easy to find on apple leaves in orchard during the flowering period (Addison et al., 2000). Cattail pollen has a high value for predatory mites mainly consisting of the family Phytoseiidae (Samaras et al., 2015), and is currently used in greenhouses (Pijnakker et al., 2015). A. graveolens produce abundant pollen, which is easily accessible to insects (Irvin et al., 2007). Pollen was sieved with fine mesh (thread diameter = 39 microns) prior to testing, ensuring that only pure pollen powder was used for the experiment.
Sugar Solutions
Sucrose, fructose, and glucose are major components of plant nectars (Baker and Baker, 1983), and their nutritional value to a given insect species varies. Not all mono- and oligo-saccharides are equally suitable for coccinellids (Niijima et al., 1997) therefore, 1 M solutions of two monosaccharides D-(+)-fructose and D-(+)-glucose (Merck KGaA, Darmstadt, Germany) and a disaccharide D-(+)-sucrose (Nordic Sugar, Copenhagen, Denmark) were also tested.
Experimental Design
A. bipunctata Larval Development
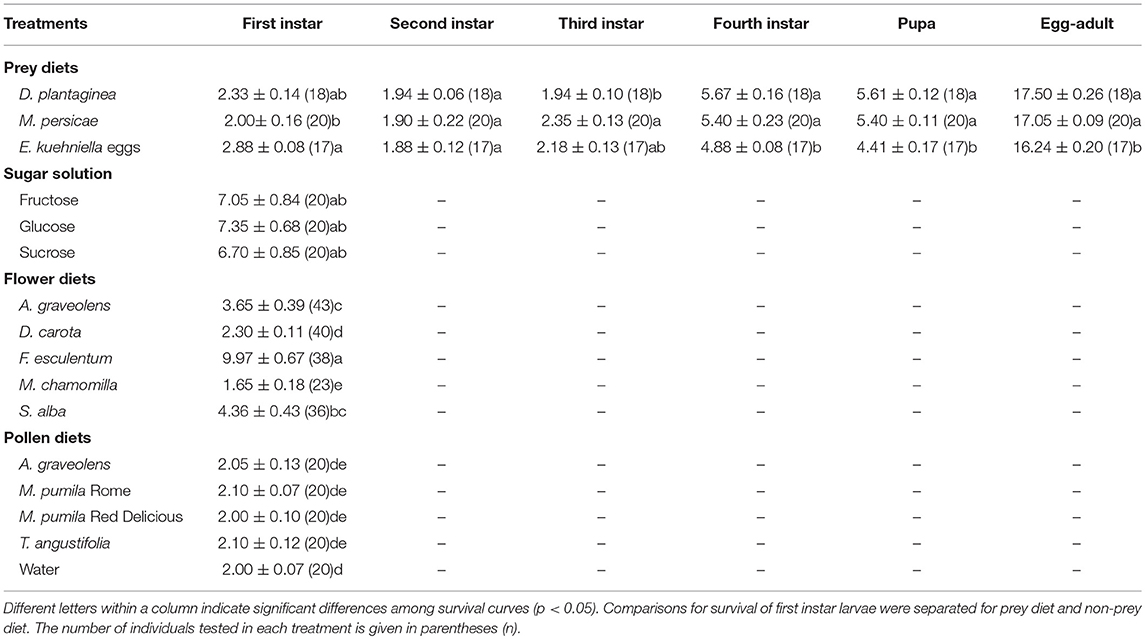
Fifteen different diets were tested: flowers of five species (F. esculentum, S. alba, D. carota, A. graveolens, and M. chamomilla), four types of pollen (M. pumila Rome, M. pumila Red Delicious, T. angustifolia, and A. graveolens), three sugar solutions (1 M glucose, fructose, and sucrose solutions), two species of aphids (D. plantaginea and M. persicae), and E. kuehniella eggs. For each treatment, 17–46 larvae were tested. Water was provided in all treatments and water-only was the starvation treatment. Larvae (<24 h) were selected randomly and placed in individual 30 ml plastic cups with a piece of fine mesh netting held in place by the rim of a lid with a hole in the center, allowing ventilation. A 1 cm layer of agar (15 g/l) in the bottom of the plastic cup provided moisture. Water and sugar solutions were provided by filling a 0.5 ml microcentrifuge tube, sealed with soaked cotton serving as a dispenser. For the aphid treatments, each cup contained a piece of a leaf from P. lanceolata infested with D. plantaginea or from C. annuum infested with M. persicae. Pollen diets were placed on a piece of filter paper on the agar layer, and E. kuehniella eggs and flower diets were placed on the agar layer directly. The agar served to support flowers and provided a water source, keeping the flowers fresh. Flowers were collected between 10.00 and 12.00 h while blooming in the greenhouse. Only flowers without any insect infestation were chosen. All food types were provided ad libitum. Flowers, pollen, aphids and E. kuehniella eggs were replaced daily while sugar diets were replaced every other day and water added if necessary. The plastic cups with the agar layer were replaced with new ones when the diets were replaced.
The developmental stage (first, second, third and fourth instar, pupa, and adult) and the survival of A. bipunctata larvae were monitored on a daily basis. The day of molting could be determined by observing the cast skins.
A. bipunctata Adult Longevity and Fecundity
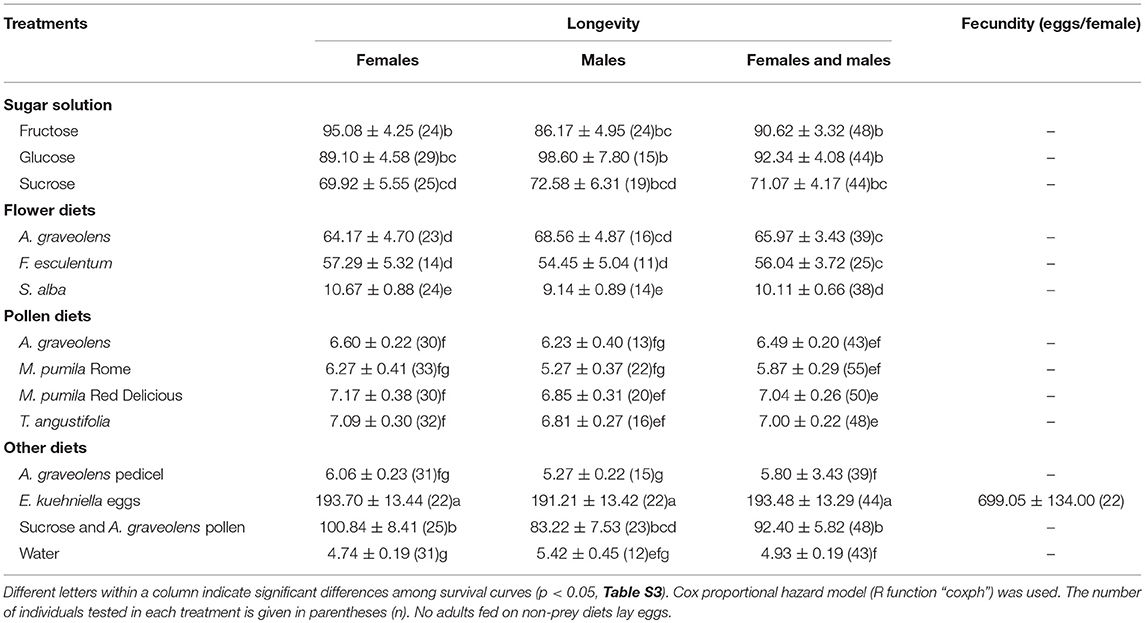
Adults (<24 h) were tested with one of the following diets: Three different flowers (A. graveolens, F. esculentum and S. alba; carrot and chamomile flowers were excluded due to a shortage of flowers), four types of pollen (as above), three types of sugar solutions (1 M glucose, fructose and sucrose solution), A. graveolens pedicels (flower removed), and a mixed diet of sucrose solution plus A. graveolens pollen. The prey diet of E. kuehniella eggs was used as a positive control. Water was provided in all treatments and water-only was the starvation treatment. Rahaman and Aniszewski (2014) reported that A.bipunctata can consume young leaves or buds of legume plants when aphids are not available. The pedicel was a plant part available in the flower diet treatments, so A. graveolens pedicels were included to test the possible value of the pedicel for A.bipunctata, and to serve as an additional negative control. One or two males and one or two females (all <24 h old) were placed together in one container (plastic cup, 6 cm in diameter 7.5 cm height; with a piece of fine mesh netting held in place by the rim of a lid with a hole in the center, as above). A few days after they emerged, adults started to mate. After mating was confirmed by observing first eggs, adults were kept individually in a new 30 ml plastic cup. For each treatment, 25–55 adults were tested, fewer adults (25) were used in the F. esculentum flower diet because of a shortage of flowers.
In each container, flowers were provided in a small cylindrical plastic vial with water, plugged with cotton wool to prevent accidental drowning of adults. Flowers were collected between 10.00 and 12.00 h in the greenhouse. Only those without any insect infestation were chosen. Pollen and E. kuehniella eggs were placed on the bottom of the container, and sugar solution and water were provided by filling a 0.5 ml microcentrifuge tube, sealed with soaked cotton serving as a dispenser. All food types were provided ad libitum. Flowers were replaced daily, E. kuehniella eggs, pollen and sugar solution diets were changed every 2–3 days to maintain good quality and avoid the growth of fungi, and water was added if necessary. Containers were replaced with new ones every 2 or 3weeks.
The survival and fecundity of adults were checked daily; Dead individuals were removed and placed in a −20°C freezer for lipid analysis. Adalia bipunctata eggs were counted and females moved to new cups. Longevity in the E. kuehniella treatment was very long, and after 241 days the remaining adults in that treatment (n = 20) were freeze-killed. Lipid content of both dead and freeze-killed adults was determined.
Lipid Analysis
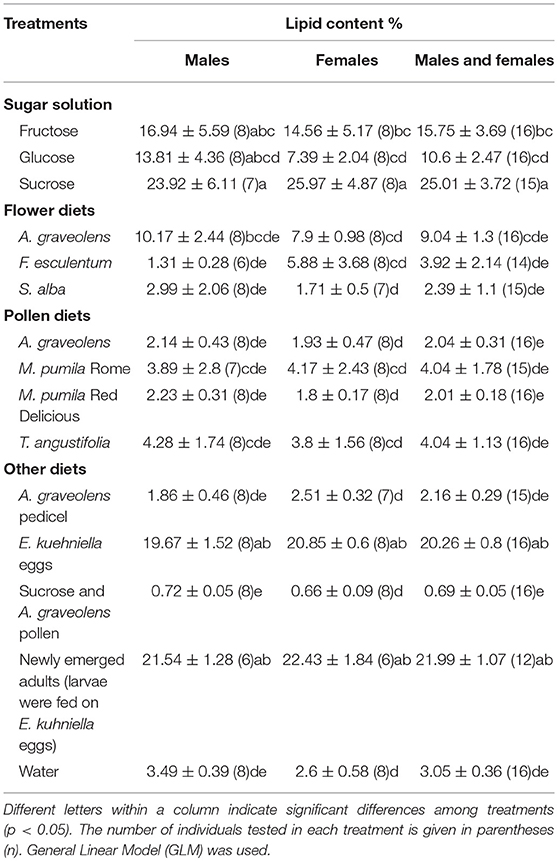
Lipid extraction of A. bipunctata adults, who fed on different diets, and newly emerged adults, who fed on E. kuehniella eggs at the larval stages, was done in a Soxhlet extractor with petroleum ether (PE) (Williams et al., 2011). Extraction time was 72 h. Prior to extraction, A. bipunctata adults were oven-dried at 50°C for 24 h and weighed. After lipid extraction, they were re-weighed to obtain the fat-extracted dry weight. Weighing was done on an XPR Micro and Ultra-Microbalance [readability down to 1 microgram, Mettler-Toledo (HK) MTCN, Hong Kong, China]. The mass of total lipids was calculated as the weight of each individual sample before extraction, minus the weight of the same sample after extraction.
Statistics
The Kaplan-Meier (KM) method was used to fit survival curves of each treatment, using the “surv” and “survfit” function from the “survival” package (Therneau, 2014) in R (R Core Team, 2014) and mean survival time and standard error were extracted from the curves using the “print.survfit” function from the same package. The effect of different diets on the longevity of larvae and adults was tested by Cox proportional hazards model (R function “coxph”). For adults, sex was included in models as well as the interaction effect of sex and diet, with cage as a random effect (using function “cluster”) (R Core Team, 2014). Models were reduced by removing higher order non-significant interactions. The “lsmean” function was used to perform pairwise comparisons for each pair of treatments. A General Linear Model (GLM) was used for lipid comparison among treatments and sex, followed by “lsmean” for pairwise comparisons for each pair of treatments. Data are presented as mean values ± standard error (SE).
Results
Larval Development
Larvae did not develop beyond the first instar in any of the non-prey diet treatments, but the survival of larval was significantly affected by diet (Cox PH, df = 15, χ2 = 287.1, p < < 0.0001). Larvae lived longer on F. esculentum and sugar diets than on other flower and pollen diets (Table 2). None of the four pollen diets differed significantly from the starvation treatment (water-only) and were all significantly poorer than all other diets with a significantly shorter survival time. Flowers of F. esculentum, S. alba, and A. graveolens increased the survival of larvae, especially F. esculentum, being slightly better than sugar diets. However, the survival of larvae fed on D. carota and M. chamomilla flowers did not differ from the starvation treatment (Figure 1).
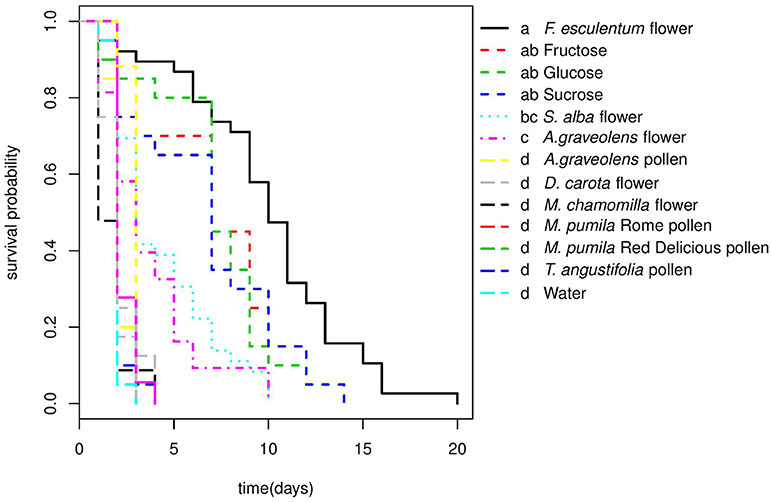
Figure 1. Survival curves of A.bipunctata larvae fed on different non-prey diets. Different letters indicate significant differences among treatments after pairwise comparisons of the survival curves (p < 0.05).
Larvae that fed on prey diets completed development in approximately 17 days (Table 2). First and second instar stages took from 2 to 3days, third instar developmental time for larvae that fed on M. persicae was significantly longer than when the same larvae fed on D. plantaginea. The fourth instar took 4–6 days and the pupal stages lasted 4–6 days before emergence. Larvae that fed on E. kuehniella eggs developed significantly faster (~1.3 days) than those on aphid diets (Table 2).
Adult Longevity and Fecundity
Adalia bipunctata adults longevity was significantly affected by diet (Cox proportional hazards, df = 13, χ2 = 1135.9, p < < 0.0001), however, no significant difference was found between females and males (Cox proportional hazards, df = 1, χ2 = 2.1, p = 0.15) and there was no interaction between sex and diet (Table S1). Adults survived on average (±SE) for 4.93 (±0.19) days in the starvation treatment (water-only). Adults' longevity on E. kuehniella eggs was significantly higher than all non-prey diets (Figure 2). Among the non-prey diet treatments, highest longevity for both sexes were obtained on sugar diets followed by A. graveolens and F. esculentum flower diets. In the presence of a 1 M sugar solution, adults survived up to 3 months. For flower diets, adults lived longer on A. graveolens (65.97 ± 3.43 days) and F. esculentum (56.04 ± 3.72 days) than on S. alba (10.11 ± 0.66 days) flowers. Compared to sugar and floral diets, pure pollen diets hardly improved A. bipunctata longevity over that of the starvation treatment. Although, for M. pumila Red Delicious and for T. angustifolia, the difference was significant (z = −4.2, p = 0.002; z = −4.4, p = 0.001) and increased longevity from < 5 days on water to around 7 days (an increase of over 40%) (Table 3). Despite adults' longevity on pure pollen diets being low, longevity on the mixed diet of A. graveolens pollen and sucrose solution was over 40% higher than on the pure sucrose solution diet, which was equivalent to the fructose diet and only surpassed by the E. kuehniella diet. There was no significant difference between females and males longevity on different diets, except for the A. graveolens pedicel diet, with slightly longer longevity of females (z = 2.3, p = 0.02; Table S2).
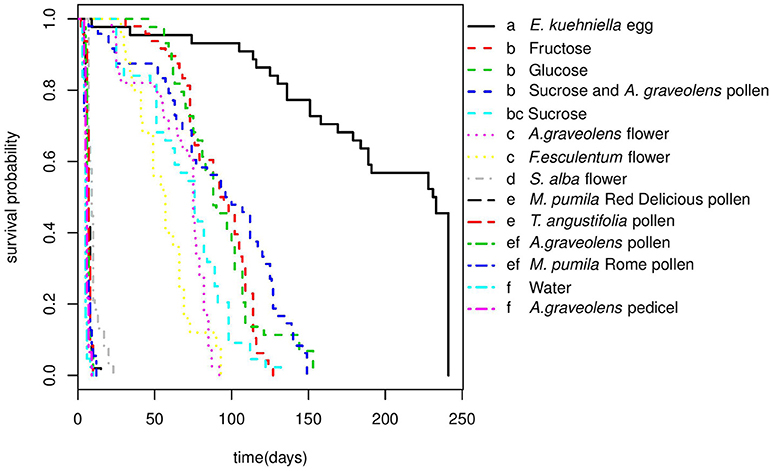
Figure 2. Survival curves of A.bipunctata adults (females and males) fed on different diets. Different letters indicate significant differences among treatments after pairwise comparisons of the survival curves (p < 0.05).
Only females that fed on E. kuhniella eggs laid eggs, with an average of 699.05 ± 134.00 eggs per female, while none of the females fed on non-prey diets laid eggs (Table 3).
Lipid Analysis
Lipid content of adults was affected by diets (χ2 = 197.5, p < < 0.0001), but no significant difference was found between females and males (χ2 = 0.2, p = 0.65). The highest lipid levels were found in individuals fed on sucrose (25%), but it was not statistically different from individuals fed on E. kuhniella eggs or newly emerged adults for both females and males (p = 0.06 and p = 0.26 for females; p = 0.06, and p = 0.26 for males; Table 4). The lipid content of females that fed on sucrose solution was significantly higher than females that fed on fructose or glucose. No significant difference in lipid contents was found in males. Lipid content of adults that fed on sucrose solution alone was much higher than those that fed on the mixed diets of sucrose solution and A. graveolens pollen (z = 9.7, p < 0.0001; Table 4). Adults that fed on fructose or glucose solution had the same lipid content as those that fed on A. graveolens flowers.
Discussion
In this study, the effect of different non-prey food sources (pollen, sugar solutions, and flowers) and prey diets on longevity and fecundity of A. bipunctata adults and survival of A. bipunctata larvae were tested. Larvae did not develop beyond the first instar in any of the non-prey diet treatments but could survive up to 10 days on a non-prey diet (Table 2). Adults fecundity was nil on all non-prey diets, but non-prey diets increased longevity up to 92 days (Table 3). High lipid content was found in adults that fed on sugar solutions (Table 4).
None of the four pollen diets differed significantly from the starvation treatment for A. bipunctata larval development (Figure 1). For adults, M. pumila (Red Delicious) and T. angustifolia pollen significantly increased longevity by 2 days over water, which was equivalent to a 40% increase in longevity, but still inferior to other non-prey diets (Table 3). The larval developmental rate of A. bipunctata is mainly dependent on the quality and quantity of food and temperature (Wratten, 1973; Omkar, 2005; Jalali et al., 2010). Hemptinne and Desprets (1986) reported that A. bipunctata larvae completed the development on pollen of Rosaceae, although the development time was longer than on a prey diet. Similar results were found for Harmonia axyridis (Pallas) provided with a frozen, moist honeybee pollen diet (Berkvens et al., 2008). Coleomegilla maculata (DeGeer) can develop and reproduce on a diet consisting solely of maize pollen (Lundgren and Wiedenmann, 2004). On the contrary, in a study conducted by Amala and Yadav (2013), the effects of five different diets on the developmental time of larval Stethorus rani (Coleoptera, Coccinellidae) were studied. They found that S. rani larvae that fed on a pollen diet had the lowest larval survival percentage compared to other diets and failed to develop to the pupal stage, but those fed on honey and extrafloral nectaries completed development. Harmonia axyridis failed to complete its development or reproduce when fed exclusively on fruit (apple, pear, and raspberries) and fungi, although larval, and adult survival were prolonged when fruit was offered compared with only water (Berkvens et al., 2010). Ladybeetles developed better when offered a mixed diet compared to pollen alone (De Clercq et al., 2005; Berkvens et al., 2008; Bonte et al., 2010; Amala and Yadav, 2013). Possible explanations for the contrasting results in this study and those of Hemptinne and Desprets (1986), may be the different nutritional value of pollen tested. Mixed pollen of Rosaceae was provided in the Hemptinne and Desprets (1986) study, but in the present study, only pollen of one plant species was provided, and the paper does not state if it was bee-pollen or hand-collected pollen. Bee-collected pollen always possesses a higher nutritional value than pollen collected by hand, because it contains larger amounts of sugars from the honey or nectar in the fluid used to cement the grains together (Lunden, 1954; Linskens and Jorde, 1997). Different nutritional requirements of the species tested could also explain inconsistencies in the results. Previous studies indicate that pollen could enable A. bipunctata females to promptly oviposit at the time of aphid population increase (Hemptinne and Desprets, 1986). Our results show that pollen diets increased adult longevity by 19–43% compared to starvation (water-only), but not as much as floral and sugar diets, pointing to the need for adults to have access to sugars. However, the value of pollen was also clearly shown by the fact that A. graveolens pollen added to a sucrose sugar diet increased adult longevity by over 30% compared to the pure sugar diet.
Sugar solutions prolonged the developmental time of larvae and improved A. bipunctata adult longevity (Tables 2, 3). Sugars are easily digestible high-energy foods, and can dramatically increase survival of coccinellids in the absence of prey (Matsuka et al., 1982; Dreyer et al., 1997). In this study, adults that fed on glucose lived longer than those that fed on sucrose. Niijima et al. (1997) mentioned that not all mono- and oligo-saccharides are equally suitable for coccinellids. Sugar-feeding did not support reproduction in this study. A similar result was reported by Smith and Krischik (1999), who found that sugar-feeding seldom supported reproduction in coccinellids on its own. However, sugar consumption can shorten pre-oviposition periods of coccinellids and help females to survive reproductive diapause (Reznik and Vaghina, 2006). The high lipid content of adults that fed on sugars (Table 4) supports this finding and also points to the value of sugars in building lipid reserves for survival in periods without prey and for overwintering.
Adalia bipunctata larval survival and adult longevity on flower diets of different species varied greatly (Table 3). The accessibility and quality of the nectar and pollen are important factors that affect the relative preference of natural enemies for specific plant species (Hogg et al., 2011; van Rijn and Wäckers, 2016). The nutritional composition differs among different plant species (Table 1). Adalia graveolens and F. esculentum flowers increased the longevity of adults compared to the starvation treatment, but did not support egg production. The results are in accordance with Togni et al. (2016), who found that when given access to coriander (Coriandrum sativum) flowers increased adult longevity but not reproduction of the coccinellid Cycloneda sanguinea. The two best-performing flowers in our study, A. graveolens and F. esculentum, both have well-exposed nectaries, making predator access easy. Sugar content is dominated by fructose and glucose in both A. graveolens and F. esculentum nectar, and A. graveolens has more total sugars than F. esculentum (Irvin et al., 2007). While we found no difference between the three sugars for larval survival, adults survived longer on fructose and glucose than on sucrose. These findings suggest that planting flowers such as A. graveolens and F. esculentum with accessible nectaries and with more fructose and glucose in the nectar would better support A. bipunctata than sucrose-dominated flowers.
Some compounds in nectar can be toxic or repellent to flower visitors (Adler, 2000; Wäckers, 2001). For example, glucosinolates (GLS) are present in all parts of Brassicaceae crop species (Merritt, 1996), even in nectar (Bruinsma et al., 2014), inducing deleterious effects on A. bipunctata (Francis et al., 2001). This may explain why A. bipunctata adults that fed on white mustard flower lived much shorter than those that fed on A. graveolens and F. esculentum.
Larvae performed poorly on all flower diets except F. esculentum (Table 2). Although considerable amounts of pollen can be found on the chamomile flower, the nectaries are hidden in a capitulum (Patt et al., 1997), so chamomile may have served as a pure pollen diet, which corresponds with the survival of larvae fed on pollen diets. Restricting the access of predators to nectar can reduce their survival and fitness considerably (Lundgren and Seagraves, 2011; Portillo et al., 2012; van Rijn and Wäckers, 2016). Feeding on D. carota flowers can selectively benefit some insects, such as the parasitoid Cotesia glomerata (Winkler et al., 2009), while some others such as the lacewing Chrysoperla carnea and the herbivore Pieris rapae (Lepidoptera: Pieridae) do not benefit (Winkler et al., 2009; Gonzalez et al., 2016). The nectar is accessible and dominated by glucose and fructose (Broussard et al., 2017) but A. bipunctata larvae did not benefit from D. carota flowers in this study. The reasons for the poor performance of A. bipunctata larvae provided with M. chamomilla and D. carota flower diets, as well as for the poor performance of larvae on pure pollen diets remain speculative but are consistent with the central role of sugars for A. bipunctata survival in the absence of prey. While pollen as a pure diet has little value for A. bipunctata larvae, and limited value for adults, adult longevity on a sucrose diet supplemented with pollen numerically exceeded the sum of the longevity on sucrose and pollen separately, pointing to a nutritional value of pollen in mixed diets. Evans et al. (1999) and Soares et al. (2004) also reported that ladybeetles benefited from mixed diets.
In this study, lipid content of newly-emerged adults, presumably stored during larval development, was also analyzed. The lipid content of adults that fed on sucrose solution did not differ from that of adults that fed on E. kuhniella eggs or newly emerged adults (larvae were fed on E. kuhniella eggs) and was significantly higher than of starved individuals (water only). On the contrary, the lipid content of adults fed on pollen diets was significantly lower than that of newly emerged adults but did not differ from that of starved individuals. The lipid reserves in adults fed on water or pollen diets were consumed in a few days, which suggests that sugars contribute to maintain lipid levels, but pollen does not (Table 4). In this study, lipid content of adults that fed on sucrose solution was much higher than those that fed on a mixed diet sucrose solution and A. graveolens pollen. On the contrary, higher lipid content has been observed in hoverflies that fed on honey and pollen, compared to those that fed on honey alone (Pinheiro et al., 2015). The inconsistency of the results could be due to the different species tested or to A. bipunctata's physiological processes. This should be addressed in future studies.
The results of the present study emphasize the importance of non-prey foods for A. bipunctata in agroecosystems such as orchards when prey populations are low. Sugar solutions and flowering plants, especially F. esculentum and A. graveolens, can prolong A. bipunctatalongevity, but floral diets did not support adult fecundity. Flowers and sugars could also increase immature survival, though they did not support molting. Whether flowering plants attract or retain A. bipunctata in a habitat per se is not known, but our results show that longevity of A. bipunctata is greatly prolonged by floral diets. In the field, flowering plants may also host alternative prey, which would support both larval development and adult fecundity. Flowering plants can enhance the effectiveness of natural enemies by increasing natural enemies' longevity, fecundity, and predation or parasitism rates (Lee and Heimpel, 2008; Russell, 2015; van Rijn and Wäckers, 2016), and have been widely used in conservation biological control (Fiedler et al., 2008; Haaland et al., 2011). Addison et al. (2000) found pollen to be abundant on apple leaves very soon after the leaves had opened, and predatory mites can use windborne pollen released from cover crops in the field (Warburg et al., 2018). Nectar is also available in apple flowers during flowering in orchards (Toth et al., 2003). However, the effects of floral diets on A. bipunctata may decrease in the field due to various abiotic and biotic conditions (Brody, 1997; Adler et al., 2006). Analyses of lipid contents of adults that fed on different diets point to the potential of sugars to contribute toward better winter survival of the adults.
In conclusion, floral diets can prolong A. bipunctata longevity, which may expand biological control services by A. bipunctata. Further studies on whether prey deprivation might affect the future ability of A. bipunctata to provide biocontrol services are needed for conservation biological control. A limitation of this study is that larvae were deprived of prey at the very first instar; however, ladybeetles would lay eggs near an aphid colony and it is rare that the first instar would face the total absence of prey. Further studies on a diet switch (from prey diet to pollen diet on the third/fourth instar) would be especially relevant, since an aphid colony may become extinct before the larvae complete their development (Dixon, 1985). Further studies on prey and non-prey mixed diets are needed in order to obtain a better understanding of the value of non-prey diets in cases where prey is limited in the field. Furthermore, because of various abiotic and biotic conditions, studies on how to provide non-prey foods in cropping systems to improve biological control agents' efficiency are needed for conservation biological control.
Data Accessibility Statement
The dataset supporting this article has been deposited in zenodo at: https://doi.org/10.5281/zenodo.2546385.
Author Contributions
XH and LS conceived the study. XH carried out the lab work and wrote the first draft of the manuscript. XH and LS interpreted the results and wrote the manuscript.
Funding
This study was financially supported by two research projects: EcoOrchard Innovative design and management to boost functional biodiversity of organic orchards 2015–2018, EU Core-organic +, funded by Green development and Demonstration Program (GUDP), Danish Ministry of Environment and Food, GUDP_ID 34009-14-0906, and Optimizing performance of biological control agents used in Integrated Pest Management 2015–2019, funded by The Danish Council for Independent Research, Technology and Production Sciences (DFF), DFF-ID: 4184-00248.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor and reviewer, AP, declared their involvement as co-editors in the Research Topic.
Acknowledgments
The China Scholarship Council (CSC) is gratefully acknowledged (grant to XH). We are grateful to Matt H. Greenstone and Jacob Weiner for helping with the text.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00006/full#supplementary-material
References
Abd, E. K. A. I., Naggar, M. E. E. I., and Marouf, A. E. (2007). Is Matricaria chamomilla a beneficial insectary plant? J. Agri. Sci. Mansoura Univ. 32, 6777–6786.
Addison, J. A., Hardman, J. M., and Walde, S. J. (2000). Pollen availability for predaceous mites on apple: spatial and temporal heterogeneity. Exp. Appl. Acarol. 24, 1–18. doi: 10.1023/A:1006329819059
Adler, L. S. (2000). The ecological significance of toxic nectar. Oikos 91, 409–420. doi: 10.1034/j.1600-0706.2000.910301.x
Adler, L. S., Wink, M., Distl, M., and Lentz, A. J. (2006). Leaf herbivory and nutrients increase nectar alkaloids. Ecol. Lett. 9, 960–967. doi: 10.1111/j.1461-0248.2006.00944.x
Amala, U., and Yadav, D. S. (2013). Effect of natural hosts and alternate food sources on the biological parameters of acarophagous predator, Stethorus rani Kapur. Pest. Manag. Hort. Ecosyst. 9, 169–172.
Baker, H. G., and Baker, I. (1983). “Floral nectar sugar constituents in relation to pollinator type,” in Handbook of Experimental Pollination Biology, ed C. E. Jones and R. J. Little (New York, NY: Van Nostrand Reinhold), 117–141.
Berkvens, N., Bonte, J., Berkvens, D., Deforce, K., Tirry, L., and Clercq, P. D. (2008). Pollen as an alternative food for Harmonia axyridis. Bio. Control 53, 201–210. doi: 10.1007/s10526-007-9128-7
Berkvens, N., Landuyt, C., Deforce, K., Berkvens, D., Tirry, L., and De Clercq, P. (2010). Alternative foods for the multicoloured Asian lady beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 107, 189–195.
Bertolaccini, I., Nunez-Perez, E., and Tizado, E. J. (2008). Effect of wild flowers on oviposition of Hippodamia variegata (Coleoptera: Coccinellidae) in the laboratory. J. Econ. Entomol. 101, 1792–1797. doi: 10.1603/0022-0493-101.6.1792
Bonte, M., Samih, M. A., and De Clercq, P. (2010). Development and reproduction of Adalia bipunctata on factitious and artificial foods. Bio Control, 55, 485–491. doi: 10.1007/s10526-010-9266-1
Brody, A. K. (1997). Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology 78, 1624–1631. doi: 10.1890/0012-9658(1997)078[1624:EOPHAS]2.0.CO;2
Broussard, M. A., Mas, F., Howlett, B., Pattemore, D., and Tylianakis, J. M. (2017). Possible mechanisms of pollination failure in hybrid carrot seed and implications for industry in a changing climate. PLoS ONE 12:e0180215. doi: 10.1371/journal.pone.0180215
Bruinsma, M., Barbosa, D. L., ten Broeke, C. J. M., van Dam, N. M., van Beek, T. A., Dicke, M., et al. (2014). Folivory affects composition of nectar, floral odor and modifies pollinator behavior. J. Chem. Ecol. 40, 39–49. doi: 10.1007/s10886-013-0369-x
De Clercq, P., Bonte, M., Van Speybroeck, K., Bolckmans, K., and Deforce, K. (2005). Development and reproduction of Adalia bipunctata (Coleoptera: Coccinellidae) on eggs of Ephestia kuehniella (Lepidoptera: Phycitidae) and pollen. Pest Manag. Sci. 61, 1129–1132. doi: 10.1002/ps.1111
Dixon, A. F. G. (1959). An experimental study of the searching behaviour of the predatory coccinellid beetle Adalia decempunctata (L.). J. Anim. Ecol. 28, 259–281. doi: 10.2307/2082
Doumbia, M., Hemptinne, J. L., and Dixon, A. F. G. (1998). Assessment of patch quality by ladybirds: role of larval tracks. Oecologia 113, 197–202. doi: 10.1007/s004420050368
Dreyer, B. S., Neuenschwander, P., Baumgartner, J., and Dorn, S. (1997). Trophic influences on survival, development and reproduction of Hyperaspis notata (Col., Coccinellidae). J. Appl. Entomol. 121, 249–256. doi: 10.1111/j.1439-0418.1997.tb01401.x
Evans, E., Stevenson, A., and Richards, D. (1999). Essential versus alternative foods of insect predators: benefits of a mixed diet. Oecologia 121, 107–112. doi: 10.1007/s004420050911
Fiedler, A. K., Landis, D. A., and Wratten, S. D. (2008). Maximizing ecosystem services from conservation biological control: the role of habitat management. Biol. Control 45, 254–271. doi: 10.1016/j.biocontrol.2007.12.009
Francis, F., Haubruge, E., Hastir, P., and Gaspar, C. (2001). Effect of aphid host plant on development and reproduction of the third trophic level, the predator Adalia bipunctata (Coleoptera: Coccinellidae). Environ. Entomol. 30, 947–952. doi: 10.1603/0046-225X-30.5.947
Free, J. B., Gennard, D., Stevenson, J. H., and Williams, I. H. (1975). Beneficial insects present on a motorway verge. Biol. Conserv. 8, 61–72. doi: 10.1016/0006-3207(75)90079-8
Giorgi, J. A., Vandenberg, N. J., McHugh, J. V., Forrester, J. A., Slipinski, S. A., Miller, K. B., et al. (2009). The evolution of food preference in Coccinellidae. Bio. Control 51, 215–231. doi: 10.1016/j.biocontrol.2009.05.019
Gonzalez, D., Nave, A., Gonçalves, F., Nunes, F. M., Campos, M., and Torres, L. (2016). Higher longevity and fecundity of Chrysoperla carnea, a predator of olive pests, on some native flowering Mediterranean plants. Agron. Sustain. Dev. 36:30. doi: 10.1007/s13593-016-0369-7
Haaland, C., Naisbit, R. E., and Bersier, L. F. (2011). Sown wildflower strips for insect conservation: a review. Insect Conserv. Divers. 4, 60–80. doi: 10.1111/j.1752-4598.2010.00098.x
Hemptinne, J. L., and Desprets, A. (1986). “Pollen as a spring food for Adalia bipunctata,” in Ecology of Aphidophaga, ed I. Hodek (Prague: Academia,and Dordrecht: Dr. W. Junk publishers), 29–35.
Hicks, D. M., Ouvrard, P., Baldock, K. C. R., Baude, M., Goddard, M. A., Kunin, W. E., et al. (2016). Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE 11:e0158117. doi: 10.1371/journal.pone.0158117
Hogg, B. N., Bugg, R. L., and Daane, K. M. (2011). Attractiveness of common insectary and harvestable floral resources to beneficial insects. Bio. Control 56, 76–84. doi: 10.1016/j.biocontrol.2010.09.007
Irvin, N. A., Hoddle, M. S., and Castle, S. J. (2007). The effect of resource provisioning and sugar composition of foods on longevity of three Gonatocerus spp., egg parasitoids of Homalodisca vitripennis. Bio. Control 40, 69–79. doi: 10.1016/j.biocontrol.2006.09.005
Jalali, M. A., Tirry, L., and Clercq, P. D. (2010). Effects of food and temperature on development, fecundity and life-table parameters of Adalia bipunctata (Coleoptera: Coccinellidae). J. Appl. Entomol. 133, 615–625. doi: 10.1111/j.1439-0418.2009.01408.x
Khan, A. A., Qureshi, J. A., Afzal, M., and Stansly, P. A. (2016). Two-Spotted ladybeetle Adalia bipunctata L. (Coleoptera: Coccinellidae): a commercially available predator to control Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae). PLoS ONE 11:e0162843. doi: 10.1371/journal.pone.0162843
Khanamani, M., Fathipour, Y., Talebi, A. A., and Mehrabadi, M. (2016). Linking pollen quality and performance of Neoseiulus californicus (Acari: Phytoseiidae) in two-spotted spider mite management programmes. Pest Manag. Sci. 73, 452–461. doi: 10.1002/ps.4305
Khodayari, S., Fathipour, Y., and Kamali, K. (2013). Life history parameters of Phytoseius plumifer (Acari: Phytoseiidae) fed on corn pollen. Acarologia 53, 185–189. doi: 10.1051/acarologia/20132087
Leather, S. R., Cooke, R. C. A., Fellowes, M. D. E., and Rombe, R. (1999). Distribution and abundance of ladybirds (Coleoptera: Coccinellidae) in non-crop habitats. Eur. J. Entomol. 96, 23–27.
Lee, J. C., and Heimpel, G. E. (2008). Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 77, 565–572. doi: 10.1111/j.1365-2656.2008.01355.x
Linskens, H. F., and Jorde, W. (1997). Pollen as food and medicine- A review. Econ. Bot. 51, 78–86. doi: 10.1007/BF02910407
Lunau, K., and Wacht, S. (1994). Optical releasers of innate proboscis extension in the hoverfly Eristalis tenax L. (Diptera: Syrphidae). J. Comp. Physiol. A 174, 575–579. doi: 10.1007/BF00217378
Lunden, R. (1954). A short introduction to the literature on pollen chemistry. Svensk kem. Tidskr 66, 201–213.
Lundgren, J. G. (2009). Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol. Control 51, 294–305. doi: 10.1016/j.biocontrol.2009.05.016
Lundgren, J. G., and Seagraves, M. P. (2011). Physiological benefits of nectar feeding by a predatory beetle. Biol. J. Linn. Soc. 104, 661–669. doi: 10.1111/j.1095-8312.2011.01729.x
Lundgren, J. G., and Wiedenmann, R. N. (2004). Nutritional suitability of corn pollen for the predator Coleomegilla maculata (Coleoptera: Coccinellidae). J. Insect Physiol. 50, 567–575. doi: 10.1016/j.jinsphys.2004.04.003
Matsuka, M., Watanabe, M., and Niijima, K. (1982). Longevity and oviposition of Vedalia beetles on artificial diets. Environ. Entomol. 11, 816–819. doi: 10.1093/ee/11.4.816
Mehrnejad, M. R., Jalali, M. A., and Mirzaei, R. (2011). Abundance and biological parameters of psyllophagous coccinellids in pistachio orchards. J. Appl. Entomol. 135, 673–681. doi: 10.1111/j.1439-0418.2010.01577.x
Merritt, S. Z. (1996). Within-plant variation in concentrations of amino acids, sugar and sinigrin in phloem sap of black mustard, Brassica nigra. Koch. J. Chem. Ecol. 22, 1133–1137. doi: 10.1007/BF02027950
Mills, N. J. (1979). Adalia bipunctata (L.) as a Generalist Predator of Aphids. Ph.D. thesis, University of East Anglia, Norwich.
Niijima, K., Abe, W., and Matsuka, M. (1997). Development of low-cost and labor-saving artificial diet for mass production of an aphidophagous coccinellid, Harmonia axyridis (Pallas). Bull. Faculty Agric. Tamag. Univ. 37, 63–74.
Nyffeler, M., Olson, E. J., and Symondson, W. O. C. (2016). Plant-eating by spiders. J. Arachnol. 44, 15–27. doi: 10.1636/P15-45.1
Omkar, P. A. (2005). Ecology of two-spotted ladybird, Adalia bipunctata: a review. J. Appl. Entomol. 129, 465–474. doi: 10.1111/j.1439-0418.2005.00998.x
Patt, J. P., Hamilton, G. C., and Lashomb, J. H. (1997). Foraging success of parasitoids wasps on flowers: interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Appl. 83, 21–30. doi: 10.1046/j.1570-7458.1997.00153.x
Pijnakker, J., Arijs, Y., Souza, S. D., Cellier, M., and Wäckers, F. (2015). “The use of Typha angustifolia (cattail) pollen to establish the predatory mites Amblyseius swirskii, Iphiseius degenerans, Euseius ovalis and Euseius gallicus in glasshouse crops,” in Conference Paper, 5th Meeting of the Working Group “Integrated Control of Mite Pest” from September 8th to 10th 2015 (Castelló de la Plana).
Pinheiro, L. A., Torres, L. M., Raimundo, J., and Santos, S. A. P. (2015). Effects of pollen, sugars and honeydew on lifespan and nutrient levels of Episyrphus balteatus. Bio. Control 60, 47–57. doi: 10.1007/s10526-014-9621-8
Pollard, S. D., Beck, M. W., and Dodson, G. D. (1995). Why do male crab spiders drink nectar? Anim. Behav. 49, 1443–1448. doi: 10.1016/0003-3472(95)90065-9
Portillo, N., Alomar, O., and Wäckers, F. L. (2012). Nectarivory by the plant-tissue feeding predator Macrolophus pigmaeus Rambur (Heteroptera: Miridae): nutritional redundancy or nutritional benefit? J. Insect Physiol. 58, 397–401. doi: 10.1016/j.jinsphys.2011.12.013
R Core Team (2014). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available online at: http://www.R-project.org/
Rahaman, S. K. M., and Aniszewski, T. (2014). Ladybird beetles Adalia bipunctata L. interaction with legume bean phaseolus species is dependent on life needs. Malays. Appl. Biol. 43, 111–118.
Resende, A. L. S., Souza, B., Ferreira, R. B., and Aguiar-Menezes, E. L. (2017). Flowers of Apiaceous species as sources of pollen for adults of Chrysoperla externa (Hagen) (Neuroptera). Bio. Control 106, 40–44. doi: 10.1016/j.biocontrol.2016.12.007
Reznik, S. Y., and Vaghina, N. P. (2006). Dynamics of fat content during induction and termination of “trophic diapause” in Harmonia sedecimnotata Fabr. females (Coleoptera, Coccinellidae). Entmol. Rev. 86, 125–132. doi: 10.1134/S0013873806020011
Riahi, E., Fathipour, Y., Talebi, A. A., and Mehrabadi, M. (2017). Linking life table and consumption rate of Amblyseius swirskii (Acari: Phytoseiidae) in presence and absence of different pollens. Ann. Entomol. Soc. Am. 110, 244–253. doi: 10.1093/aesa/saw091
Russell, M. (2015). A meta-analysis of physiological and behavioral responses of parasitoid wasps to flowers of individual plant species. Bio. Control 82, 96–103. doi: 10.1016/j.biocontrol.2014.11.014
Samaras, K., Pappas, M. L., Fytas, E., and Broufas, G. D. (2015). Pollen suitability for the development and reproduction of Amblydromalus limonicus (Acari: Phytoseiidae). Bio. Control 60, 773–782. doi: 10.1007/s10526-015-9680-5
Seagraves, M. P., Kajita, Y., Weber, D. C., Obrycki, J. J., and Lundgren, J. G. (2011). Sugar feeding by coccinellids under field conditions: the effects of sugar sprays in soybean. Bio. Control 56, 305–314. doi: 10.1007/s10526-010-9337-3
Smith, S. F., and Krischik, V. A. (1999). Effects of systemic imidacloprid on Coleomegilla maculata (Coleoptera: Coccinellidae). Environ. Entomol. 28, 1189–1195. doi: 10.1093/ee/28.6.1189
Soares, A. O., Coderre, D., and Schanderl, H. (2004). Dietary self-selection behaviour by the adults of the aphidophagous ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae). J. Anim. Ecol. 73, 478–486. doi: 10.1111/j.0021-8790.2004.00822.x
Somerville, D. C., and Nicol, H. I. (2006). Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust. J. Exp. Agr. 46, 141–149. doi: 10.1071/EA03188
Therneau, T. (2014). A Package for Survival Analysis in S. R package version 2.37-7. Available online at: http://CRAN.R-project.org/package=survival
Togni, P. H. B., Venzon, M., Muniz, C. A., Martins, E. F., Pallini, A., and Sujii, E. R. (2016). Mechanisms underlying the innate attraction of an aphidophagous coccinellid to coriander plants: implications for conservation biological control. Bio. Control 92, 77–84. doi: 10.1016/j.biocontrol.2015.10.002
Toth, E. N., Szabo, L. G., Botz, L., and Orose-Kovacs, Z. (2003). Effect of rootstocks on floral nectar composition in apple cultivars. Plant Syst. Evol. 238, 43–45. doi: 10.1007/s00606-002-0274-1
van Rijn, P. C. J., and Wäckers, F. L. (2016). Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 53, 925–933. doi: 10.1111/1365-2664.12605
Vattala, H. D., Wratten, S. D., Phillips, C. B., and Wäckers, F. L. (2006). The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Bio. Control 39, 179–185. doi: 10.1016/j.biocontrol.2006.06.003
Wäckers, F. L. (2001). A comparison of nectar- and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata. J. Insect Physiol. 47, 1077–1084. doi: 10.1016/S0022-1910(01)00088-9
Warburg, S., Inbar, M., Gal, S., Salomon, M., Palevsky, E., and Sadeh, A. (2018). The effects of a windborne pollen-provisioning cover crop on the phytoseiid community in citrus orchards in Israel. Pest Manag. Sci. 75, 405–412. doi: 10.1002/ps.5129
Williams, C. M., Thomas, R. H., MacMillan, H. A., Marshall, K. E., and Sinclair, B. J. (2011). Triacylglyceride measurement in small quantities of homogenised insect tissue: Comparisons and caveats. J. Insect Physiol. 57, 1602–1613. doi: 10.1016/j.jinsphys.2011.08.008
Winkler, K., Wäckers, F. L., Kaufman, L. V., Larraz, V., and Van Lenteren, J. C. (2009). Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Bio. Control 50, 299–306. doi: 10.1016/j.biocontrol.2009.04.009
Wratten, S. D. (1973). The effectiveness of the coccinellid beetle Adalia bipunctata (L.) as a predator of lime aphid, Eucallipterus tiliae L. J. Anim. Ecol. 42, 785–802. doi: 10.2307/3139
Keywords: non-prey food, Adalia bipunctata, conservation biological control, functional biodiversity, lipid content
Citation: He X and Sigsgaard L (2019) A Floral Diet Increases the Longevity of the Coccinellid Adalia bipunctata but Does Not Allow Molting or Reproduction. Front. Ecol. Evol. 7:6. doi: 10.3389/fevo.2019.00006
Received: 15 June 2018; Accepted: 09 January 2019;
Published: 05 February 2019.
Edited by:
Maria Pappas, Democritus University of Thrace, GreeceReviewed by:
Alberto Pozzebon, University of Padova, ItalyAntónio Onofre Soares, Universidade dos Açores, Portugal
Copyright © 2019 He and Sigsgaard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lene Sigsgaard, les@plen.ku.dk; orcid.org/0000-0001-6478-5079
†Xueqing He, orcid.org/0000-0002-9530-2030
 Xueqing He
Xueqing He Lene Sigsgaard
Lene Sigsgaard