As Israel goes vaccine-wild, will the homegrown version lose its shot?
Phase II of Brilife trial launches, but success of current immunization drive in quashing coronavirus may make effective testing impossible, and some who get placebo could drop out
Nathan Jeffay is The Times of Israel's health and science correspondent
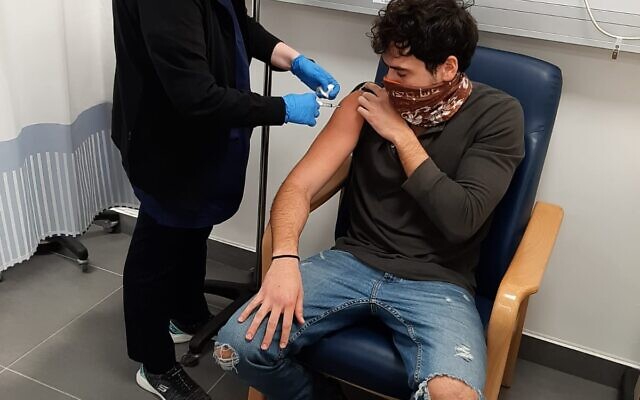
Israel’s full-steam-ahead vaccination drive has thrown a wrench in the works for development of the country’s own COVID shot, which has just started Phase II trials.
Israel leads the world in per capita vaccination and is hoping to have much of the country inoculated by early spring — around the same time developers of the Brilife vaccine are planning to launch a large-scale trial to test the shot’s efficacy. But if the country is essentially coronavirus free by then, or close to it, it will be impossible to know if the test works.
Beyond that, there is a sticky ethical question regarding keeping thousands of people from being vaccinated and then giving them a placebo that puts them at unnecessary risk of infection when doses of a tried-and-tested vaccine are being given across the country.
As the Phase II trial for Israel’s Brilife vaccine got underway on Monday at Sheba Medical Center, Dr. Eytan Ben Ami, who is overseeing the testing, admitted that Israel’s move toward COVID-19 safety is creating new challenges.
“For the time being we’re continuing with the original trial design, but I really can’t tell what will be by end of the trial,” said Ben Ami.

He spoke as the first volunteers for the Phase II trial were being given the shot, which was developed by the Institute for Biological Research, a Defense Ministry body.
The first round of tests found only minor side effects, and Phase II will see a total of 1,000 volunteers injected at several hospitals with either the vaccine candidate or a saline placebo to test for safety and effectiveness. It is scheduled to run for a year, though researchers are hoping to have enough data within a few months to begin the much larger Phase III trial.
Despite the availability of the Pfizer-BioNTech shot in Israel, Ben Ami said he has still been inundated with volunteer applications for the Brilife trial.
Israel is racing to become the first country in the world with herd immunity, with some 10 million doses of Pfizer and Moderna vaccines reportedly scheduled to arrive in coming months.
Millions of doses of the Pfizer vaccine have already arrived and almost 500,000 people have been vaccinated in just over a week. For now, only those 60 years or older or in a high-risk category are supposed to be getting the shot, but healthcare providers are preparing to soon expand the campaign to the general public.

But the faster the country heals, the longer it will take to gauge whether the Brilife vaccine works, since potential exposure to the coronavirus will be limited. In the US, a resurgence of the virus in the fall helped speed up trials by pharmaceutical companies there.
Ben Ami said recruiting 30,000 healthy unvaccinated people for the Phase III trial will likely prove impossible anyway, and the only option may be to work with hospitals abroad.
“In order to have the vaccine in a timely manner, I think it would have to go overseas,” Ben Ami said.
A spokesperson from the Defense Ministry told The Times of Israel on Tuesday that the location of the third phase was still being evaluated.
Prof. Jonathan Gershoni, a vaccine expert from Tel Aviv University who is unconnected to the Brilife trials, said developers around the world are grappling with the challenge of how to test new vaccines as more people get protected and as the number of coronavirus cases falls.
He said he was confident that companies would find scientifically sound ways to complete trials despite new challenges.
“I’m certain that the reality is there are many vaccines that still need testing, and I’m sure companies will devise means to run reliable trials and not need to disqualify them,” he said. “They will figure out how to do it.”

Some have also questioned whether there is any sense in pursuing the development of a drug when there are many other candidates already in late-stage development.
“I have a great belief in the Israeli vaccine,” Jonathan Halevy, president of Jerusalem’s Shaare Zedek Medical Center, said in a press briefing recently. “But I don’t think it’s going to be ready for approval or use before a year or two year’s time, and the question is what will happen with COVID-19 by then, and I am the eternal optimist and think it will not be with us.”
Ben Ami said, however, that developing Brilife is still important, given that researchers do not know how long the vaccines provide immunity for. Having a homemade shot will ensure a steady supply should patients need to boost immunity in the future.
In addition, the Israeli shot is being developed as a single dose, making it potentially more attractive than the other vaccines, which must be given in two separate doses. Developing countries in particular may find the logistics of the current two-shot vaccines difficult, Ben Ami said.
Some other doctors have pointed to the value of Brilife as it could be tweaked to inoculate against other viruses in the future.
Researchers are also wrestling with how to deal with trial participants who, wishing to make sure they are vaccinated, get a separate dose outside the test — potentially skewing efficacy and safety results.

In the United States, doctors debated what should happen in such scenarios during a US Food and Drug Administration panel, and some argued that the trial should continue as if nothing has changed.
“You disclose to them at the outset that it’s possible they’re going to receive a placebo,” said Harvard Law professor Glenn Cohen. “It’s not considered unethical.”
But in Israel, volunteers are being told that as soon as they have the chance to get an approved vaccine, they can report to the hospital administering the Brilife trial, where they will be told whether they have been given the actual shot or a placebo. The 75% of volunteers receiving a dose of Brilife will likely rely on it as their COVID-19 protection and not take another shot, but some placebo patients are expected to bow out and get protected.
Their general health data will still be collected in case it turns out to have any relevance, but they won’t provide the comparison they are supposed to bring to the trial, namely how many infections placebo patients are picking up compared to patients given a prospective vaccination.
“It does mess up the results a little,” Ben Ami said. “But patient safety comes first. If they received the placebo they have the right to go to get vaccinated. We have to work within the reality that there is an available vaccine.”
The Defense Ministry spokesperson said that the trial will be adjusted as necessary, while maintaining its scientists’ “ability to draw conclusions about the safety and effectiveness of the institute’s vaccine.”
Ben Ami doesn’t expect the challenge to derail the Brilife trial, but admitted it could undermine the blinded nature of the trial. Currently, participants and doctors do not know who has been given the actual shot or a placebo, but that data will be necessary to know whether someone can or needs to be immunized.

Gershoni said one possibility for keeping the study blinded that’s under discussion in professional circles is not revealing to volunteers what they have initially been injected with — if they show up and report that they have a chance to get an approved vaccine — but rather giving placebo recipients a real shot, and vaccine recipients a placebo shot.
The idea was mooted by Dr. Anthony S. Fauci, the director of the National Institute of Allergy and Infectious Diseases in the US, but Pfizer has questioned the practicality of such a possibility.
Ben Ami insisted that even if all placebo patients end up taking a foreign vaccine, the overall research would still be reliable enough to progress to the next round of testing,
In the absence of Israeli placebo data, Brilife participants could be compared to a placebo cohort from a similar vaccine trial in another country, he said.
And if infection levels from volunteers who receive the actual shot are low, it will be adequate to move on to a Phase III trial in a country that has an unvaccinated population and, by extension, active coronavirus cases.