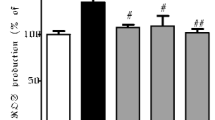
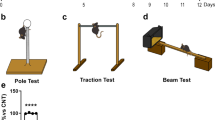
Abstract
Besides motor disorder, cognitive dysfunction is also common in Parkinson’s disease (PD). Essentially no causal therapy for cognitive dysfunction of PD exists at present. In this study, a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of PD was used to analyze the neuroprotective potential of orally administered silibinin, a proverbial hepatoprotective flavonoid derived from the herb milk thistle (Silybum marianum). Results demonstrated that silibinin administration significantly attenuated MPTP-induced cognitive impairment in behavioral tests. Nissl staining results showed that MPTP injection significantly increases the loss of neurons in the hippocampus. However, these mice were protected by oral administration of silibinin, accompanying reduction in the cell apoptosis in the hippocampus. The hippocampal aggregates of α-synuclein (α-syn) appeared in MPTP-injected mice, but were significantly decreased by silibinin treatment. MPTP injection induced oxidative stress, as evidenced by increased malondialdehyde (MDA) and decreased superoxide dismutase (SOD). The oxidative stress was alleviated by silibinin treatment. Mitochondrial disorder including the decline of mitochondrial membrane potential (MMP) was another signature in the hippocampus of MPTP-treated mice, accompanying increased mitochondrial fission and decreased fusion. Silibinin administration restored these mitochondrial disorders, as expected for the protection against MPTP injury. These findings suggest that silibinin has a potential to be further developed as a therapeutic candidate for cognitive dysfunction in PD.











Similar content being viewed by others
References
Hoehn MM, Yahr MD (2001) Parkinsonism: onset, progression, and mortality. Neurology 57:11–26
Yilmaz NH, Calisoglu P, Guntekin B, Hanoglu L (2020) Correlation between alpha activity and neuropsychometric tests in Parkinson’s disease. Neurosci Lett 738:135346
Bostantjopoulou S, Katsarou Z, Papadimitriou A, Veletza V, Hatzigeorgiou G, Lees A (2001) Clinical features of parkinsonian patients with the alpha-synuclein (G209A) mutation. Mov Disord 16:1007–1013
Litvan I, Mohr E, Williams J, Gomez C, Chase TN (1991) Differential memory and executive functions in demented patients with Parkinson’s and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 54:25–29
Orgeta V, McDonald KR, Poliakoff E, Hindle JV, Clare L, Leroi I (2020) Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2:CD011961
Disbrow E, Carmichael O, He J, Lanni K, Dressler E, Zhang L, Malhado-Chang N, Sigvardt KJJ (2014) Resting state functional connectivity is associated with cognitive dysfunction in non-demented people with Parkinson’s disease. J Parkinson Dis 4:453–465
Braak H, Del Tredici K, Rub U, de Vos RAI, Steur ENHJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Del Tredici K, Braak H (2016) Review: Sporadic Parkinson’s disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol 42:33–50
Ibarretxe-Bilbao N, Ramirez-Ruiz B, Tolosa E, Marti MJ, Valldeoriola F, Bargallo N, Junque C (2008) Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol 255:1324–1331
Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D (2014) Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 137:2493–2508
Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, Davatzikos C, Clark CM, Siderowf A (2012) Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 135:170–180
Dinter E, Saridaki T, Diederichs L, Reichmann H, Falkenburger BH (2020) Parkinson’s disease and translational research. Transl Neurodegener 9:43
Cheng GX, Yin SB, Yang YH, Hu YH, Huang CY, Yao QM, Ting WJ (2021) Effects of bilateral subthalamic nucleus deep brain stimulation on motor symptoms in Parkinson’s disease: a retrospective cohort study. Neural Regen Res 16:905–909
Campos FL, Carvalho MM, Cristovao AC, Je G, Baltazar G, Salgado AJ, Kim YS, Sousa N (2013) Rodent models of Parkinson’s disease: beyond the motor symptomatology. Front Behav Neurosci 7:175
Schrag A, Jahanshahi M, Quinn NP (2001) What contributes to depression in Parkinson’s disease? Psychol Med 31:65–73
Klepac N, Trkulja V, Relja M, Babic T (2008) Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol 15:128–133
Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108:3017–3022
Lee Y, Chun HJ, Lee KM, Jung YS, Lee J (2015) Silibinin suppresses astroglial activation in a mouse model of acute Parkinson’s disease by modulating the ERK and JNK signaling pathways. Brain Res 1627:233–242
Lee Y, Park HR, Chun HJ, Lee J (2015) Silibinin prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease via mitochondrial stabilization. J Neurosci Res 93:755–765
Song X, Zhou B, Cui L, Lei D, Zhang P, Yao G, Xia M, Hayashi T, Hattori S, Ushiki-Kaku Y, Tashiro SI, Onodera S, Ikejima T (2017) Silibinin ameliorates Abeta25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem Res 42:1073–1083
Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, Hayashi T, Xia M, Tashiro S, Onodera S, Ikejima T (2016) Protective effect of silibinin on learning and memory impairment in LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res 41:1662–1672
Bove J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2:484–494
Bus JS, Gibson JE (1984) Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect 55:37–46
Kopin IJ (1987) MPTP: an industrial chemical and contaminant of illicit narcotics stimulates a new era in research on Parkinson’s disease. Environ Health Perspect 75:45–51
Trudler D, Sanz-Blasco S, Eisele YS, Ghatak S, Bodhinathan K, Akhtar MW, Lynch WP, Pina-Crespo JC, Talantova M, Kelly JW, Lipton SA (2021) Alpha-synuclein oligomers induce glutamate release from astrocytes and excessive extrasynaptic NMDAR activity in neurons, thus contributing to synapse loss. J Neurosci. https://doi.org/10.1523/jneurosci.1871-20.2020
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311
Kwon S-H, Kim H-C, Lee S-Y, Jang C-G (2009) Loganin improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol 619:44–49
Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Methods in molecular biology 594:57–72
Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW (2004) Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 56:173–181
Ressler KJ, Mayberg HS (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10:1116–1124
Kadar A, Wittmann G, Liposits Z, Fekete C (2009) Improved method for combination of immunocytochemistry and Nissl staining. J Neurosci Methods 184:115–118
Bolner A, Micciolo R, Bosello O, Nordera GP (2016) A panel of oxidative stress markers in Parkinson’s disease. Clin Lab 62:105–112
Zheng J, Lu C (2020) Oxidized LDL causes endothelial apoptosis by inhibiting mitochondrial fusion and mitochondria autophagy. Front Cell Dev Biol 8:600950
Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159:931–938
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844
Aarsland D, Brønnick K, Fladby T (2011) Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep 11:371–378
Kujawska M, Jodynis-Liebert J (2018) Polyphenols in Parkinson’s disease: a systematic review of in vivo studies. Nutrients 10:642
Song X, Liu B, Cui L, Zhou B, Liu L, Liu W, Yao G, Xia M, Hayashi T, Hattori S, Ushiki-Kaku Y, Tashiro SI, Ikejima T (2018) Estrogen receptors are involved in the neuroprotective effect of silibinin in abeta1-42-treated rats. Neurochem Res 43:796–805
Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA (2013) The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 84:1258–1264
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of parkinson disease: a review. JAMA 323:548–560
Miyamoto M, Miyamoto T (2020) Relationship of substantia nigra hyperechogenicity to risk of Lewy body disease in idiopathic REM sleep behavior disorder patients: a longitudinal study. Sleep Med 68:31–34
Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK (2013) Curcumin modulates alpha-synuclein aggregation and toxicity. ACS Chem Neurosci 4:393–407
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48
Brazdis RM, Alecu JE, Marsch D, Dahms A, Simmnacher K, Lorentz S, Brendler A, Schneider Y, Marxreiter F, Roybon L, Winner B, Xiang W, Prots I (2020) Demonstration of brain region-specific neuronal vulnerability in human iPSC-based model of familial Parkinson’s disease. Hum Mol Genet 29:1180–1191
McGlinchey RP, Lacy SM, Huffer KE, Tayebi N, Sidransky E, Lee JC (2019) C-terminal alpha-synuclein truncations are linked to cysteine cathepsin activity in Parkinson’s disease. J Biol Chem 294:9973–9984
Vasquez V, Mitra J, Wang H, Hegde PM, Rao KS, Hegde ML (2020) A multi-faceted genotoxic network of alpha-synuclein in the nucleus and mitochondria of dopaminergic neurons in Parkinson’s disease: Emerging concepts and challenges. Prog Neurobiol 185:101729
Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E (2000) α-Synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol 157:401–410
Alim MA, Ma QL, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T, Kaji H, Yoshii M, Hisanaga S, Ueda K (2004) Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J Alzheimers Dis 6:435–442; discussion 443 – 439
Hashimoto M, Kawahara K, Bar-On P, Rockenstein E, Crews L, Masliah E (2004) The Role of alpha-synuclein assembly and metabolism in the pathogenesis of Lewy body disease. J Mol Neurosci 24:343–352
Ma P, Yun J, Deng H, Guo M (2018) Atg1-mediated autophagy suppresses tissue degeneration in pink1/parkin mutants by promoting mitochondrial fission in Drosophila. Mol Biol Cell 29:3082–3092
Tatsuta T, Langer T (2008) Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J 27:306–314
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20:31–42
Chandra G, Shenoi RA, Anand R, Rajamma U, Mohanakumar KP (2019) Reinforcing mitochondrial functions in aging brain: An insight into Parkinson’s disease therapeutics. J Chem Neuroanat 95:29–42
Jayaraj RL, Beiram R, Azimullah S, Meeran MFN, Ojha SK, Adem A, Jalal FY (2019) Lycopodium attenuates loss of dopaminergic neurons by suppressing oxidative stress and neuroinflammation in a rat model of Parkinson’s disease. Molecules 24:2182
Ambani LM, Van Woert MH, Murphy S (1975) Brain peroxidase and catalase in Parkinson disease. Arch Neurol 32:114–118
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Wang, C., Liu, W. et al. Oral Administration of Silibinin Ameliorates Cognitive Deficits of Parkinson’s Disease Mouse Model by Restoring Mitochondrial Disorders in Hippocampus. Neurochem Res 46, 2317–2332 (2021). https://doi.org/10.1007/s11064-021-03363-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03363-5