This article was last updated on April 16, 2022
Canada: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
USA: ![]() Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
Oye! Times readers Get FREE $30 to spend on Amazon, Walmart…
While Big Pharma, governments and the media would have us all believing that the COVID-19 vaccines are going to be the only solution to the spread of the SARS-CoV-2 virus, a recent paper from Ronald Brown at the School of Public Health and Health Systems at the University of Waterloo puts the actual effectiveness of the experimental and unprecedented mRNA vaccines into perspective by differentiating the concepts of relative risk reduction and absolute risk reduction.
Let's start by looking at the definition for both risk reduction terms knowing that CER is the control group event rate, EER is the experimental group rate.
1.) Relative risk reduction (RRR) – tells you by how much the treatment reduced the risk of bad outcomes relative to the control group who did not have the treatment. The formula for calculating RRR is CER-EER/CER.
2.) Absolute risk reduction (ARR) – is the absolute difference in outcome rates between the control and treatment groups. The formula for calculating ARR is CER-EER.
If you want a very simple way of thinking about absolute risk reduction, it can be thought of as the number of people that need to be vaccinated to prevent one more case of a disease (in the current reality, COVID-19).
Let's look at an example. Neuropathy occurred in 9.6 percent of a group that received no care and 2.8 percent of a group that took medications to reduce the risk of neuropathy. In this case, the relative risk reduction is (0.096-0.028)/0.096 = 0.71 or 71 percent in the treated group compared to the non-treated group. Absolute risk reduction would be (0.096-0.028) = 0.068 or 6.8 percent. Note that the relative risk reduction is far larger than the absolute risk reduction.
Here is a quote about the two types of risk:
"One problem with the relative risk measure is that without knowing the level of risk in the control group, one cannot assess the effect size in the treatment group. Treatments with very large relative risk reductions may have a small effect in conditions where the control group has a very low bad outcome rate. On the other hand, modest relative risk reductions can assume major clinical importance if the baseline (control) rate of bad outcomes is large."
Now, let's look at a quote from a November 2020 article by Peter Doshi posted on the BMJ Opinion website which suggests that Brown's research was needed (all bolds are mine):
"In the United States, all eyes are on Pfizer and Moderna. The topline efficacy results from their experimental covid-19 vaccine trials are astounding at first glance. Pfizer says it recorded 170 covid-19 cases (in 44,000 volunteers), with a remarkable split: 162 in the placebo group versus 8 in the vaccine group. Meanwhile Moderna says 95 of 30,000 volunteers in its ongoing trial got covid-19: 90 on placebo versus 5 receiving the vaccine, leading both companies to claim around 95% efficacy.
Let’s put this in perspective. First, a relative risk reduction is being reported, not absolute risk reduction, which appears to be less than 1%. Second, these results refer to the trials’ primary endpoint of covid-19 of essentially any severity, and importantly not the vaccine’s ability to save lives, nor the ability to prevent infection, nor the efficacy in important subgroups (e.g. frail elderly). Those still remain unknown. Third, these results reflect a time point relatively soon after vaccination, and we know nothing about vaccine performance at 3, 6, or 12 months, so cannot compare these efficacy numbers against other vaccines like influenza vaccines (which are judged over a season). Fourth, children, adolescents, and immunocompromised individuals were largely excluded from the trials, so we still lack any data on these important populations….
There may be much more complexity to the “95% effective” announcement than meets the eye—or perhaps not. Only full transparency and rigorous scrutiny of the data will allow for informed decision making. The data must be made public."
You will note that Peter Doshi, Associate Editor of the British Medical Journal notes that relative risk reduction is being reported by Pfizer and Moderna rather than absolute risk reduction.
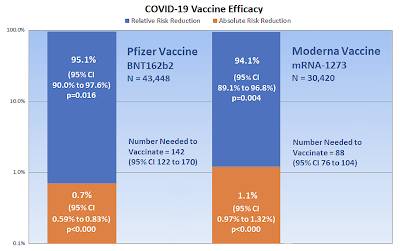
Now, let's go back to Brown's paper. Here is a chart from the paper showing the critical appraisal results of Pfizer's and Moderna's COVID-19 vaccines:
As you can see, Big Pharma has publicly touted its 95.1 percent and 94.1 percent relative risk reduction data which makes both the BNT162b2 vaccine and mRNA-1273 vaccine look like miraculous pharmaceuticals when it comes to defeating the SARS-CoV-2 virus. That said, if you look at the absolute risk reduction for both vaccines, they are far less impressive, coming in at 0.7 percent for Pfizer's vaccine and 1.1 percent for Moderna's vaccine. In the case of Pfizer's vaccine, 142 people need to be vaccinated to reduce the number of COVID-19 cases by one and in the case of Moderna's vaccine, 88 people would have to be vaccinated to reduce the number of COVID-19 cases by one. For every million people vaccinated with both doses as per the manufacturers' recommendations, on average, both vaccines would reduce the number of cases by 8,696 in total. In other words, neither vaccine should be expected to actually reduce the risk of contracting COVID-19 in the real world.
The author also notes the following issues with the COVID-19 vaccine trails:
"Vaccine clinical trial case definitions for SARS-CoV-2 infection included COVID-19 clinical symptoms; thus the trials were not designed to provide evidence of vaccine efficacy for protection against asymptomatic infections. In addition to outcome reporting bias, information bias may have also affected COVID-19 vaccine trial outcomes due to misclassification of SARS-CoV-2 infections as mild adverse effects of the vaccines. For example, several COVID-19 clinical symptoms are similar to the vaccines’ adverse effects such as fever, pain, and fatigue, which could potentially lead to missed diagnoses of viral infections."
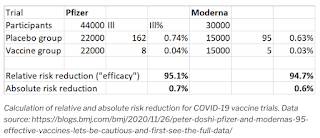
As an aside, here is an analysis of the same risk issues that appeared on the Ryerson Geographic Information Science and Systems website by Dr. Claus Rinner with my bold:
"The concept of risk, and our ability to assess risk, has also made the headlines in the context of the COVID-19 vaccine trials. Using data from a Nov 26 opinion piece in the British Medical Journal (BMJ), we can see that vaccine efficacy in terms of the relative reduction of the risk of getting ill is around 95%. For example, in the Pfizer trial, assuming an equal split of the 44,000 participants into the vaccine and placebo groups, 0.74% of the placebo group fell ill but only 0.04% of the vaccinated participants did. The relative risk reduction is calculated as the difference between these two incidences (0.7%) divided by the placebo value (0.74%), arriving at the conclusion that 95% of COVID-19 could be avoided if people got immunized. However, there is another way of looking that the same data: The risk reduction in absolute terms is only 0.7%, from an already very low risk of 0.74% to a minimal risk of 0.04%. Thus, risk reduction is 95%, but it also is just 0.7%."
Here is a table summarizing Dr. Rinner's analysis:
Let's close with this conclusion from Ronald Brown's analysis:
"A critical appraisal of phase III clinical trial data for the Pfizer/BioNTech vaccine BNT162b2 and Moderna vaccine mRNA-1273 shows that absolute risk reduction measures are very much lower than the reported relative risk reduction measures. Yet, the manufacturers failed to report absolute risk reduction measures in publicly released documents. As well, the U.S FDA Advisory Committee (VRBPAC) did not follow FDA published guidelines for communicating risks and benefits to the public, and the committee failed to report absolute risk reduction measures in authorizing the BNT162b2 and mRNA-1273 vaccines for emergency use. Such examples of outcome reporting bias mislead and distort the public’s interpretation of COVID-19 mRNA vaccine efficacy and violate the ethical and legal obligations of informed consent."
I couldn't have said it better myself. Unfortunately, governments and their mainstream media lapdogs have completely failed to report this critical information to a public that is practically clambering over itself to be vaccinated, believing that it is the ONLY solution for a return to a "normal life". Obviously, governments are going to find it far easier to sell the experiment using a risk reduction of 95 percent than they are trying to sell it using a risk reduction of less than 1 percent.
Addendum
If you wish to read more on the subject of risk reduction, please click on this link which will take you to an article recently published on the Lancet website entitled "COVID-19 vaccine efficacy and effectiveness – the elephant (not) in the room".
You can publish this article on your website as long as you provide a link back to this page.

Be the first to comment