Abstract
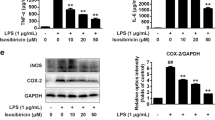
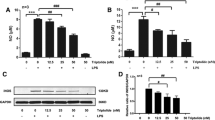
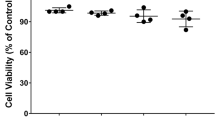
Dopamine (DA) is important in the maintenance of normal nervous system function. DA is the target of multiple drugs, and it induces critical alterations in immune cells. However, these impacts are controversial, and the mechanism remains unclear. In the present study, we treated BV-2 microglial cells and primary microglia with DA and measured the changes in cytokines. We also identified the expression of DA receptors (DRs) using confocal and immunofluorescent microscopy. Specific agonists and antagonists of D1-like DRs (D1DR and D5DR) were used to observe alterations in cytokines. Western blot and siRNA interference were performed to investigate the involvement of the downstream signaling molecules of DRs. We also measured changes in mitogen-activated protein kinases (MAPKs) and the nuclear factor-kappa B (NF-κB) signaling pathway and assessed their involvement using inhibitors. We found that DA alone produced no effects on IL-6, TNF-α or nitric oxide (NO) production, and it inhibited lipopolysaccharide (LPS)-induced NO in microglial cells. Microglia expressed a high abundance of D1-like DRs (D1DR and D5DR). The agonists inhibited NO production, and antagonists reversed the DA-induced suppression of NO. Adenylatec cyclase (AC), cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) mediated DA function, and cAMP-response element binding protein (CREB) was not involved. ERK1/2 and NF-κB, but not p-38 or JNK, played roles in DA-suppressed NO generation via altering inducible nitric oxide synthase (iNOS) transcription. These data illustrate that DA modulates LPS-induced NO production via the AC/cAMP-PKA-ERK1/2-NF-κB-iNOS axis in mouse microglia, and D1-like DRs are involved. The present study provides functional evidence for an essential role of DA in immunoregulation.







Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, Hsueh B, Crow AK, Martens H, Schwindling C, Calvigioni D, Bains JS, Mate Z, Szabo G, Yanagawa Y, Zhang MD, Rendeiro A, Farlik M, Uhlen M, Wulff P, Bock C, Broberger C, Deisseroth K, Hokfelt T, Linnarsson S, Horvath TL, Harkany T (2017) Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20(2):176–188. https://doi.org/10.1038/nn.4462
Milienne-Petiot M, Groenink L, Minassian A, Young JW (2017) Blockade of dopamine D1-family receptors attenuates the mania-like hyperactive, risk-preferring, and high motivation behavioral profile of mice with low dopamine transporter levels. J Psychopharmacol (Oxford England) 31(10):1334–1346. https://doi.org/10.1177/0269881117731162
Torres-Rosas R, Yehia G, Pena G, Mishra P, del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L (2014) Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20(3):291–295. https://doi.org/10.1038/nm.3479
Miyagi J, Oshibuchi H, Kasai A, Inada K, Ishigooka J (2014) Valproic acid inhibits excess dopamine release in response to a fear-conditioned stimulus in the basolateral complex of the amygdala of methamphetamine-sensitized rats. Eur J Pharmacol 730:20–25. https://doi.org/10.1016/j.ejphar.2014.01.020
Rangel-Barajas C, Coronel I, Floran B (2015) Dopamine receptors and neurodegeneration. Aging Dis 6(5):349–368. https://doi.org/10.14336/ad.2015.0330
Jiang S, Gao H, Luo Q, Wang P, Yang X (2017) The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: a meta-analysis. Neurol Sci 38(8):1373–1380. https://doi.org/10.1007/s10072-017-2988-4
Mikulak J, Bozzo L, Roberto A, Pontarini E, Tentorio P, Hudspeth K, Lugli E, Mavilio D (2014) Dopamine inhibits the effector functions of activated NK cells via the upregulation of the D5 receptor. J Immunol 193(6):2792–2800. https://doi.org/10.4049/jimmunol.1401114
Cosentino M, Ferrari M, Kustrimovic N, Rasini E, Marino F (2015) Influence of dopamine receptor gene polymorphisms on circulating T lymphocytes: a pilot study in healthy subjects. Hum Immunol 76(10):747–752. https://doi.org/10.1016/j.humimm.2015.09.032
Saha B, Mondal AC, Basu S, Dasgupta PS (2001) Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. Int Immunopharmacol 1(7):1363–1374
Menassa DA, Gomez-Nicola D (2018) Microglial dynamics during human brain development. Front Immunol 9:1014. https://doi.org/10.3389/fimmu.2018.01014
Xie F, Zhang F, Min S, Chen J, Yang J, Wang X (2018) Glial cell line-derived neurotrophic factor (GDNF) attenuates the peripheral neuromuscular dysfunction without inhibiting the activation of spinal microglia/monocyte. BMC Geriatr 18(1):110. https://doi.org/10.1186/s12877-018-0796-1
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2(1):a006346. https://doi.org/10.1101/cshperspect.a006346
Biber K, Owens T, Boddeke E (2014) What is microglia neurotoxicity (Not)? Glia 62(6):841–854. https://doi.org/10.1002/glia.22654
Zhu C, Kros JM, van der Weiden M, Zheng P, Cheng C, Mustafa DA (2017) Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta neuropathol Commun 5(1):4. https://doi.org/10.1186/s40478-016-0405-5
Sasaki A (2017) Microglia and brain macrophages: an update. Neuropathology 37(5):452–464. https://doi.org/10.1111/neup.12354
Farber K, Pannasch U, Kettenmann H (2005) Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci 29(1):128–138. https://doi.org/10.1016/j.mcn.2005.01.003
Yoshioka Y, Sugino Y, Tozawa A, Yamamuro A, Kasai A, Ishimaru Y, Maeda S (2016) Dopamine inhibits lipopolysaccharide-induced nitric oxide production through the formation of dopamine quinone in murine microglia BV-2 cells. J Pharmacol Sci 130(2):51–59. https://doi.org/10.1016/j.jphs.2015.11.002
Wang B, Chen T, Wang J, Jia Y, Ren H, Wu F, Hu M, Chen Y (2018) Methamphetamine modulates the production of interleukin-6 and tumor necrosis factor-alpha via the cAMP/PKA/CREB signaling pathway in lipopolysaccharide-activated microglia. Int Immunopharmacol 56:168–178. https://doi.org/10.1016/j.intimp.2018.01.024
Zhao W, Huang Y, Liu Z, Cao BB, Peng YP, Qiu YH (2013) Dopamine receptors modulate cytotoxicity of natural killer cells via cAMP-PKA-CREB signaling pathway. PLoS ONE 8(6):e65860. https://doi.org/10.1371/journal.pone.0065860
Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160(1–2):62–73. https://doi.org/10.1016/j.cell.2014.11.047
Basu B, Sarkar C, Chakroborty D, Ganguly S, Shome S, Dasgupta PS, Basu S (2010) D1 and D2 dopamine receptor-mediated inhibition of activated normal T cell proliferation is lost in jurkat T leukemic cells. J Biol Chem 285(35):27026–27032. https://doi.org/10.1074/jbc.M110.144022
Shao QH, Zhang XL, Chen Y, Zhu CG, Shi JG, Yuan YH, Chen NH (2018) Anti-neuroinflammatory effects of 20C from Gastrodia elata via regulating autophagy in LPS-activated BV-2 cells through MAPKs and TLR4/Akt/mTOR signaling pathways. Mol Immunol 99:115–123. https://doi.org/10.1016/j.molimm.2018.04.014
Hu W, Shi L, Li MY, Zhou PH, Qiu B, Yin K, Zhang HH, Gao Y, Kang R, Qin SL, Ning JZ, Wang W, Zhang LJ (2017) Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-kappaB signalling pathways. Sci Rep 7(1):16479. https://doi.org/10.1038/s41598-017-16008-x
Deb DK, Bao R, Li YC (2017) Critical role of the cAMP-PKA pathway in hyperglycemia-induced epigenetic activation of fibrogenic program in the kidney. FASEB J 31(5):2065–2075. https://doi.org/10.1096/fj.201601116R
Somalwar AR, Choudhary AG, Sharma PR, Sagarkar BN, Sakharkar S, Subhedar AJ, Kokare NK DM (2018) Cocaine- and amphetamine-regulated transcript peptide (CART) induced reward behavior is mediated via Gi/o dependent phosphorylation of PKA/ERK/CREB pathway. Behav Brain Res 348:9–21. https://doi.org/10.1016/j.bbr.2018.03.035
Moon SK, Cha BY, Kim CH (2004) ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: involvement of the ras dependent pathway. J Cell Physiol 198(3):417–427. https://doi.org/10.1002/jcp.10435
Yang CC, Hsiao LD, Yang CM, Lin CC (2017) Thrombin enhanced matrix metalloproteinase-9 expression and migration of SK-N-SH cells via PAR-1, c-Src, PYK2, EGFR, Erk1/2 and AP-1. Mol Neurobiol 54(5):3476–3491. https://doi.org/10.1007/s12035-016-9916-0
Kefaloyianni E, Gaitanaki C, Beis I (2006) ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal 18(12):2238–2251. https://doi.org/10.1016/j.cellsig.2006.05.004
Natarajan K, Abraham P, Kota R, Isaac B (2018) NF-kappaB-iNOS-COX2-TNF alpha inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol 118:766–783. https://doi.org/10.1016/j.fct.2018.06.040
Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA (2004) Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J Biol Chem 279(2):1323–1329. https://doi.org/10.1074/jbc.M307521200
Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM (2017) Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124:52–61. https://doi.org/10.1016/j.neuropharm.2017.04.033
McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ (2002) Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol 132(1–2):34–40. https://doi.org/10.1016/S0165-5728(02)00280-1
Wedel J, Hottenrott MC, Stamellou E, Breedijk A, Tsagogiorgas C, Hillebrands JL, Yard BA (2014) N-Octanoyl dopamine transiently inhibits T cell proliferation via G1 cell-cycle arrest and inhibition of redox-dependent transcription factors. J Leukoc Biol 96(3):453–462. https://doi.org/10.1189/jlb.3A0813-455R
Ferreira TB, Barros PO, Teixeira B, Cassano T, Centuriao N, Kasahara TM, Hygino J, Vasconcelos CC, Filho HA, Alvarenga R, Wing AC, Andrade RM, Andrade AF, Bento CA (2014) Dopamine favors expansion of glucocorticoid-resistant IL-17-producing T cells in multiple sclerosis. Brain Behav Immun 41:182–190. https://doi.org/10.1016/j.bbi.2014.05.013
Matsuyama T, Kawano M, Takagi R, Nakagome K, Chikamatsu K, Matsushita S (2018) Interleukin-8 produced by T cells is under the control of dopamine signaling. Clin Exp Neuroimmunol 9(4):251–257. https://doi.org/10.1111/cen3.12472 doi
Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MBP (2006) A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 6(6):903–907. https://doi.org/10.1016/j.intimp.2005.12.007
Lee D (2015) Global and local missions of cAMP signaling in neural plasticity, learning, and memory. Front Pharmacol 6:161. https://doi.org/10.3389/fphar.2015.00161
Ruggiero C, Doghman-Bouguerra M, Ronco C, Benhida R, Rocchi S, Lalli E (2018) The GRP78/BiP inhibitor HA15 synergizes with mitotane action against adrenocortical carcinoma cells through convergent activation of ER stress pathways. Mol Cell Endocrinol 474:57–64. https://doi.org/10.1016/j.mce.2018.02.010
Nelson M, Adams T, Ojo C, Carroll MA, Catapane EJ (2018) Manganese toxicity is targeting an early step in the dopamine signal transduction pathway that controls lateral cilia activity in the bivalve mollusc Crassostrea virginica. Comp Biochem Physiol C Toxicol Pharmacol 213:1–6. https://doi.org/10.1016/j.cbpc.2018.07.002
Selvaraj P, Shen Q, Yang F, Naqvi NI (2017) Cpk2, a catalytic subunit of cyclic AMP-PKA, regulates growth and pathogenesis in rice blast. Front Microbio 8:2289. https://doi.org/10.3389/fmicb.2017.02289
Zhu T, Wu XL, Zhang W, Xiao M (2015) Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase A (PKA)-dependent nuclear factor-kappaB (NF-kappaB) signaling pathway in mice. Int J Mol Sci 16(9):20195–20211. https://doi.org/10.3390/ijms160920195
Vijay K (2018) Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol 59:391–412. https://doi.org/10.1016/j.intimp.2018.03.002
Pires BRB, Silva R, Ferreira GM, Abdelhay E (2018) NF-kappaB: two sides of the same coin. Genes. https://doi.org/10.3390/genes9010024
Guo Z, Shao L, Du Q, Park KS, Geller DA (2007) Identification of a classic cytokine-induced enhancer upstream in the human iNOS promoter. FASEB J 21(2):535–542. https://doi.org/10.1096/fj.06-6739com
Chu LF, Wang WT, Ghanta VK, Lin CH, Chiang YY, Hsueh CM (2008) Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res 1239:24–35. https://doi.org/10.1016/j.brainres.2008.08.087
Filip GA, Postescu ID, Bolfa P, Catoi C, Muresan A, Clichici S (2013) Inhibition of UVB-induced skin phototoxicity by a grape seed extract as modulator of nitrosative stress, ERK/NF-kB signaling pathway and apoptosis, in SKH-1 mice. Food Chem Toxicol 57:296–306. https://doi.org/10.1016/j.fct.2013.03.031
Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8(10):766–775. https://doi.org/10.1038/nrn2214
Basudhar D, Somasundaram V, de Oliveira GA, Kesarwala A, Heinecke JL, Cheng RY, Glynn SA, Ambs S, Wink DA, Ridnour LA (2017) Nitric oxide synthase-2-derived nitric oxide drives multiple pathways of breast cancer progression. Antioxid Redox Signal 26(18):1044–1058. https://doi.org/10.1089/ars.2016.6813
Adams L, Franco MC, Estevez AG (2015) Reactive nitrogen species in cellular signaling. Exp Biol Med (Maywood) 240(6):711–717. https://doi.org/10.1177/1535370215581314
Dietz AK, Robinson RR, Forsthuber T (2018) Protective effect of IFN-γ during experimental autoimmune encephalomyelitis is associated with the induction of inducible nitric oxide synthase. J Immunol 200(1 Supplement):54.10
Acknowledgements
The authors thank Drs. Wu Feng and Ren Huixun in the Department of Immunology and Pathogenic Biology of Xi’an Jiaotong University for the great help in the modification of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers: 81273196, 81430048, 81772034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics Approval and Consent to Participate
All protocols were approved by the Ethics Committee of Xi’an Jiaotong University.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Chen, T., Li, G. et al. Dopamine Alters Lipopolysaccharide-Induced Nitric Oxide Production in Microglial Cells via Activation of D1-Like Receptors. Neurochem Res 44, 947–958 (2019). https://doi.org/10.1007/s11064-019-02730-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02730-7