A British teenager has made a remarkable recovery after being the first patient in the world to be given a genetically engineered virus to treat a drug-resistant infection.
Isabelle Holdaway, 17, nearly died after a lung transplant left her with an intractable infection that could not be cleared with antibiotics. After a nine-month stay at Great Ormond Street hospital, she returned to her home in Kent for palliative care, but recovered after her consultant teamed up with a US laboratory to develop the experimental therapy.
The scientists behind the breakthrough have said bacteria-killing viruses, known as phages, have the potential to be used as an alternative treatment to counter the growing crisis of resistance to antibiotics.
Isabelle’s mother, Jo, who made the initial suggestion of phage therapy to doctors at Great Ormond Street after reading about it online, said her daughter was “the luckiest child on earth” to have received the treatment in time.
“It’s incredible medical science. It’s been a miracle,” she said.
Isabelle has cystic fibrosis, a genetic disease that results in frequent infections clogging up the lungs with mucus. By summer 2017, her lungs had less than a third of their normal function and she had been plagued by two stubborn bacterial strains for eight years. She and her doctors decided a double lung transplant was the best option, even though it meant her existing infections could spread.
After the transplant, this fear became a reality: a bacterial strain similar to TB took hold, colonising her surgical wound, then her liver, until eventually pockets of bacteria known as nodules began pushing through the skin on her arms, legs and buttocks.
“She lost so much weight. She was literally like a skeleton,” Jo said. “The bacteria was just coming through her skin. There was nothing they could do to make her comfortable. It was horrific.”
She searched the internet for alternative treatments and on reading about phage therapies asked Isabelle’s consultant, Helen Spencer, whether this might be an option.
By chance, a Great Ormond Street colleague was in touch with Prof Graham Hatfull, a scientist who had spent more than three decades amassing a collection of phages, stored in 15,000 frozen vials at the University of Pittsburgh. Hatfull agreed to help find a treatment for Isabelle and another teenage patient at Great Ormond Street who had a life-threatening infection.
Phages work by infecting bacteria cells and killing them, but they are very specific in which infections they can target. Hatfull and colleagues identified dozens of phages known to infect bacterial relatives of the patients’ strains, and tested thousands of combinations of them in petri dishes to see which wiped out the patients’ bacteria.
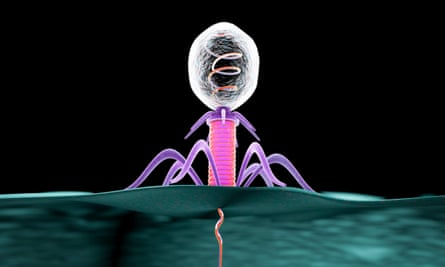
“The idea is to use [phages] as antibiotics – as something we could use to kill bacteria that cause infection,” Hatfull said.
In January 2018 they found a phage that could hit the strain that infected one of the teenagers. But they were too late: the patient had died earlier that month. Around the same time, Jo had been told her daughter was unlikely to survive.
Each experiment took a week because the bacteria grow slowly. Shortly after Isabelle was sent home on a palliative care plan, Hatfull’s lab identified a phage that wiped out the infection, and another two phages that could infect it but not kill it efficiently. By removing a single gene, they were able to increase the efficiency of these two phages, making a cocktail that they believed could kill the infection. A combination was used to avoid the possibility of the infection becoming resistant to the phage.
In June 2018, Isabelle returned to Great Ormond Street and after some safety tests, was given the cocktail twice daily via an intravenous drip and on her skin. Six weeks later a liver scan revealed the infection had essentially disappeared.
Jo said: “The open wounds on her wrist healed. You could see her sparkle coming back to the point where she was getting up, eating, arguing with her sister.”
There were almost no side effects.
Hatfull said: “We didn’t think we’d ever get to a point of using these phages therapeutically. It’s a brilliant outcome.”
Isabelle is still on the treatment and it has been a slow, steady recovery. All but one of her skin nodules have cleared. She has returned to school, is studying for A-levels, has started a part-time job and is learning to drive.
Her case, outlined in the journal Nature Medicine, follows the successful treatment last year of a US patient infected with a drug-resistant superbug and comes as the looming crisis of antibiotic resistance is fuelling a growing interest in phage research.
As Isabelle’s treatment was not part of a clinical trial, it is impossible to know how effective the therapy would be for others; the authors want larger clinical trials to establish this.
Spencer said: “The bigger question is whether it could be used to treat other resistant bacteria.”
Finding the right phages for each patient is a big challenge. In the future, scientists hope it may be possible to conduct automated searches of phage libraries to identify personalised treatments. Some infections, such as the hospital superbug Staphylococcus aureus, are known to be genetically homogeneous enough that a few phages could treat almost all strains of the infection, raising the prospect of phage therapy becoming routine.
“We’re sort of in uncharted territory,” Hatfull said.
Jason Gill, a senior scientist at the centre for phage technology at Texas A&M University, said phages could have huge potential to tackle drug-resistant infections.
“It’s probably going to turn out that the phages are going to be really effective for some conditions, and others they won’t work that well,” he said.
