The Government has today revealed a detailed plan of how its Covid-19 vaccine strategy will be implemented in the weeks and months ahead.
Here is all you need to know about what happens next:
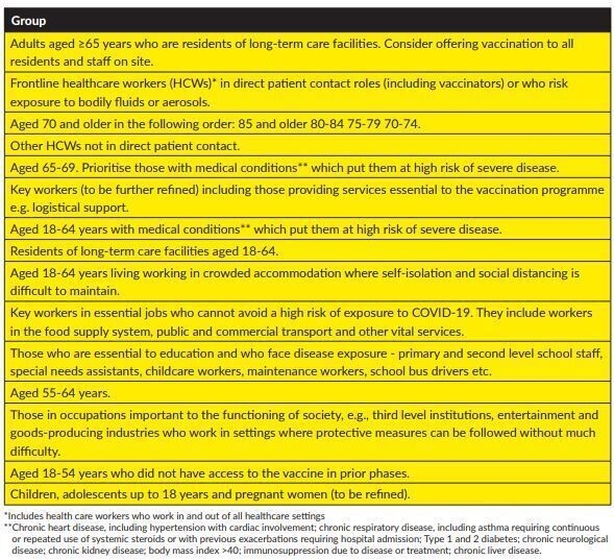
Who can avail of vaccines first?
The highest priority groups, those over the age of 65 living in long-term care facilities and frontline healthcare workers in direct patient contact, will be first to receive the vaccine.
Next in line for the vaccine will be those aged over 70 – of these, the 85 plus age group will be first, followed by those aged 80 to 84; then 75 to 79-year olds and finally people aged 70 to 74.
Other healthcare workers will be next in line for inoculation, followed by those in the 65 to 69 age bracket, prioritising people with pre-existing medical conditions.
Key workers will receive the vaccine next, followed by those in the 18 to 64 age group who are deemed high risk, are resident in long-term resident facilities or live/work in crowded conditions.
When will vaccination begin?
Minister for Health Stephen Donnelly said the decision by the European Medicines Agency (EMA) to bring forward its possible approval of the Pfizer/BioNTech vaccine to 21 December could allow roll-out to begin here before the new year.
The High Level Task Force on vaccination states that once approved by the EMA, "the earliest delivery from the vaccination production site to Ireland is within days".
The vaccines will be delivered in three phases - the initial roll-out, a mass ramp-up and open access.
In the early stages, vaccine supply will be limited as only a small number of vaccines will be approved.
As time progresses, vaccine production will expand and become more widely available to the Irish public.
During this mass ramp-up (the second phase), the Department of Health said it expects that Mass Vaccination Centres will be introduced.
Where will the vaccines be available?
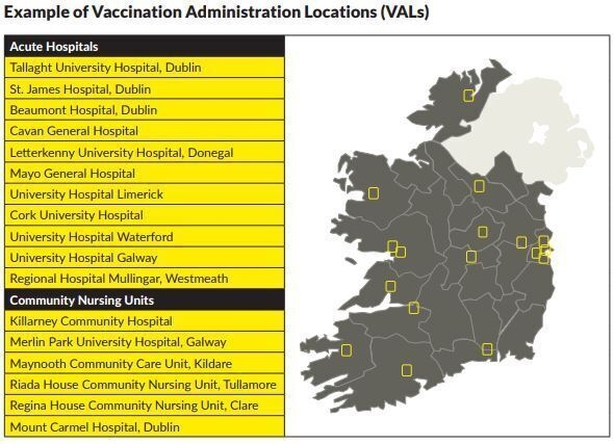
Five types of Vaccination Administration Locations (VALs) have been considered. It is likely that all delivery options will be used at various stages, according to the Department of Health.
The five types of VAL being considered are:
- Long-Term Residential Care Facilities
- Large Scale Healthcare Sites
- Mass Vaccination Centres (MVC)
- General Practice
- Community Pharmacy
Initially, Covid-19 community vaccination teams will be deployed to administer vaccines to the residents and staff of Long-Term Care Facilities.
The Department has identified a number of large scale healthcare sites that will be used as hubs for mobile vaccination teams to collect vaccines from storage.
There also has been "positive engagement" with Nursing Homes Ireland, to identify a number of private nursing homes to be among the locations for the early phase of the vaccination programme.
Mass Vaccination Centres (MVCs) will be located regionally and designed to cater for large numbers of recipients in an efficient and timely manner.
Discussions are under way with the relevant authorities to ensure that a geographical distribution of such MVCs is provided.
As more vaccines are approved and a broader population is targeted for vaccination, General Practice and community pharmacies will play an increasing role in vaccine administration.
Will I have to pay for a coronavirus vaccine?
No, Vaccines will be available free of charge to everyone in Ireland.
How will the vaccination process work?
Members of nominated groups for vaccination will be invited to register for, and consent to, vaccination and then offered scheduled appointments.
At the vaccination site, a person's details will be recorded alongside the batch details and a time/date stamp.
The recipient will be asked to wait for 15 minutes post-vaccination to monitor for any immediate adverse reaction.
Follow-up reminders will be issued to ensure that a person returns for their second dose three weeks later. The recipient will be able to report any suspected side effects experienced on a portal on the Health Products Regulatory Authority website.
Who will administer the vaccine?
All vaccinators will be qualified and registered healthcare professionals who will receive "comprehensive and specialist" training relevant to the types of Covid-19 vaccines they will administer.
The trained vaccination workforce currently identified includes up to 180 dedicated community-based specialist vaccinators and approximately 1,500 healthcare workers who were trained as peer vaccinators, in the recent flu vaccination campaign.
GPs and pharmacists "can offer enhanced capacity for this programme subject to agreement".
Options being considered to scale up the vaccination workforce include rehiring recently retired medical and nursing staff, asking staff to work extra hours and contracting private vaccination service.
Is the vaccine safe?
The EMA is the expert regulatory body tasked with approving all vaccines for the EU. It will not give any drug the go-ahead if there are safety concerns.
The World Health Organization recently reassured people that the process behind developing a Covid-19 vaccine was accelerated but "no corners have been cut".
Here in Ireland, the Deputy Chief Medical Officer Ronan Glynn issued a statement this week, explaining that it is understandable that people would have questions and concerns about the quick delivery of the vaccine.
Dr Glynn said the shorter timeline was due to research and scientific endeavour alongside global investment "on a scale never seen before" in relation to vaccines.
He said that the manufacturing process also began in parallel with the phase three trials, so that it would be on the ground and ready to deploy in some numbers if approved.
Professor Karina Butler, consultant paediatrician and Chair of the National Immunisation Advisory Committee, and Dr Cillian De Gascun, director of the National Virus Reference Laboratory, recently answered several important questions about Covid-19 vaccines.
