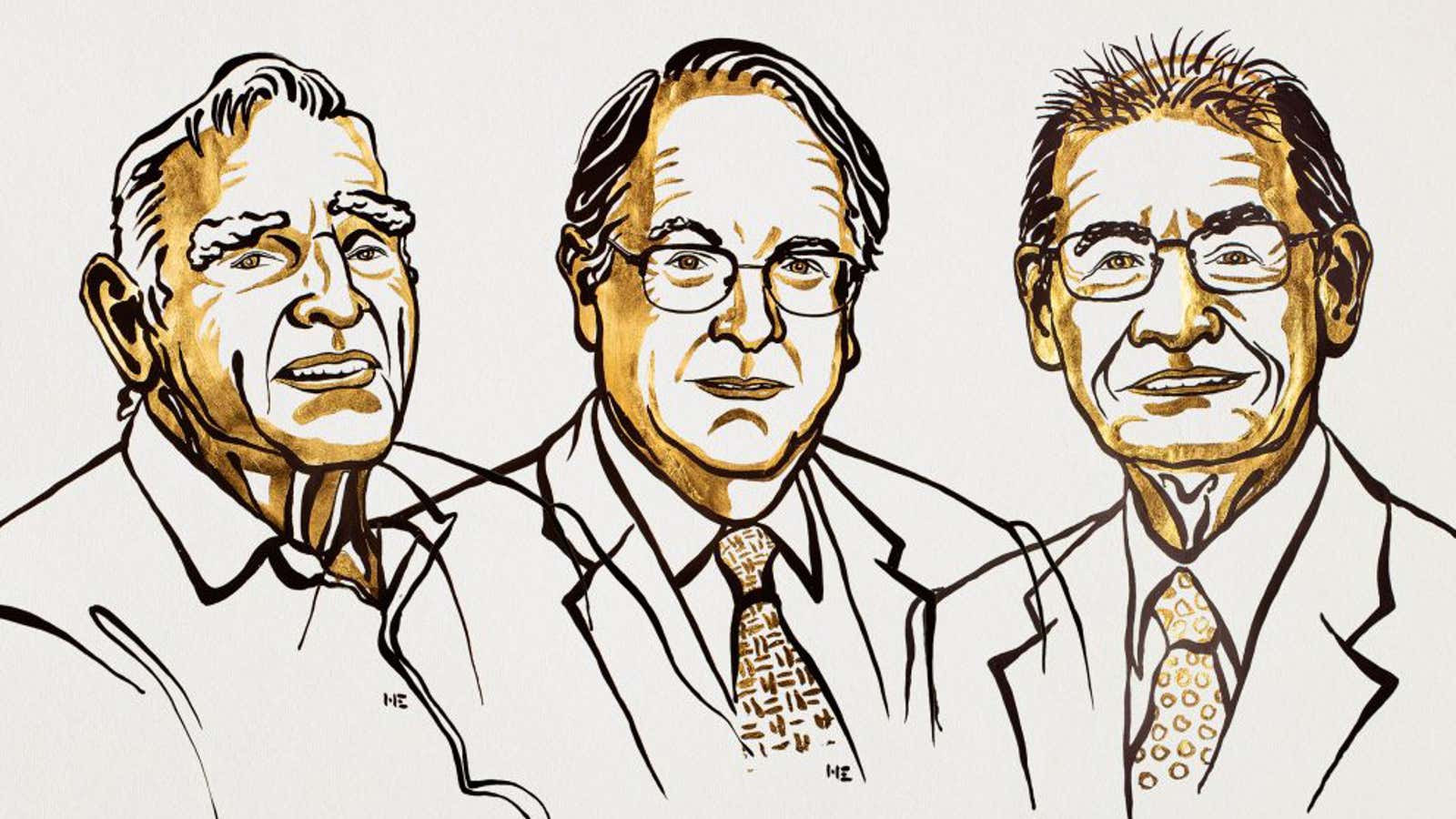
You’re likely reading this news story on a device powered by the invention that won this year’s Nobel Prize in chemistry: lithium-ion batteries. The award went to John Goodenough, professor at the University of Texas at Austin; Stanley Whittingham, professor at the Binghamton University; and Akira Yoshino, professor at Meijo University, each of whom made significant contributions to the development of the world’s most powerful battery.
The lithium-ion battery story started during the oil crises of the 1970s, when companies like Exxon began investing in oil alternatives and new energy sources. Whittingham, a materials scientist, was hired to develop batteries.
Every battery, from the first one invented in 1799, is made of two electrodes—an anode and a cathode—dipped in a conductive solution (called an electrolyte) with a separator that controls the flow of the electric charge. At Exxon, Whittingham realized that lithium metal could be used as an anode, as long as the highly reactive metal was encased and not brought in contact with air or water. And his earlier work had showed titanium disulphide could be used as a cathode to store charged lithium atoms, called lithium ions.
After tweaking and testing, in 1976, Whittingham and his colleagues showed that combining lithium metal and titanium disulphide could not just make a battery, but one that was rechargeable. It brought lighter lithium-based batteries in competition with the bulky rechargeable lead-acid batteries that were used in cars to power electrical needs.
The trouble was, Whittingham’s battery didn’t last very long. During its cycles of charging and discharging, lithium metal had a tendency to form dendrites—long string-like structures—that could cause a short circuit and blow up the battery.
At about the same time, the oil crises ended and a glut of cheap fuel was back. Exxon shut down its battery research. But Whittingham’s working prototype and other work on battery materials ignited wider interest in the field.
One of the researchers inspired by the work was John Goodenough, then at the University of Oxford, who found that instead of titanium disulfide as a cathode, it was better to use cobalt oxide. The cobalt-based battery was able to store more energy and do so more safely.
Meanwhile, on the other side of the world, Yoshino was working in Japan for the chemicals company Asahi Kasei. He found that, instead of using lithium metal, it was better to use graphite as an anode. The material stored fewer lithium atoms, but it also substantially reduced fire risks. (While the use of lithium metal was dropped, the battery still shuttled lithium ions between the electrodes—thus the name lithium-ion battery.)
Yoshio Nishi and Keizaburo Tozawa, both working at Sony, put in the hard work to convert the scientific ideas of Whittingham, Goodenough, and Yoshino into the world’s first commercial lithium-ion battery in 1992. It proved an instant success: Sony sold 3 million units in 1993 and 15 million in 1994.
Since then, engineering and materials developments have made lithium-ion batteries both cheaper and more versatile. They are now in use in everything from tiny devices like wireless earphones to giant ones like electric ships—and, of course, smartphones and electric cars. They can also be used to store renewable energy for later use and to power electric planes.
Crucially, lithium-ion batteries are helping lower greenhouse-gas emissions and tackle humanity’s greatest challenge in climate change. Ironically, that which began its life in an oil company has now become a threat for the entire oil industry.
You can read an in-depth analysis of the lithium-ion battery revolution in Quartz’s field guide published in April. You can read all of Quartz’s coverage of the 2019 Nobel Prizes here.