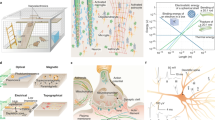
Abstract
Neural probes are among the most widely applied tools for studying neural circuit functions and treating neurological disorders. Given the complexity of the nervous system, it is highly desirable to monitor and modulate neural activities simultaneously at the cellular scale. In this review, we provide an overview of recent developments in multifunctional neural probes that allow simultaneous neural activity recording and modulation through different modalities, including chemical, electrical, and optical stimulation. We will focus on the material and structural design of multifunctional neural probes and their interfaces with neural tissues. Finally, future challenges and prospects of multifunctional neural probes will be discussed.
Similar content being viewed by others
Introduction
A central goal in both basic and translational neuroscience is to understand the neural circuit mechanisms underlying complex behaviors1,2. In the brain, neural circuits process information through a temporal sequence of bioelectrical pulses from neurons3, which involves the inward and outward flow of ions through transmembrane protein channels4. Neural probes, which transduce extracellular ionic currents into electrical signals, provide a bridge between the biotic and abiotic world and have been one of the most applied tools in neural activity recording5. Over the past decades, neural probes with different materials and structures have been developed to record activity from large groups of neurons at single-cell resolution6,7,8. In particular, the transition from rigid to soft neural probes has greatly improved the interface between neural probes and brain tissues, enabling stable neuronal activity recordings over long periods of time9,10,11,12.
The central nervous system of mammals consists of various types of neurons. To unravel the causal relationships between neural circuit activity and brain function, it is necessary to simultaneously record and manipulate the activity of specific types of neurons in the brain. Over the past few years, a variety of multifunctional neural probes with integrated recording and stimulating units have been developed13,14,15,16. Multifunctional neural probes provide a powerful platform to simultaneously monitor the activities of neurons and their responses to well-controlled stimuli17,18,19. In this review, we summarize recent developments in multifunctional neural probes, with an emphasis on their structural and material designs. Multifunctional neural probes with different modulation modalities, including chemical, electrical, and optical stimulation, will be highlighted.
Multifunctional probes for simultaneous neural recording and chemical delivery
Neuromodulator administration has been widely used to modulate neural activities and treat neurological disorders in medicine. Neuromodulators are generally infused by intravenous and oral administrations, which have a limited ability to reach a targeted brain region due to the presence of the blood‒brain barrier20. Moreover, neuromodulator drugs in the bloodstream may cause deleterious side effects and affect important physiological functions, such as memory and learning21. Direct brain infusion is the most effective method to deliver neuromodulators into the brain. Direct brain infusion has been accomplished by microinjection through glass/steel capillaries22,23. However, this technique cannot record neural activity responses to neuromodulators and thus lacks the capability to reveal the functional roles of neuromodulators.
To address the above issue, drug delivery systems have been integrated with recording electrodes to achieve simultaneous neuromodulator delivery and neural activity monitoring. Initial efforts were made by integrating microfluidic channels into neural probes for localized delivery of neuromodulators into targeted brain regions24,25,26. For example, Shin et al. developed a multifunctional neural probe by integrating a 3-inlet polydimethylsiloxane (PDMS) microfluidic device with a silicon-based neural probe (Fig. 1a)25. To achieve a rapid mixing of drugs from different inlets, they included a staggered herringbone mixer into the microfluidic device for the delivery and mixing of multiple drugs into the brain. The multifunctional probe allowed real-time recording of neural activity responses to neuromodulators.
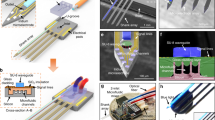
Neural activity modulation with chemical delivery. a Schematic of a multifunctional neural probe with integrated microfluidic chips. Reproduced with permission from ref. 25. Copyright 2015, The Royal Society of Chemistry. b A wireless fluidic probe driven by Joule heating-induced polymer expansion. Reproduced with permission from ref. 33. Copyright 2015, Cell press. c Surface coating for sustained drug release. Reproduced with permission from ref. 36. Copyright 2019, John Wiley and Sons. d Electrodes with dexamethasone/PEDOT:PSS hybrid nanotubes and drug release with electrical stimulation. Reproduced with permission from ref. 38. Copyright 2006, John Wiley and Sons. e Schematic and structure of an organic electronic ion pump-based delivery/sensing neural interface. Reproduced with permission from ref. 41. Copyright 2016, National Academy of Sciences
Although multifunctional probes based on rigid materials, such as silicon, can allow for simultaneous neural activity recording and local drug delivery, the rigidity of the probes can cause chronic inflammatory responses in the brain. For example, activated microglial and glial cells can form a compact and insulating sheath around rigid probes, resulting in signal degradation in long-term studies27. Compared to rigid probes, flexible probes that are integrated on polymer substrates, such as polyimide (PI)28,29, SU-830,31, and PDMS32, can form more compliant probe-brain interfaces, resulting in improved long-term stability. For example, Altuna et al. developed a flexible multifunctional neural probe by combining microfluidic channels with an SU-8-based flexible neural probe24. The fluidic outlets were located in close proximity to the recording electrodes, which enabled simultaneous drug delivery and electrical recording of neural activity responses in the rat hippocampus at a resolution of a few hundred microns.
Fluid delivery is generally achieved by using a pump equipped with connection tubes, which may limit their applications in freely moving animals. To address this issue, Jeong et al. developed a wireless flexible fluidic probe that could be remotely controlled in awake, freely moving mice (Fig. 1b)33. The device consisted of two bonded PDMS layers to form a microchannel with a cross-sectional area of only 10 × 10 µm2. Fluid pumping was wirelessly controlled by a battery-powered infrared module. Upon activation, a thermally responsive layer beneath the reservoir was heated and expanded, resulting in drug delivery in freely moving animals. Recently, Qazi et al. developed a multifunctional probe integrated with refillable and disposable plug-n-play cartridges, which allowed prolonged drug delivery for over 4 weeks34.
Chemicals and drugs can also be directly loaded onto implantable neural probes for sustained release in the brain35. For example, Huang et al. fabricated a multifunctional neural probe by coating anti-inflammatory/anti-fouling nanogels on the surface of a neural probe (Fig. 1c)36. The nanogel-coated neural probe showed improved mechanical compliance with the brain tissue and thus allowed stable neural recording from rat thalamic nuclei for over 4 weeks. In addition, drugs could be selectively deposited together with conducting polymers, such as poly(pyrrole) (PPy)37 and poly (3,4-ethylenediocythiophene) (PEDOT)38, onto the recording sites of neural probes. These polymers could be electrically actuated, enabling controlled release of the loaded drugs (Fig. 1d). Nevertheless, it is difficult to control when and where the drugs are released.
Organic electronic ion pumps have been recently integrated into multifunctional neural probes for controlled drug delivery. An organic electronic ion pump consisted of two electrolyte reservoirs and a separating ion-conductive PEDOT:PSS film39. When a voltage was applied to the device, K+ ions were electrophoretically transported from the source electrolyte to the target reservoir through the PEDOT:PSS film. The local increase in K+ ions depolarizes the cell membrane and activates the Ca2+ channel to stimulate cell activity40. Jonsson et al. developed a 32-channel multifunctional neural probe by integrating a PEDOT:PSS-based recording electrode with organic electronic ion pumps (Fig. 1e)41. The size of the recording electrodes was 20 µm × 20 µm, and each of the electrodes was connected to an organic electronic ion pump with a 20 µm-wide outlet. Since the sizes of the recording electrodes and the outlets were on the same scale as single neuronal cells, an in vitro demonstration showed that the multifunctional probe allowed chemical modulation and electrical recording at a single-cell resolution. Nevertheless, the size of several hundred micrometers makes it challenging for in vivo neural recording and modulation with minimal insertion footprints and inflammatory responses. Another limitation is that the 32 outlets shared one reservoir, and it is difficult to individually control each sensing/delivery site.
Multifunctional neural probes for neural recording and electrical stimulation
Electrical stimulation has been widely used in the restoration of sensory and motor functions42, as well as in the construction of brain–computer interfaces43. During electrical stimulation, electrical pulses are sent through stimulating electrodes to the targeted regions in the brain44. Electrical stimulation can induce charge redistribution at the electrode/neural interfaces through non-Faradic reactions or Faradaic reactions, leading to the depolarization or hyperpolarization of neurons.
The stimulation efficiency of an electrode is determined by its charge injection capacity (CIC), which is the amount of charge that can be injected into brain tissue without inducing any irreversible chemical reactions at the surface of the stimulating electrode. To achieve increased CIC, attempts have been made to improve the specific surface areas of stimulating electrodes by coating them with porous nanomaterials, such as Pt28,45, gold46, IrO247, TiN48, carbon nanotubes (CNTs)49, and graphene50 (Fig. 2a). In addition, pseudocapacitive conducting polymers, such as PPy51 and PEDOT52, have also been exploited as the coating layer to improve the CIC of stimulating electrodes (Fig. 2a). The introduction of these rough layers can increase the effective surface area of the stimulating electrodes, resulting in improved CIC. Another strategy to improve the CIC is through structural engineering of stimulating electrodes. For example, stimulating electrodes consisting of arrays of CNTs53, carbon nanofibers54 and porous Pt nanorods55 have been developed and exhibited improved stimulation efficiency compared to their planar counterparts (Fig. 2b). In addition, fiber electrodes, such as graphene fibers56,57 and CNT fibers58,59, have also been developed for electrical stimulation. The numerous nanowrinkles on these fibers could significantly increase their effective surface area, leading to an improved stimulation efficiency. Moreover, the small cross-sectional areas of the fiber-based stimulating electrodes also led to reduced traumatic implantation damage to the brain tissue56.
a Increasing CIC with surface coating of metal, metal oxide, conducting polymers, and carbon nanomaterials. Reproduced with permission from ref. 45. (Pt), copyright 2020, American Chemical Society; ref. 46. (Au), copyright 2017, John Wiley and Sons; ref. 47. (IrO2), copyright 2016, American Chemical Society; ref. 48. (TiN), copyright 2018, Elsevier; ref. 51. (Ppy). Copyright 2011, The Royal Society of Chemistry; ref. 52. (PEDOT). Copyright 2019, American Chemical Society; ref. 53. (CNT), copyright 2008, Nature Publishing Group; ref. 50. (PEDOT/GO), copyright 2019, Elsevier. b Increasing CIC with surface engineering to fabricate nanopillar arrays or microfibers with nanowrinkles. Reproduced with permission from ref. 53. (CNT array), copyright 2006, American Chemical Society; ref. 55. (Pt nanorods), copyright 2019, American Chemical Society; ref. 56. (Graphene microfiber), copyright 2020, Nature Publishing Group; ref. 59. (CNT yarn), copyright 2018, Nature Publishing Group
To investigate the response of neurons to electrical stimulation, multifunctional neural electrodes have been developed by integrating stimulating electrodes with recording electrodes. For example, Shi et al. developed an ultrathin multifunctional neural probe with integrated recording and stimulating electrodes and used a resorbable silk substrate as a temporary carrier17. After the dissolution of the silk substrate, the ultrathin multifunctional neural probe formed a biocompatible and conformal interface with the brain, enabling simultaneous neural activity modulation and recording with high spatiotemporal resolution. In addition, electrical modulation/recording interfaces with other novel functions have been developed, including transient electronics that can degrade after use60, self-healable electronics that can self-assemble on the static nerve61 and morphing electronics that can adapt their structure to a growing static nerve62.
Although neural activity can be effectively modulated by electrical stimulation, there are still several major challenges to be addressed: (1) Electrical stimulation leads to an electrical artifact in the recording channels that has a signal strength orders of magnitude higher than that of the recorded action potentials. (2) Electrical stimulation does not have cell type specificity. (3) During repeated stimulations, electrodes may undergo irreversible chemical reactions, resulting in decreased stimulation efficiency.
Multifunctional neural probes for simultaneous neural recording and optical modulation
Optogenetics can enable millisecond-scale neural modulation in defined types of cells in behaving animals and has greatly advanced our understanding of neural circuit function63. In optogenetics, genes encoding light-activated ion channels/pumps, i.e., opsins, are expressed in specific types of cells64. Upon the absorption of light with the correct wavelength, these ion channels/pumps open and allow the inflow of Na+ or Cl−, leading to the depolarization (activation) or hyperpolarization (silencing) of neurons. This section discusses multifunctional neural interfaces for combined electrophysiological recording and optogenetic modulation.
Multifunctional neural probes for electrocorticography (ECoG) recording/modulation
ECoG electrodes can record from a large cortical surface6 and are less invasive than depth electrodes65. Therefore, combining ECoG electrodes with optogenetic techniques can provide large-scale mapping of physiological neural signals under optical stimulation. However, the opaque metallic conductive materials in conventional devices prevent direct optical stimulation at electrode-tissue interfaces66. To address this issue, indium tin oxide (ITO) has been used to fabricate transparent ECoG electrodes for optogenetic modulations because of its high transparency and conductivity. For example, Kwon et al. fabricated a transparent ITO electrode array on a parylene C substrate and integrated it with microLED-based light delivery units (Fig. 3a)67. The ITO electrode array could record light-evoked signals from the rat visual cortex transfected with AAV-hSyn-hChR2(H134R)-mCherry.
a A transparent ITO electrode array for optical stimulation and neural recording. Reproduced with permission from ref. 67. Copyright 2013, IEEE Xplore. b A transparent graphene electrode array for simultaneous optical stimulation and neural recording. Reproduced with permission from ref. 68. Copyright 2014, Nature Publishing Group. c Gold mesh electrode arrays fabricated by using sacrificial polystyrene spheres as templates. Reproduced with permission from ref. 71. Copyright 2018, American Association for the Advancement of Science. d Gold NW mesh electrodes and their stable recording performance under mechanical deformation. Reproduced with permission from ref. 72. Copyright 2020, John Wiley and Sons. e Transparent CNT spider-web electrode array for neural recording under optogenetic modulation. Reproduced with permission from ref. 73. Copyright 2018, American Chemical Society
ITO-based electrodes usually suffer from rapidly decreased performance under mechanical deformation because of the brittleness of ITO. On the other hand, graphene has been considered to be a promising material for transparent ECoG electrodes because of its high transparency, excellent flexibility, and good biocompatibility68,69,70. Park et al. fabricated a transparent ECoG electrode array that was composed of four stacked graphene monolayers. The device was termed the carbon-layered electrode array (CLEAR) (Fig. 3b)68. The CLEAR device exhibited a light transmittance of approximately 90%, which enabled effective stimulation of opsin-expressing neurons underneath the graphene electrodes. Thunemann et al. further developed a graphene microelectrode array with no light-induced artifact by using residue- and contamination-free single-layer graphene as the electrode material69.
Graphene has limited conductivity compared to metals. Therefore, graphene electrodes with reduced sizes usually have high impedance, limiting their applications in high spatial resolution neural recording and manipulation. Recently, transparent metallic electrodes with a mesh- or grid-type structure design have been developed for simultaneous optogenetic stimulation and electrical recording. The large void areas in these mesh- and grid-type metallic electrodes allow efficient light transmission, leading to high transparence of the electrodes. Qiang et al. fabricated a transparent mesh electrode by patterning gold electrodes and connection lines with polystyrene spheres (Fig. 3c)71. The mesh-type gold electrodes showed no artifact under optical stimulation, enabling high-fidelity electrical recordings of light-evoked activities in an awake mouse. Seo et al. fabricated a transparent Au nanowire (NW) microelectrode array with a polymer fiber network as the shading mask (Fig. 3d)72. The microelectrode array had a high transparency of 87% and a low impedance of 18.6 Ω at 1 kHz, which enabled 2D mapping of light-evoked signals in transgenic mice. However, metal-based microelectrode arrays usually suffer from low stretchability, which limits their applications in mechanically active environments. To address this issue, Zhang et al. developed a transparent and stretchable ECoG electrode array with a CNT spider-web network as the electrode material and PDMS as the substrate (Fig. 3e)73. The ECoG electrode array exhibited a high stretchability, and the impedance increased by only 26% at a tensile strain of 20%. As a result, the device showed no performance change even after being hit by a falling rod.
Multifunctional neural probes for depth recording/modulation
Compared to ECoG electrodes, depth electrodes can allow neural activity recordings at single spike resolution. This section discusses depth electrode-based recording/optical modulation interfaces with various light delivery units, including optical fiber, LEDs, upconverting nanoparticles (UCNPs), and thermally drawn fibers.
Neural modulation with optical fiber and LEDs
Optical fibers have been widely used as the light source for neural modulation in both in vitro and in vivo studies74,75,76. In 2012, Anikeeva et al. developed an optrode device by manually attaching four tetrode bundles of nickel and chromium alloy wire onto an optical fiber (Fig. 4a)18. The device had a light weight of only 2 g and a height of 22 mm and could record light-evoked responses of medial prefrontal cortex neurons in freely moving mice.
a A microwire electrode bundle surrounded by four optical fibers for optical neural modulation of freely behaving mice. Reproduced with permission from ref. 19. Copyright 2012, Nature Publishing Group. b A transparent ZnO microprobe array for optogenetic neural manipulation. Reproduced with permission from ref. 77. Copyright 2015, Nature Publishing Group. c A soft alginate-PAAm hydrogel optical fiber with low Young’s modulus and reduced inflammatory response of the brain tissue. Reproduced with permission from ref. 79. Copyright 2018, John Wiley and Sons. d A multilayer, injectable optical modulation/recording interface based on µ-ILEDs for optical neural modulation. Reproduced with permission from ref. 80. Copyright 2013, AAAS
Optical modulation/recording interfaces with assembled optical fibers and recording microelectrodes typically involve manual assembly of two or more separate material blocks, resulting in a large physical size. In addition, these assemblies have limited spatial resolution. To address these issues, Lee et al. developed a 4 × 4 micro-optoelectrode array (MOA) with transparent ZnO micropillars as both waveguides and conductive recording electrodes (Fig. 4b)77. Owing to the micrometer-sized output apertures, the MOA devices detected spiking responses at light power as low as 1.76 ± 1.79 μW. Moreover, the MOA enabled efficient neural modulation at high spatial resolution. In another example, Zou et al. developed a multifunctional neural probe by immersing an array of ultraflexible microelectrode filaments and an optical fiber into a viral vector containing a polyethylene glycol (PEG) bath and withdrawing it into ambient air78. During the withdrawing process, the PEG quickly solidified, resulting in an implantable AAV delivery optrode. After implantation into the brain, the virus vectors were released after PEG dissolution, resulting in highly localized transduction of neurons near the microelectrodes. The AAV-delivery optrode allowed simultaneous neural activity recording and modulation of spatially defined neuronal populations for 3 months.
Conventional optical fibers are mainly based on silica, whose Young’s modulus is approximately six orders of magnitude higher than that of the brain tissue. This large mechanical mismatch causes significant implantation damage to the brain tissue, which subsequently induces neuronal loss at the implantation sites. To address this issue, Wang et al. developed a soft alginate-polyacrylamide hydrogel optical fiber for neural modulation (Fig. 4c)79. The Young’s modulus of the hydrogel optical fiber was only tens of kPa, similar to that of brain tissues (≈1 kPa). As a result, the soft device elicited a greatly reduced chronic inflammatory response compared with its rigid counterpart. However, swollen hydrogel optical fibers usually show lower light delivery efficiency at increased depth than conventional optical fibers due to increased propagation loss. Therefore, it is difficult for hydrogel optical fibers to be used in deep brain stimulation.
Although optical fiber-based light stimulation allows neural activity modulation with high temporal resolution, the large sizes of the optical fibers can cause greater damage upon insertion into the brain tissue. In addition, the tethered nature of the optical fibers can restrict animals’ natural behavior. Compared to optical fibers, microLEDs are smaller, less expensive, and available at various wavelengths. In particular, the small sizes of microLEDs can allow their precise integration onto neural probes80. In addition, microLEDs can be wirelessly powered and allow tetherless light delivery81. Therefore, multifunctional neural interfaces with integrated microLEDs can allow precise neural modulation in awake, behaving rodents through optogenetic manipulation. Kim et al. developed a wireless, flexible, and layered multifunctional interface that consists of four microscale GaN LEDs (µ-ILEDs), a Pt microelectrode, a photodetector, and a temperature sensor (Fig. 4d)80. The cross-section of µ-ILEDs was only 50 μm × 50 μm. Therefore, light could be delivered to the targeted brain region at cellular-scale precision. In addition, the small physical size and mechanical flexibility of the multifunctional probe resulted in greatly reduced neuronal loss compared to a metal cannula and fiber optics.
Neural modulation with UCNPs
Most photosensitive opsins can only be activated with visible light. However, visible light has limited transmission depth in the brain. As a result, light-delivery devices, such as optical fibers or microLEDs, need to be implanted into deep brain tissues. By contrast, near-infrared (NIR) (650–1450 nm) light can penetrate deep brain tissues. Therefore, the development of systems that can convert highly tissue-penetrative NIR light to visible light represents a promising strategy for low-invasive optical neural stimulation. UCNPs are composed of rare-earth components and can absorb low-energy NIR light and emit high-energy visible light through a multiphoton absorption process82. Therefore, UCNPs are promising candidates for low-invasive neural modulation. In particular, lanthanide-doped UCNPs with emission spectra from blue to yellow have been synthesized and used to manipulate the neural activities of cells83,84, worms85, and living rodents86,87,88,89,90. For example, Hososhima et al. synthesized NaYF4:Sc/Yb/Er and NaYF4:Sc/Yb/Tm@NaYF4 nanorods, which could absorb NIR light and emit green and blue light, respectively (Fig. 5a)83. The emitted visible light-induced the photoreactive response of the nearby ND7/23 cells expressing CHR opsins. Chen et al. injected UCNPs into ventral tegmental area (VTA) neurons that expressed light-sensitive proteins89. Tissue-penetrating NIR light could be locally converted by UCNPs to visible light to evoke dopamine release in VTA DA neurons.
a Lanthanide-doped nanorods with different upconversion emission spectra. Reproduced with permission from ref. 83. Copyright 2015, Nature Publishing Group. b Core–shell UCNPs for neural activity modulation in living animals. Reproduced with permission from ref. 86. Copyright 2017, John Wiley and Sons. c Dye-sensitized core-shell UCNPs with enhanced upconversion emission efficiency for neural activity manipulation. Reproduced with permission from ref. 84. Copyright 2016, American Chemical Society. d Core-shell-shell UCNPs with enhanced upconversion emission efficiency for neural activity inhibition. Reproduced with permission from ref. 88. Copyright 2018, American Chemical Society
UCNPs usually have a low light emission efficiency due to surface quenching. To address this issue, Lin et al. synthesized core-shell UCNPs with Tm3+- or Er3+-doped NaYF4 as the core and NaYF4 as the shell and applied the UCNPs to NIR-based optogenetic modulation in rodents (Fig. 5b)86. The core-shell structure design could effectively block surface quenchers, leading to improved emission efficiency. In another example, they further developed a robotic laser projection system for tetherless optogenetic modulation in different rat brain regions and complex animal behavior control87. Wu et al. further synthesized a type of dye-sensitized core-shell UCNP with NaYF4:Yb3+:Er3+ as the core, NaYF4:Yb3+ as the shell and IR-806 as the dye sensitizer (Fig. 5c)84. The dye-sensitized core-shell UCNPs showed ∼1000 fold upconversion luminescence enhancement compared to the same UCNPs without dye sensitizing. As a result, the dye-sensitized core-shell UCNPs enabled robust activation of ReaChR-expressing hippocampal neurons. Lin et al. synthesized NaYF4-based UCNPs with a unique core-shell-shell structure88. The UCNPs consisted of a β-NaYF4 core, a β-NaYF4:Yb/Er shell layer, and an outer β-NaYF4 shell layer (Fig. 5d). The outer β-NaYF4 provided spatial confinement to the Yb lattice, which enabled the elimination of Yb-associated concentration quenching. Compared to core-shell UCNPs, the core-shell-shell UCNPs exhibited an almost threefold enhanced emission intensity at ~540–570 nm, enabling reliable wireless modulation of eNpHR-expressing neurons in rats.
Fiber-based multifunctional neural probes
Multifunctional fiber probes have been demonstrated for low-invasive neural activity recording and modulation. For example, Canales et al. developed an all-polymer fiber probe by a thermal drawing process for combined optical stimulation, drug delivery and neural recording91. The multifunctional fiber consisted of a polycarbonate (PC) waveguide, a cyclic olefin copolymer light confinement layer, an additional PC encapsulation layer, and conductive polyethylene recording electrodes (Fig. 6a). The PC waveguide was surrounded with conductive polyethylene recording electrodes and microfluidic channels for drug delivery. Owing to its small cross-section, the multifunctional fiber probe elicited minimal inflammatory responses of the brain tissue and allowed optogenetic stimulation/electrical recording in freely moving transgenic mice for over 2 months.
a A thermally drawn fiber-based multifunctional neural interface and its optical image. Reproduced with permission from ref. 91. Copyright 2015, Nature Publishing Group. b Fiber-based multifunctional neural interfaces for one-step optogenetics and chronic neural activity recording. Reproduced with permission from ref. 92. Copyright 2017, Nature Publishing Group. c A spatially expandable fiber-based multifunctional neural interface for simultaneous neural activity recording and modulation across distant brain regions. Reproduced with permission from ref. 93. Copyright 2020, Nature Publishing Group. d A hydrogel matrix-held fiber-based multifunctional neural interface for chronic neural activity recording and modulation. Reproduced with permission from ref. 94. Copyright 2021, Nature Publishing Group
Park et al. further introduced graphite into conductive polyethylene to reduce the resistance of the recording electrodes in thermally drawn multifunctional fibers92. As a result, the size of the recording electrodes was reduced to 20–25 µm in diameter. Moreover, the microchannels in the multifunctional fiber allowed the delivery of virus vectors into the mouse brain. Thus, the multifunctional probes could allow virus injection and optrode implantation through a single-step surgery (Fig. 6b). Recently, Jiang et al. developed a spatially expandable multifunctional fiber electrode by inserting an array of multifunctional fibers into the hollow channels of a helical scaffolding fiber93. After implantation, the multifunctional fiber electrode extruded out of the scaffold and expanded to reach the multiple targeted regions through a single implantation (Fig. 6c). As a result, the device allowed simultaneous recording and optical/chemical neural modulation across distant regions. Park et al. further fabricated a compliant multifunctional probe by integrating fiber microelectrodes within a hydrogel matrix (Fig. 6d)94. The bending stiffness of the hybrid probe was only 0.42 N m−1. Therefore, the hybrid probe induced minimal foreign body responses and enabled stable tracking of single neurons in freely moving mice for over 6 months after implantation.
Conclusion and prospects
Multifunctional neural probes have greatly advanced our understanding of brain functions. This review summarizes the progress of multifunctional neural probes with different neural modulation modalities, including chemical stimulation, electrical stimulation, and optogenetic stimulation. Although substantial progress has been made in multifunctional neural probes, there are still significant challenges to be addressed, including the following: (i) Modern multifunctional neural probes are mainly composed of rigid stimulation units with relatively large footprints (e.g., microfluidics and optical fibers). The rigid nature and large footprints of the stimulation units can induce elevated tissue damage and inflammatory responses in the brain, limiting the use of these multifunctional probes in chronic studies. Therefore, it is highly desirable to develop mechanically compliant and miniature multifunctional neural probes for long-term stable neural recording and modulation. (ii) It is still challenging for modern neural modulation techniques to achieve type-specific modulation at single-neuron resolution. For instance, optogenetic modulation and chemical modulation can achieve cell-specific modulation but are limited in spatial resolution due to light propagation or chemical diffusion. Therefore, the development of new neural modulation techniques with combined high spatiotemporal resolution and cell specificity is highly desirable, especially for the study of neural circuit mechanisms. (iii) Neural modulation, including electrical stimulation and light stimulation, can result in changes in the microenvironment surrounding neurons, including temperature and pH. It is thus desirable to integrate multifunctional neural probes with other functionalities, such as temperature monitors and pH sensors. Addressing these challenges relies on not only the materials and structure optimization of neural interfaces but also multidisciplinary scientific collaborations from scientists and engineers of materials science, electronics, mechanical engineering, and neuroscience.
References
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Sousa, A. M. M., Meyer, K. A., Santpere, G., Gulden, F. O. & Sestan, N. Evolution of the human nervous system function, structure, and development. Cell 170, 226–247 (2017).
He, F., Lycke, R., Ganji, M., Xie, C. & Luan, L. Ultraflexible neural electrodes for long-lasting intracortical recording. iScience 23, 101387 (2020).
Buzsáki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Kook, G., Lee, S. W., Lee, H. C., Cho, I. & Lee, H. J. Neural probes for chronic applications. Micromachines 7, 179 (2016).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Sahasrabuddhe, K., et al. The Argo: A 65,536 channel recording system for high-density neural recording in vivo. Biorxiv, https://doi.org/10.1101/2020.07.17.209403
Hong, G. S. & Lieber, C. M. Novel electrode technologies for neural recordings. Nat. Rev. Neurosci. 20, 339–345 (2019).
Song, E. M., Li, J. H., Won, S. M., Bai, W. B. & Rogers, J. A. Materials for flexible bioelectronics, systems as chronic neural interfaces. Nat. Mater. 19, 590–603 (2020).
Li, H. B., Wang, J. F. & Fang, Y. Bioinspired flexible electronics for seamless neural interfacing and chronic recording. Nanoscale Adv. 2, 3095–3102 (2020).
Ledesma, H. A. et al. An atlas of nano-enabled neural interfaces. Nat. Nanotech. 14, 645–657 (2019).
Chapman, C. A. R., Goshi, N. & Seker, E. Multifunctional neural interfaces for closed-loop control of neural activity. Adv. Funct. Mater. 28, 1703523 (2018).
Sunga, C. H. et al. Multimaterial and multifunctional neural interfaces: from surface-type and implantable electrodes to fiber-based devices. J. Mater. Chem. B 8, 6624–6666 (2020).
Vázquez-Guardado, A., Yang, Y. Y., Bandodkar, A. J. & Rogers, J. A. Recent advances in neurotechnologies with broad potential for neuroscience research. Nat. Neurosci. 23, 1522–1536 (2020).
Tian, H. H., Xu, K., Zou, L. & Fang, Y. Multimodal neural probes for combined optogenetics and electrophysiology. iScience 25, 103612 (2022).
Shi, Z. F. et al. Silk-enabled conformal multifunctional bioelectronics for investigation of spatiotemporal epileptiform activities and multimodal neural encoding/decoding. Adv. Sci. 6, 1801617 (2020).
Berényi, A., Belluscio, M., Mao, D. & Buzsáki, G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science 337, 736–737 (2012).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2012).
Pardridge, W. M. Blood-brain barrier delivery. Drug Discov. Today 12, 54–61 (2007).
Kinderen, R. J. A. et al. Side-effects of antiepileptic drugs: The economic burden. Seizure 23, 184–190 (2014).
Spataro, L. et al. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp. Neurol. 194, 289–300 (2005).
Gill, S. S. et al. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595 (2003).
Altuna, A. et al. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab Chip 13, 1422–1430 (2013).
Shin, H. et al. Neural probes with multi-drug delivery capability. Lab Chip 15, 3730–3737 (2015).
Wen, X. M. et al. Flexible, multifunctional neural probe with liquid metal enabled, ultra-large tunable stiffness for deep-brain chemical sensing and agent delivery. Biosen. Bioelectron. 131, 37–45 (2019).
Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
Guan, S. L. et al. Elastocapillary self-assembled neurotassels for stable neural activity recordings. Sci. Adv. 5, eaav2842 (2019).
Chung, J. E. et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 101, 21–31 (2019).
Luan, L. et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 3, e1601966 (2017).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–637 (2015).
Tybrandt, K. et al. High-density stretchable electrode grids for chronic neural recording. Adv. Mater. 30, 1706520 (2018).
Jeong, J. W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Qazi, R. et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat. Biomed. Eng. 3, 655–669 (2019).
Kim, H. J. et al. Biological assessment of multifunctional hydrogel-decorated implantable neural cuff electrode for clinical neurology application. Sci. Rep. 7, 15245 (2017).
Huang, W. C. et al. Multifunctional 3D patternable drug-embedded nanocarrier-based interfaces to enhance signal recording and reduce neuron degeneration in neural implantation. Adv. Mater. 27, 4186–4193 (2015).
Wadhwa, R., Lagenaur, C. F. & Cui, X. Y. T. Electrochemically controlled release of dexamethasone from conducting polymer polypyrrole coated electrode. J. Control. Release 110, 531–541 (2006).
Abidian, M. R., Kim, D. H. & Martin, D. C. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 18, 405–409 (2006).
Isaksson, J. et al. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 6, 673–679 (2007).
Simon, D. T. et al. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 8, 742–746 (2009).
Jonsson, A. et al. Bioelectronic neural pixel: chemical stimulation and electrical sensing at the same site. PANS 113, 9440–9445 (2016).
Luo, S. Y., Xu, H. N., Zuo, Y., Liu, X. G. & All, A. H. A Review of functional electrical stimulation treatment in spinal cord injury. NeuroMol. Med. 22, 447–463 (2020).
Caldwell, D. J., Ojemann, J. G. & Rao, R. P. N. Direct electrical stimulation in electrocorticographic brain-computer interfaces: enabling technologies for input to cortex. Front. Neurosci. 13, 804 (2009).
Kozai, T. D. Y. et al. Nanostructured Coatings for Improved Charge Delivery to Neurons. Nanotechnology and Neuroscience: Nano-electronic, Photonic and Mechanical Neuronal Interfacing. 71–134 (Springer, 2014).
Sunwoo, S. H. et al. Stretchable low-Impedance nanocomposite comprised of Ag-Au core-shell nanowires and Pt black for epicardial recording and stimulation. Adv. Mater. Technol. 5, 1900768 (2020).
Chapman, C. A. R. et al. Nanoporous gold biointerfaces: modifying nanostructure to control neural cell coverage and enhance electrophysiological recording performance. Adv. Funct. Mater. 27, 1604631 (2017).
Kim, Y. H., Kim, G. H., Kim, M. S. & Jung, S. D. Iridium oxide-electrodeposited nanoporous gold multielectrode array with enhanced stimulus efficacy. Nano Lett. 16, 7163–7168 (2016).
Meijs, S., et al. Diamond/porous titanium nitride electrodes with superior electrochemical performance for neural interfacing. Front. Bioeng. Biotechnol. 6, 171 (2018).
Leefer, E. W., Botterman, B. R., Romero, M. I., Rossi, A. F. & Gross, G. W. Carbon nanotube coating improves neuronal recordings. Nat. Nanotech. 3, 434–439 (2008).
Lee, S., Eom, T., Kim, M. K., Yang, S. G. & Shim, B. S. Durable soft neural micro-electrode coating by an electrochemical synthesis of PEDOT:PSS/graphene oxide composites. Electrochim. Acta 313, 79–90 (2019).
Higgins, T. M., Moulton, S. E., Gilmore, K. J., Wallace, G. G. & Panhuis, M. Gellan gum doped polypyrrole neural prosthetic electrode coatings. Soft Matter 7, 4690–4695 (2011).
Schander, A., Teßmann, T., Strokov, S., Stemmann, H., Kreiter, A. K. & Lang, W. In-vitro evaluation of the long-term stability of PEDOT:PSS coated microelectrodes for chronic recording and electrical stimulation of neurons. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 6174–6177 (2016).
Wang, K., Fishman, H. A., Dai, H. J. & Harris, J. S. Neural stimulation with a carbon nanotube microelectrode array. Nano Lett. 6, 2043–2048 (2006).
Asis, E. et al. High efficient electrical stimulation of hippocampal slices with vertically aligned carbon nanofiber microbrush array. Biomed Microdevices 11, 801–808 (2009).
Ganji, M. et al. Selective formation of porous Pt nanorods for highly electrochemically efficient neural electrode interfaces. Nano Lett. 19, 6244–6254 (2019).
Zhao, S. Y. et al. Full activation pattern mapping by simultaneous deep brain stimulation and fMRI with graphene fiber electrodes. Nat. Commun. 11, 1788 (2020).
Wang, K. Z. et al. High-performance graphene-fiber-based neural recording microelectrodes. Adv. Mater. 31, 1805867 (2019).
Vitale, F., Summerson, S. R., Aazhang, B., Kemere, C. & Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 9, 4465 (2015).
McCallum, G. A. et al. Chronic interfacing with the autonomic nervous system using carbon nanotube (CNT) yarn electrodes. Sci. Rep. 7, 11723 (2017).
Yu, K. J. et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 15, 782–791 (2016).
Song, K. et al. Adaptive self-healing electronic epineurium for chronic bidirectional neural interfaces. Nat. Commun. 11, 4195 (2020).
Liu, Y. X. et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotech. 38, 1031–1036 (2020).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Ganji, M. et al. Development and translation of PEDOT:PSS microelectrodes for intraoperative monitoring. Adv. Funct. Mater. 28, 1700232 (2017).
Park, A. H. et al. Optogenetic mapping of functional connectivity in freely moving mice via insertable wrapping electrode array beneath the skull. ACS Nano 10, 2791–2802 (2016).
Kwon, K. Y., Sirowatka, B., Weber, A. & Li, W. Opto-µECoG array: a hybrid neural interface with transparent µECoG electrode array and integrated LEDs for optogenetics. IEEE Trans. Biomed. Circuits Syst. 7, 593–600 (2013).
Park, D. W. et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 5, 5258 (2014).
Thunemann, M. et al. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat. Commun. 9, 2035 (2018).
Lu, Y. C. et al. Ultralow impedance graphene microelectrodes with high optical transparency for simultaneous deep two-photon imaging in transgenic mice. Adv. Funct. Mater. 28, 1800002 (2018).
Qiang, Y. et al. Transparent arrays of bilayer-nanomesh microelectrodes for simultaneous electrophysiology and two-photon imaging in the brain. Sci. Adv. 4, eaat0626 (2018).
Seo, J. W. et al. Artifact-free 2D mapping of neural activity in vivo through transparent gold nanonetwork array. Adv. Funct. Mater. 30, 2000896 (2020).
Zhang, J. et al. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett. 18, 2903–2911 (2018).
Tamura, K. et al. A glass-coated tungsten microelectrode enclosing optical fibers for optogenetic exploration in primate deep brain structures. J. Neurosci. Methods 211, 49–57 (2012).
Ozden, I. et al. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J. Neurosci. Methods 219, 142–154 (2013).
LeChasseur, Y. et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. Nat. Methods 9, 319–324 (2011).
Lee, J., Ozden, I., Song, Y. K. & Nurmikko, A. V. Transparent intracortical microprobe array for simultaneous spatiotemporal optical stimulation and multichannel electrical recording. Nat. Methods 12, 1157–1162 (2015).
Zou, L. et al. Self-assembled multifunctional neural probes for precise integration of optogenetics and electrophysiology. Nat. Commun. 12, 5871 (2021).
Wang, L. L. et al. Ultrasoft and highly stretchable hydrogel optical fibers for in vivo optogenetic modulations. Adv. Optical Mater. 6, 1800427 (2018).
Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Kim, K. et al. Artifact-free and high-temporal-resolution in vivo opto-electrophysiology with microLED optoelectrodes. Nat. Commun. 11, 2063 (2020).
Gnach, A. & Bednarkiewicz, A. Lanthanide-doped up-converting nanoparticles: merits and challenges. Nano Today 7, 532–563 (2012).
Hososhima, S. et al. Near-infrared (NIR) up-conversion optogenetics. Sci. Rep. 5, 16533 (2015).
Wu, X. et al. Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS Nano 10, 1060–1066 (2016).
Bansal, A., Liu, H. C., Jayakumar, M. K. G., Andersson-Engels, S. & Zhang, Y. Quasi-continuous wave near-infrared excitation of upconversion nanoparticles for optogenetic manipulation of C. elegans. Small 12, 1732–1743 (2016).
Lin, X. D. et al. Multiplexed optogenetic stimulation of neurons with spectrum-selective upconversion nanoparticles. Adv. Healthc. Mater. 6, 1700446 (2017).
Wang, Y. et al. Tetherless near-infrared control of brain activity in behaving animals using fully implantable upconversion microdevices. Biomaterials 142, 136–148 (2017).
Lin, X. D. et al. Core-shell-shell upconversion nanoparticles with enhanced emission for wireless optogenetic inhibition. Nano Lett. 18, 948–956 (2018).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684 (2018).
Ai, X. Z. et al. Remote regulation of membrane channel activity by site-specific localization of lanthanide-doped upconversion nanocrystals. Angew. Chem. Int. Ed. 56, 3031–3035 (2017).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical, and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Jiang, S. et al. Spatially expandable fiber-based probes as a multifunctional deep brain interface. Nat. Commun. 11, 6115 (2020).
Park, S. J. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 12, 3435 (2021).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos. 51972073, 61971150, 21790393) and the Strategic Priority Research Program of Chinese Academy of Science (Grant no. XDB32030100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Wang, J. & Fang, Y. Recent developments in multifunctional neural probes for simultaneous neural recording and modulation. Microsyst Nanoeng 9, 4 (2023). https://doi.org/10.1038/s41378-022-00444-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-022-00444-5
This article is cited by
A self-stiffening compliant intracortical microprobe
Biomedical Microdevices (2024)