Abstract
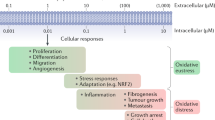
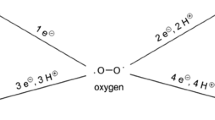
Oxidative stress resulting from accumulation of reactive oxygen, nitrogen, and halogen species (ROS, RNS, and RHS, respectively) causes the damage of cells and biomolecules. However, over the long evolutionary time, living organisms have developed the mechanisms for adaptation to oxidative stress conditions including the activity of the antioxidant system (AOS), which maintains low intracellular levels of RONS (ROS and RNS) and RHS. Moreover, living organisms have adapted to use low concentrations of these electrophiles for the regulation of cell functions through the reversible posttrans lational chemical modifications of redoxsensitive amino acid residues in intracellular effectors of signal transduction path ways (protein kinases and protein phosphatases), transcription factors, etc. An important finetuning mechanism that ensures involvement of RONS and RHS in the regulation of physiological processes is interconversion between different reactive species. This review focuses on the complex networks of interacting RONS and RHS types and their endogenous sources, such as NOX family of NADPH oxidases, complexes I and III of the mitochondrial electron transport chain, NO synthases, cytochrome P450 containing monooxygenase system, xanthine oxidoreductase, and myeloperoxidases. We high light that kinetic parameters of reactions involving RONS and RHS determine the effects of these reactive species on cell functions. We also describe the functioning of enzymatic and nonenzymatic AOS components and the mechanisms of RONS and RHS scavenging under physiological conditions. We believe that analysis of interactions between RONS and relationships between different endogenous sources of these compounds will contribute to better understanding of their role in the maintenance of cell redox homeostasis as well as initiation and progression of diseases.
Similar content being viewed by others
Abbreviations
- COX:
-
cyclooxygenase or prostaglandin G/H synthase
- CYP:
-
cytochrome P450
- ETC:
-
electron transport chain
- GPx:
-
glutathione peroxidase
- GR:
-
glutathione reductase
- GSH:
-
reduced glutathione
- LOX:
-
lipoxygenase
- NOS:
-
nitric oxide synthase
- NOX:
-
proteins
- NADPH:
-
oxidase family proteins
- Prx:
-
peroxiredoxin
- RHS:
-
reactive halogen species
- RNS:
-
reactive nitrogen species
- RONS:
-
reactive oxygen and nitrogen species
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- Srx:
-
sulfiredoxin
- Trx:
-
thioredoxin
- TrxR:
-
thioredoxin reductase
- XOR:
-
xanthine oxidoreductase
References
Sies, H. Berndt, C., and Jones, D. P. (2017) Oxidative stress, Ann. Rev. Biochem., 86, 715–748.
Di Meo, S. Reed, T. T. Venditti, P., and Victor, V. M. (2016) Role of ROS and RNS sources in physiological and pathological conditions, Oxid. Med. Cell. Longev., 2016 1245049.
Ma, M. W. Wang, J. Zhang, Q. Wang, R. Dhandapani, K. M. Vadlamudi, R. K., and Brann, D. W. (2017) NADPH oxidase in brain injury and neurodegenerative disorders, Mol. Neurodegen., 12, 7.
Forman, H. J. (2016) Redox signaling: an evolution from free radicals to aging, Free Radic. Biol. Med., 97, 398–407.
Moldogazieva, N. T. Lutsenko, S. V., and Terentiev, A. A. (2018) Reactive oxygen and nitrogen species-induced protein modifications: implication in carcinogenesis and anticancer therapy, Cancer Res., 78, 6040–6047.
Forman, H. J. Ursini, F., and Maiorino, M. (2014) An overview of mechanisms of redox signaling, J. Mol. Cell. Cardiol., 73, 2–9.
Adams, L. Franco, M. C., and Estevez, A. G. (2015) Reactive nitrogen species in cellular signaling, Exp. Biol. Med., 240, 711–717.
Ray, P. D. Huang, B. W., and Tsuji, Y. (2012) Reactive oxygen species (ROS) homeostasis and redox regulation and signaling, Cell Signal., 24, 981–990.
Forman, H. J. Maiorino, M., and Ursini, F. (2010) Signaling functions of reactive oxygen species, Biochemistry, 49, 835–842.
Adams, L. Franco, M. C., and Estevez, A. G. (2015) Reactive nitrogen species in cellular signaling, Exp. Biol. Med., 240, 711–717.
Fridovich, I. (1997) Superoxide anion radical, superoxide dismutases, and related matters, J. Biol. Chem., 272, 18515–18517.
Halliwell, B., and Gutteridge, J. M. C. (1999) Free Radicals in Biology and Medicine, Oxford University Press, Oxford.
Parke, D. V. (1996) Chemical toxicity and reactive oxygen species, Int. J. Occup. Med. Environ. Health, 9, 331–340.
Valko, M. Leibfritz, D. Moncol, J. Cronin, M. T. Mazur, M., and Telser, J. (2007) Free radicals and antioxidants in normal physiological functions and human diseases, Int. J. Biochem. Cell Biol., 39, 44–84.
Pacher, P. Beckman, J. S., and Liaudet, L. (2007) Nitric oxide and peroxynitrite in health and disease, Phys. Rev., 87, 315–424.
Wagner, B. A. Reszka, K. J. McCormick, M. L. Britigan, B. E. Evig, C. B., and Burns, C. P. (2004) Role of thiocyanate, bromide and hypobromous acid in hydrogen peroxide-induced apoptosis, Free Radic. Res., 38, 167–175.
Daiber, A. Di Lisa, F. Oelze, M. Kroller-Schon, S. Steven, S. Schulz, E., and Munzel, T. (2017) Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signaling and its role for vascular function, Br. J. Pharmacol., 174, 1670–1689.
Forstermann, U., and Sessa, W. C. (2012) Nitric oxide synthases: regulation and function, Eur. Heart J., 33, 829–837.
Luo, S. Lei, H. Qin, H., and Xia, Y. (2014) Molecular mechanisms of endothelial NO synthase uncoupling, Curr. Pharm. Des., 20, 3548–3553.
Morgan, M. J., and Liu, Z.-G. (2011) Crosstalk of reactive oxygen species and NF-?B signaling, Cell Res., 21, 103–115.
Finkel, T. (2012) Signal transduction by mitochondrial oxidants, J. Biol. Chem., 287, 4434–4440.
Brand, M. D. (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling, Free Radic. Biol. Med., 100, 14–31.
Bedard, K., and Krause, K. H. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology, Phys. Rev., 87, 245–313.
Fialkow, L. Wang, Y., and Downey, G. P. (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function, Free Radic. Biol. Med., 42, 153–164.
Garaude, J. Acin-Perez, R. Martinez-Cano, S. Enaomorado, M. Ugolini, M. Nistal-Villan, E. HervasStubbs, S. Pelegrin, P. Sander, L. E. Enriques, J. A., and Sancho, D. (2016) Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense, Nat. Immunol., 17, 1037–1045.
Vignais, P. V. (2002) The superoxide-generating NADPH oxidase: structural aspects and activation mechanism, Cell. Mol. Life Sci., 59, 1428–1459.
Meitzler, J. L., and Ortiz de Montellano, P. R. (2009) Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains, J. Biol. Chem., 284, 18634–18643.
Brandes, R. P. Weissmann, N., and Schroder, K. (2014) NOX family NADPH oxidases: molecular mechanisms of activation, Free Radic. Biol. Med., 76, 208–226.
Purushothaman, D., and Sarin, A. (2009) Cytokinedependent regulation of NADPH oxidase activity and the consequences for activated T cell homeostasis, J. Exp. Med., 206, 1515–1523.
Miyano, K., and Suminoto, H. (2012) Assessment of the role for Rho family GTPases in NADPH oxidase activation, Methods Mol. Biol., 827, 195–212.
Pick, E. (2014) Role of the Rho GTPase Rac in the activation of phagocyte NADPH oxidase: outsourcing a key task, Small GTPases, 5, e27952.
Bustelo, X. R. Sauzeau, V., and Berenjeno, I. (2007) GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo, Bioessays, 29, 356–370.
Dhaunsi, G. S. Alsaeid, M., and Akhtar, S. (2016) Phytanic acid activates NADPH oxidase through transactivation of epidermal growth factor receptor in vascular smooth muscle cells, Lipids Health Dis., 15, 105.
Yang, D. Elner, S. G. Bian, Z. M. Till, G. O. Petty, H. R., and Elner, V. M. (2007) Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells, Exp. Eye Res., 85 462–472.
Damaino, S. Fusco, R. Morano, A. De Mizio, M. Paterno, R. De Rosa, A. Spinelli, R. Amente, S. Frunzio, R. Mondola, P. Miot, F. Laccetti, P. Santillo, M., and Avvedimento, E. V. (2012) Reactive oxygen species regulate the levels of dual oxidase (Duox1-2) in human neuroblastoma cells, PLoS One, 7, e34405.
Panday, A. Sahoo, M. K. Osorio, D., and Batra, S. (2015) NADPH oxidases: an overview from structure to innate immunity-associated pathologies, Cell. Mol. Immunol., 12 5023.
Tyurin-Kuzmin, P. A. Zhdanovskaya, N. D. Sukhova, A. A. Sagaradze, G. D. Albert, E. A. Ageeva, L. V. Sharonov, G. V. Vorotnikov, A. V., and Tkachuk, V. A. (2016) Nox4 and Duox1/2 mediate redox activation of mesenchymal cell migration by PDGF, PLoS One, 11, e0154157.
Colin, F. (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases, Int. J. Mol. Sci., 20, 2407.
Wardman, P. (1989) Reduction potentials of one-electron couples involving free radicals in aqueous solution, J. Phys. Chem. Ref. Data, 18, 1637–1755.
Haber, F., and Weiss, J. (1934) The catalytic decomposition of hydrogen peroxide, Proc. Royal Soc., 147, 332–351.
Fenton, H. J. H. (1894) Oxidation of tartaric acid in presence of iron, J. Chem. Soc. Transac., 65, 899–910.
Semenov, N. N. (1959) Some Problems in Chemical Kinetics and Reactivity, Princeton University Press, Princeton USA.
Weidinger, A., and Kozlov, A. V. (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction, Biomolecules, 5, 472–484.
Heppner, D. E. Dustin, C. M. Liao, C. Hristova, M. Veith, C. Little, A. C. Ahlers, B. A. White, S. L. Deng, B. Lam, Y. W. Li, J., and van der Vliet, A. (2018) Direct cysteine sulfenylation drives activation of the Src kinase, Nat. Commun., 9, 4522.
Nelson, K. J. Bolduc, J. A. Wu, H. Collins, J. A. Burke, E. A. Reisz, J. A. Klomsiri, C. Wood, S. T. Yammani, R. R. Poole, L. B. Furdui, C. M., and Loeser, R. F. (2018) H2O2 oxidation of cysteine residues in c-Jun N-terminal kinase 2 (JNK2) contributes to redox regulation in human articular chondrocytes, J. Biol. Chem., 293, 16376–16389.
Luanpitpong, S. Chanvorachote, P. Stehlik, C. Tse, W. Callery, P. S. Wang, L., and Rojanasakul, Y. (2013) Regulation of apoptosis by Bcl-2 cysteine oxidation in human lung epithelial cells, Mol. Biol. Cell, 24, 858–869.
Wong, H.-S. Dighe, P. A. Mezera, V. Monternier, P.-A. and Brand, M. D. (2017) Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions, J. Biol. Chem., 292 16804–16809.
Grivennikova, V. G., and Vinogradov, A. D. (2013) Mitochondrial production of reactive oxygen species, Biochemistry (Moscow), 78, 1490–1511.
Alberts, B. Johnson, A. Lewis, J. Raff, M. Roberts, K. and Walter, P. (2002) Molecular biology of the cell, in The Mitochondrion (4th Edn.), Garland Science, New York.
Van Boxel, G. I. Doherty, W. L., and Parmar, M. (2012) Cellular oxygen utilization in health and sepsis, Contin. Educ. Anaesth. Crit. Care Pain, 12, 207–212.
Murphy, M. P. (2009) How mitochondria produce reactive oxygen species, Biochem. J., 417, 1–13.
James, A. M. Smith, R. A., and Murphy, M. P. (2004) Antioxidant and prooxidant properties of mitochondrial coenzyme Q, Arch. Biochem. Biophys., 423, 47–56.
Maranzana, E. Barbero, G. Falasca, A. I. Lenaz, G., and Genova, M. L. (2013) Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I, Antioxid. Redox Signal., 19, 1469–480.
Lenaz, G. Tioli, G. Falasca, A. I., and Genova, M. L. (2016) Complex I function in mitochondrial supercomplexes, Biochim. Biophys. Acta, 1857, 991–1000.
Lenaz, G., and Genova, M. L. (2009) Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly, Int. J. Biochem. Cell Biol., 41 1750–1772.
Acin-Perez, R., and Enriquez, J. A. (2014) The function of the respiratory supercomplexes: the plasticity model, Biochim. Biophys. Acta, 1837, 444–450.
Stone, J. R., and Yang, S. (2006) Hydrogen peroxide: a signaling messenger, Antioxid. Redox Signal., 8, 243–270.
Van der Vliet, A., and Janssen-Heininger, Y. M. (2014) Hydrogen peroxide as a damage signal in tissue injury and inflammation: murderer, mediator, or messenger? J. Cell Biochem., 115, 427–435.
Bienert, G. P. Schjoerring, J. K., and Jagn, T. (2006) Membrane transport of hydrogen peroxide, Biochim. Biophys. Acta, 1758, 994–1003.
Bienert, G. P., and Chaumont, F. (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide, Biochim. Biophys. Acta, 1840, 1596–1604.
Bestetti, S. Medrano-Fernandez, I. Galli, M. Ghitti, M. Bienert, G. Musco, G. Orsi, A. Rubartelli, A., and Sitia, R. (2018) A persulfidation-based mechanism controls aquaporin-8 conductance, Sci. Adv., 4, eaar5770.
Forman, H. J. Bernardo, A., and Davies, K. J. A. (2016) What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys., 603, 48–53.
Augusto, O., and Miyamoto, S. (2011) Oxygen radicals and related species, in Principles of Free Radical Biomedicine Nova Science Publishers, Inc., Chap. II, pp. 1–23.
Sies, H. (2014) Role of metabolic H2O2 generation: redox signaling and oxidative stress, J. Biol. Chem., 289, 8735–8741.
Ma, Q. (2013) Role of Nrf2 in oxidative stress and toxicity, Ann. Rev. Pharmacol. Toxicol., 53, 401–426.
Schmidtlin, C. J. Dodson, M. B. Madhavan, L., and Zhang, D. D. (2019) Redox regulation by NRF2 in aging and disease, Free Radic. Biol. Med., 34, 702–707.
Itoh, K. Wakabayashi, N. Katoh, Y. Ishii, T. Igarashi, K. Engel, J. D., and Yamamoto, M. (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain, Genes Dev., 13, 76–86.
Suzuki, T., and Yamamoto, M. (2017) Stress-sensing mechanisms and physiological roles of the Keap1–NRF2 system during cellular stress, J. Biol. Chem., 292, 16817–16824.
Fourquet, S. Guerois, R. Biard, D., and Toledano, M. B. (2010) Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation, J. Biol. Chem., 285 8463–8471.
Rouzer, C. A., and Marnett, L. J. (2009) Cyclooxygenases: structural and functional insights, J. Lipid Res.., 50, S29–S34.
Yun, M. R. Park, H. M. Seo, K. W. Lee, S. J. Im, D. S. and Kim, C. D. (2010) 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase, Free Radic. Res., 44, 742–750.
Cho, K. J. Seo, J.-M., and Kim, J. H. (2011) Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species, Mol. Cell, 32, 1–5.
Ricciotti, E., and FitzGerald, G. A. (2011) Prostaglandins and inflammation, Arterioscler. Thromb. Vasc. Biol., 32 986–1000.
Fitzpatrick, F. A. (2004) Cyclooxygenase enzymes: regulation and function, Curr. Pharm. Des., 10, 577–588.
Uchida, K. M., and Shibata, T. (2008) 15-δ(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses, Chem. Res. Toxicol., 21, 138–144.
Hong, H. Y. Jeon, W. K., and Kim, B. C. (2008) Up-regulation of heme oxygenase-1 expression through the Rac1/NADPH oxidase/ROS/p38 signaling cascade mediates the anti-inflammatory effect of 15-deoxy-δ12,14-prostaglandin J2 in murine macrophages, FEBS Lett., 582 861–868.
Andreou, A., and Feussner, I. (2009) Lipoxygenases–structure and reaction mechanism, Phytochemistry, 70 1504–1510.
De Carvalho, D. D. Sadok, A. Bourgarel-Rey, V. Gattacceca, F. Penel, C. Lehmann, M., and Kovacic, H. (2008) Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells, Int. J. Cancer, 122, 1757–1764.
Mahipal, S. V. Subhashini, J. Reddy, M. C. Reddy, M. M. Anilkumar, K. Roy, K. R. Reddy, G. V., and Reddanna, P. (2007) Effect of 15-lipoxygenase metabolites 15-(S)-HPETE and 15-(S)-HETE on chronic myelogenous leukemia cell line K-562: reactive oxygen species (ROS) mediate caspase-dependent apoptosis, Biochem. Pharmacol., 74, 202–214.
Li, Q. Mao, M. Qiu, Y. Liu, G. Sheng, T. Yu, X. Wang, S., and Zhu, D. (2016) Key role of ROS in the process of 15-lipoxygenase/15-hydroxy eicosatetraenoic acidinduced pulmonary vascular remodeling in hypoxia pulmonary hypertension, PLoS One, 11, e0149164.
Soumya, S. J. Binu, S. Helen, A. Reddanna, P., and Sudhakaran, P. R. (2013) 15(S)-HETE-induced angiogenesis in adipose tissue is mediated through activation of PI3K/Akt/mTOR signaling pathway, Biochem. Cell Biol., 91, 498–505.
Harrison, R. (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic. Biol. Med., 33, 774–797.
Kostic, D. A. Dimitrievich, D. S. Stoyanovich, G. S. Palic, I. R. Dordevich, A. S., and Ickovski, J. D. (2015) Xanthine oxidase: isolation, assays of activity, and inhibition, J. Chem., 2015, 294858; doi: 10.1155/2015/294858.
Enroth, C. Eger, B. T. Okamoto, K. Nishino, T. Nishino, T., and Pai, E. F. (2000) Crystal structure of bovine milk dehydrogenase and xanthine oxidase: structure-based mechanism of conversion, Proc. Natl. Acad. Sci. USA, 97, 10723–10728.
Battelli, M. G. Polito, L. Bortolotti, M., and Bolognesi, A. (2016) Xanthine oxidoreductase-derived reactive species: physiological and pathological effects, Oxid. Med. Cell. Longev., 2016, 3527579.
Matsumoto, S. Koshiishi, I. Inoguchi, T. Nawata, H. and Utsumi, H. (2003) Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice, Free Radic. Res., 37, 767–772.
Cantu-Medellin, N., and Kelley, E. E. (2013) Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation, Redox Biol., 1, 353–358.
Bielski, B. H. J. Cabelli, D. E., and Arudi, R. L. (1985) Reactivity of HO2/O2 radicals in aqueous solution, J. Phys. Chem. Ref. Data, 14, 1041–1100.
Kelley, E. E. Khoo, N. K. Hundley, N. J. Malik, U. Z. Freeman, B. A., and Tarpey, M. M. (2010) Hydrogen peroxide is the major product of xanthine oxidase, Free Radic. Biol. Med., 48, 493–498.
Winterbourn, C. C., and Metodiewa, D. (1994) The reaction of superoxide with reduced glutathione, Arch. Biochem. Biophys., 314, 284–290.
Gonzalez, F. J. (2005) Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1, Mutat. Res., 569, 101–110.
Mokhosoev, I. M. Kuznetsova, G. P. Al’terman, M. A. Bachmanova, G. I., and Archakov, A. I. (1987) Inactivation of sodium dithionite reduced cytochromes P-450-of different origins, Biokhimiya, 52, 1649–1658.
Singh, S. Rajendran, R. Kuroda, K. Isogai, E. KrsticDemonacos, M., and Demonacos, C. (2016) Oxidative stress and breast cancer biomarkers: the case of the cytochrome P450 2E1, J. Cancer Metastas. Treat., 2, 268–276.
Poulos, T., and Johnson, E. (2015) Structures of cytochrome P450 enzymes, in Cytochrome P450 (Ortiz de Montellano, P., ed.) Springer, Cham.
Mak, P. J., and Denisov, I. G. (2018) Spectroscopic studies of the cytochrome P450 reaction mechanisms, Biochim. Biophys. Acta, 1866, 178–204.
Batabyal, D. Richards, L. S., and Poulos, T. L. (2017) Effects of redox partner binding on cytochrome P450 conformational dynamics, J. Am. Chem. Soc., 139, 13193–13199.
Hrycay, E. G., and Bandiera, S. M. (2015) Involvement of cytochrome P450 in reactive oxygen species formation and cancer, Adv. Pharmacol., 74, 35–84.
Shumyantseva, V. V. Makhova, A. A. Bulko, T. V. Bernhardt, R. Kuzikov, A. V. Shich, E. V. Kukes, V. G. and Archakov, A. I. (2015) Taurine modulates catalytic activity of cytochrome P-450 3A4, Biochemistry (Moscow), 80, 366–373.
Archakov, A. I., and Mokhosoev, I. M. (1989) Modification of proteins by active oxygen and their degradation, Biokhimiya, 54, 179–186.
Daiber, A. Bachschmid, M. Beckman, J. S. Munzel, T. and Ullrich, V. (2004) The impact of metal catalysis on protein tyrosine nitration by peroxynitrite, Biochem. Biophys. Res. Commun., 317, 873–881.
Daiber, A. Schoneich, C. Schmidt, P. Jung, C., and Ullrich, V. (2000) Autocatalytic nitration of P450CAM by peroxynitrite, J. Inorg. Biochem., 81, 213–220.
Daiber, A. Herold, S. Schoneich, C. Namgaladze, D. Peterson, J. A., and Ullrich, V. (2000) Nitration and inactivation of cytochrome P450BM-3 by peroxynitrite. Stopped-flow measurements prove ferryl intermediates, Eur. J. Biochem., 267, 6729–6739.
Rosen, G. M. Tsai, P., and Pou, S. (2002) Mechanism of free-radical generation by nitric oxide synthase, Chem. Rev., 102, 1191–1200.
Zhou, L., and Zhu, D.-Y. (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation and clinical implications, Nitric Oxide, 20, 223–230.
Green, S. J. Mellouk, S. Hoffman, S. L. Meltzer, M. S. and Nacy, C. A. (1990) Cellular mechanisms of nonspecific immunity to intracellular infection: cytokineinduced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes, Immunol. Lett., 25 15–19.
Green, S. J. Scheller, L. F. Marletta, M. A. Seguin, M. C. Klotz, F. W. Slayter, M. Nelson, B. J., and Nacy, C. A. (1994) Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens, Immunol. Lett., 43, 87–94.
Wallace, J. L. Ianaro, A. Flannigan, K. L., and Cirino, G. (2015) Gaseous mediators in resolution of inflammation, Semin. Immunol., 27, 227–233.
Forstermann, U., and Munzel, T. (2006) Endothelial nitric oxide synthase in vascular disease, Circulation, 113, 1708–1714.
Costa, E. D. Rezende, B. A. Cortes, S. F., and Lemos, V. S. (2016) Neuronal nitric oxide synthase in vascular physiology and diseases, Front. Physiol., 7, 206.
Lind, M. Hayes, A. Capmda, M. Petrovic, D. Rodrigo, L. Kruzliak, P., and Zulli, A. (2017) Inducible nitric oxide synthase: good or bad? Biomed. Pharmacother., 93, 370–375.
Lacza, Z. Pankotai, E., and Busija, D. W. (2009) Mitochondrial nitric oxide synthase: current concepts and controversies, Front. Biosci. (Landmark Ed.), 14, 4436–4443.
Klatt, P. Pfeiffer, S. List, B. M. Lehner, D. Glatter, O. Bachinger, H. P. Werner, E. R. Schmidt, K., and Mayer, B. (1996) Characterization of heme-deficient neuronal nitric oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin, J. Biol. Chem., 271, 7336–7342.
Schmidt, P. P. Lange, R. Gorren, A. C. Werner, E. R. Mayer, B., and Andersson, K. K. (2001) Formation of a protonated trihydrobiopterin radical cation in the first reaction cycle of neuronal and endothelial nitric oxide synthase detected by electron paramagnetic resonance spectroscopy, J. Biol. Inorg. Chem., 6, 151–158.
List, B. M. Klosch, B. Volker, C. Gorren, A. C. Sessa, W. C. Werner, E. R. Kukovetz, W. R. Schmidt, K., and Mayer, B. (1997) Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of heme in dimerization, Biochem. J., 323, 159–165.
Piazza, M. Guillemette, J. G., and Dieckmann, T. (2015) Dynamics of nitric oxide synthase–calmodulin interactions at physiological calcium concentrations, Biochemistry, 54, 1989–2000.
Spratt, D. E. Taiakina, V. Palmer, M., and Guillemette, J. G. (2007) Differential binding of calmodulin domains to constitutive and inducible nitric oxide synthase enzymes, Biochemistry, 46, 8288–8300.
Hemmens, B. Goessler, W. Schmidt, K., and Mayer, B. (2000) Role of bound zinc in dimer stabilization but not enzyme activity of neuronal nitric oxide synthase, J. Biol. Chem., 275, 35786–35791.
Hinchee-Rodriguez, K. Garg, N. Venkatakrishnan, P. Roman, M. G. Adamo, M. L. Masters, B. S., and Roman, L. J. (2013) Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle, Biochem. Biophys. Res. Commun., 345, 501–505.
Su, Y. (2014) Regulation of endothelial nitric oxide synthase activity by protein–protein interaction, Curr. Pharm. Des., 20, 3514–3520.
Luo, S. Lei, H. Qin, H., and Xia, Y. (2014) Molecular mechanisms of endothelial NO synthase uncoupling, Curr. Pharm. Des., 20, 3548–3553.
Berka, V. Liu, W. Wu, G., and Tsai, A. L. (2014) Comparison of oxygen-induced radical intermediates in iNOS oxygenase domain with those from nNOs and eNOS, J. Inorg. Biochem., 139, 93–105.
Krzyaniak, M. D. Cruce, A. A. Vennam, P. Lockart, M. Berka, V. Tsai, A. L., and Bowman, M. K. (2016) The tetrahydrobiopterin radical interacting with high- and lowspin heme in neuronal nitric oxide synthase–a new indicator of the extent of NOS coupling, Free Radic. Biol. Med., 101, 367–377.
Chen, C. A. Druhan, L. J. Varadharaj, S. Chen, Y. R. and Zweier, J. L. (2008) Phosphorylation of endothelial nitric oxide synthase regulates superoxide generation from the enzyme, J. Biol. Chem., 283, 27038–27047.
Chen, C. A. Wang, T. Y. Varadharaj, S. Reyes, L. A. Hemann, C. Talukder, M. A. Chen, Y. R. Druhan, L. J. and Zweier, J. L. (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function, Nature, 468, 1115–1118.
Wu, F. Szczepaniak, W. S. Shiva, S. Liu, H. Wang, Y. Wang, L. Wang, Y. Kelly, E. E. Chen, A. F. Gladwin, M. T., and McVerry, B. J. (2014) Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury, Am. J. Physiol.-Lung Cell. Mol. Physiol., 307, L987-L997.
Xia, N. Daiber, A. Habermeier, A. Closs, E. I. Thum, T. Spanier, G. Lu, Q. Oelze, M. Torzewski, M. Lackner, K. J. Munzel, T. Forstermann, U., and Li, H. (2010) Resveratrol reverse endothelial nitric oxide synthase uncoupling in apolipoprotein E knockout mice, J. Pharmacol. Exp. Ther., 335, 149–154.
Roe, N. D., and Ren, J. (2012) Nitric oxide synthase uncoupling: a therapeutic target in cardiovascular diseases, Vasc. Pharmacol., 57, 168–172.
De Pascali, F. Hemann, C. Samons, K. Chen, C. A., and Zweier, J. L. (2014) Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation, Biochemistry, 53, 3679–3688.
Pryor, W. A., and Squadrito, G. L. (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide and superoxide, Am. J. Physiol., 268, L699-L722.
Squadrito, G. L., and Pryor, W. A. (1998) Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite and carbon dioxide, Free Radic. Biol. Med., 25, 392–403.
Goldstein, S., and Czapski, G. (1995) The reaction of NO⊙ with O2-. and HO2⊙: a pulse radiolysis study, Free Radic. Biol. Med., 19, 505–510.
Radi, R. (2013) Peroxynitrite, a stealthy biological oxidant, J. Biol. Chem., 288, 26464–26472.
Sturzbecher-Hohne, M. Nauser, T. Kissner, R., and Koppenol, W. H. (2009) Photon-initiated homolysis of peroxynitrous acid, Inorg. Chem., 48, 7307–7312.
Lymar, S. V. Khairutdinov, R. F., and Hurst, J. K. (2003) Hydroxyl radical formation by O–O bond homolysis in peroxynitrous acid, Inorg. Chem., 42, 5259–5266.
Ford, E. Hughes, M. N., and Wardman, P. (2002) Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH, Free Radic. Biol. Med., 32, 1314–1323.
Denicola, A. Souza, J. M., and Radi, R. (1998) Diffusion of peroxynitrite across erythrocyte membranes, Proc. Natl. Acad. Sci. USA, 95, 3566–3571.
Meli, R. Nauser, T. Latal, P., and Koppenol, W. H. (2002) Reaction of peroxynitrite with carbon dioxide: intermediates and determination of the yield of CO3-. and .NO2, J. Biol. Inorg. Chem., 7, 31–36.
Yakovlev, V. A., and Mikkelsen, R. B. (2010) Protein tyrosine nitration in cellular signal transduction pathways, J. Recept. Signal Transduct. Res., 30, 420–429.
Herold, S. Exner, M., and Boccini, F. (2003) The mechanism of the peroxynitrite-mediated oxidation of myoglobin in the absence and presence of carbon dioxide, Chem. Res. Toxicol., 16, 390–402.
Boccini, F., and Herold, S. (2004) Mechanistic studies of the oxidation of oxyhemoglobin by peroxynitrite, Biochemistry, 28, 16393–16404.
Osipov, A. N. Borisenko, G. G., and Vladimirov, Y. A. (2007) Biological activity of hemoprotein nitrosyl complexes, Biochemistry (Moscow), 72, 1491–1504.
Davies, M. J. (2010) Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention, J. Clin. Biochem. Nutr., 48, 8–19.
Kussendrager, K. D., and van Hooijdonk, A. C. (2000) Lactoperoxidase: physicochemical properties, occurrence, mechanism of action and applications, Br. J. Nutr., 84, S19–25.
Ruf, J., and Carayon, P. (2006) Structural and functional aspects of thyroid peroxidase, Arch. Biochem. Biophys., 445, 269–277.
Wang, J., and Slungaard, A. (2006) Role of eosinophil peroxidase in host defense and disease pathology, Arch. Biochem. Biophys., 445, 256–260.
Bathish, B. Turner, R. Paumann-Page, M. Kettle, A. J. and Winterbourn, C. C. (2018) Characterization of peroxidasin activity in isolated extracellular matrix and direct detection of hypobromous acid formation, Arch. Biochem. Biophys., 646, 120–127.
Furtmuller, P. G. Jantschko, W. Zederbauer, M. Jakopitsch, C. Arnhold, J., and Obinger, C. (2004) Kinetics of interconversion of redox intermediates of lactoperoxidase, eosinophil peroxidase and myeloperoxidase, Japan. J. Infect. Dis., 57, S30–S31.
Winterbourn, C. C. Kettle, A. J., and Hampton, M. B. (2016) Reactive oxygen species and neutrophil function, Ann. Rev. Biochem., 85, 765–792.
Klebanoff, S. J. Kettle, A. J. Rosen, H. Winterbourn, C. C., and Nauseef, W. M. (2013) Myeloperoxidase: a frontline defender against phagocytosed microorganisms, J. Leuk. Biol., 93, 185–198.
Senthilmohan, R., and Kettle, A. J. (2006) Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride, Arch. Biochem. Biophys., 445, 235–244.
Davies, M. J. Hawkins, C. L. Pattinson, D. I., and Rees, M. D. (2008) Mammalian heme peroxidases: from molecular mechanisms to health implications, Antioxid. Redox Signal., 10, 1199–1234.
Tlili, A. Erard, M. Faure, M.-C. Baudin, X. Piolot, T. Dupre-Crochet, S., and Nüße, O. (2012) Stable accumulation of p67phox at the phagosomal membrane and production with the phagosome, J. Leuk. Biol. Med., 91 83–95.
Panasenko, O. M. Gorudko, I. V., and Sokolov, A. V. (2013) Hypochlorous acid as a precursor of free radicals in living systems, Biochemistry (Moscow), 78, 1466–1489.
Segal, B. H. Leto, T. L. Gallin, J. I. Malech, H. L., and Holland, S. M. (2000) Genetic, biochemical, and clinical features of chronic granulomatous disease, Medicine, 79 70–200.
Vethanayagam, R. R. Almyroudis, N. G. Grimm, M. J. Lewandowski, D. C. Pham, C. T. Blackwell, T. S. Petraitiene, R. Petraitis, V. Walsh, T. J. Urban, C. F., and Segal, B. H. (2011) Role of NADPH oxidase versus neutrophil proteases in antimicrobial host defense, PLoS One, 6, e28149.
Shilon, M. U. MacMickong, J. D. Nicholson, S. Brause, J. E. Potter, S. Marino, M. Fang, F. Dinauer, M., and Nathan, C. (1999) Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase, Immunity, 10, 29–38.
Van der Vliet, A. Eiserich, J. P. Halliwell, B., and Cross, C. E. (1997) Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrate. A potential additional mechanism of nitric oxide-dependent toxicity, J. Biol. Chem., 272, 7617–7625.
Gaut, J. P. Byun, J. Tran, H. D. Lauber, W. M. Carroll, J. A. Hotchkiss, R. S. Belaaouaj, A., and Heinecke, J. W. (2002) Myeloperoxidase produces nitrating oxidants in vivo, J. Clin. Invest., 109, 1311–1319.
Summers, F. A. Morgan, P. E. Davies, M. J., and Hawkins, C. L. (2008) Identification of plasma proteins that are susceptible to thiol oxidation by hypochlorous acid and N-chloramines, Chem. Res. Toxicol., 21, 1832–1840.
Storkey, C. Davies, M. J., and Pattison, D. I. (2014) Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach, Free Radic. Biol. Med., 73, 60–66.
Degrossoli, A. Muller, A. Xie, K. Schneider, J. F. Bader, V. Winkhofer, K. F. Meyer, A. J., and Leichert, L. I. (2019) Neutrophil-generated HOCl leads to non-specific thiol oxidation in phagocytized bacteria, eLife, 7, 332288.
Nybo, T. Dietrich, S. Gamon, L. F. Chuang, C. Y. Hammer, A. Hoefer, G. Malle, E. RogowskaWrzesinska, A., and Davies, M. J. (2019) Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase, Redox Biol., 20, 496–513.
Pattison, D. I., and Davies, M. J. (2006) Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous acid-mediated damage, Biochemistry, 45, 8152–8162.
Verrastro, I. Tveen-Jensen, K., and Spickett, C. M. (2018) The effect of HOCl-induced modifications on phosphatase and tensin homologue (PTEN) structure and function, Free Radic. Res., 52, 232–247.
Colon, S. Page-McCaw, P., and Bhave, G. (2017) Role of hypohalous acids in basement membrane homeostasis, Antioxid. Redox Signal., 27, 839–854.
Van der Veen, B. S. de Winther, M. P., and Heeringa, P. (2009) Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease, Antioxid. Redox Signal., 11, 2899–2937.
Nicholls, S. J., and Hazen, S. L. (2005) Myeloperoxidase and cardiovascular disease, Arterioscler. Thromb. Vasc. Biol., 25, 1102–1111.
Nicholls, S. J. Wilson Tang, W. H. Brennan, D. Brennan, M. L. Mann, S. Nissen, S. E., and Hazen, S. L. (2011) Risk prediction with serial myeloperoxidase monitoring in patients with acute chest pain, Clin. Chem., 57 1762–1770.
Henderson, J. P. Byun, J. Takeshita, J., and Heinecke, J. W. (2003) Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue, J. Biol. Chem., 278, 23522–23528.
Takeshita, J. Byun, J. Nhan, T. Q. Pritchard, D. K. Pennathur, S. Schwartz, S. M. Chait, A., and Heinecke, J. W. (2005) Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages, J. Biol. Chem., 281 3096–3104.
Ismael, F. O. Barrett, T. J. Sheipouri, D. Brown, B. E. Davies, M. J., and Hawkins, C. L. (2016) Role of myeloperoxidase oxidants in the modulation of cellular lysosomal enzyme function: a contributing factor to macrophage dysfunction in atherosclerosis?, PLoS One, 11 e0168844.
Nybo, T. Cai, H. Gaman, L. F. Rogowska-Wrzesinska, A., and Davies, M. J. (2018) Chlorination and oxidation of human plasma fibronectin by myeloperoxidase-derived oxidants, and its consequences for smooth muscle cell function, Redox Biol., 19, 388–400.
Colombo, G. Clerici, M. Altomare, A. Rusconi, F. Giustarini, D. Portimaro, N. Garavaglia, M. L. Rossi, R. Dalle-Donne, I., and Milzani, A. (2017) Thiol oxidation and dityrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid, J. Proteom., 152, 22–32.
He, L. He, T. Farrar, S. Ji, L. Liu, T., and Ma, X. (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species, Cell. Physiol. Biochem., 44, 532–553.
Ursini, F. Maiorino, M., and Forman, H. J. (2016) Redox homeostasis: the golden mean of healthy living, Redox Biol., 8, 205–215.
Niki, E. (2016) Oxidative stress and antioxidants: distress or eustress? Arch. Biochem. Biophys., 595, 19–24.
Inupakutika, M. A. Sengupta, S. Devireddy, A. R. Azad, R. K., and Mittler, R. (2016) The evolution of reactive oxygen species metabolism, J. Exp. Botany, 67, 5933–5943.
Egea, J. Fabregat, I. Frapart, Y. M. Ghezzi, P. Gorlach, A., et al. (2017) European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS), Redox Biol., 13, 94–162.
Moldogazieva, N. T., and Terentiev, A. A. (2006) Alphafetoprotein and growth factors. Structure-function relationships and analogies, Usp. Biol. Chim.(Rus), 46, 99–148.
Chaudiere, J., and Ferrari-Iliou, R. (1999) Intracellular antioxidants: from chemical to biochemical mechanisms, Food Chem. Toxicol., 37, 949–962.
Irshad, M., and Chaudhuri, P. S. (2002) Oxidant–antioxidant system: role and significance in human body, Indian J. Exp. Biol., 40, 1233–1239.
Pillai, C. K., and Pillai, K. S. (2002) Antioxidants in health, Indian J. Physiol. Pharmacol., 46, 1–5.
Andreyev, A. Y. Kushnareva, Y. E. Murphy, A. N., and Starkov, A. A. (2015) Mitochondrial ROS metabolism: 10 years later, Biochemistry (Moscow), 80, 517–531.
Gupta, R. K. Patel, A. K. Shah, N. Chaudhary, A. K. Jha, U. K. Yadav, U. C. Gupta, P. K., and Pakuwal, U. (2014) Oxidative stress and antioxidants in disease and cancer: a review, Asian Pacific J. Cancer Prevent., 15, 4405–4409.
Fridovich, I. (1997) Superoxide anion radical (O22-.), superoxide dismutases, and related matters, J. Biol. Chem., 272 18515–18517.
Fukai, T., and Ushio-Fukai, M. (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases, Antioxid. Redox Signal., 15, 1583–1606.
Li, Q. Sato, E. F. Kira, Y. Nishikawa, M. Utsumi, K. and Inoue, M. (2006) A possible cooperation of SOD1 and cytochrome c in mitochondria-dependent apoptosis, Free Radic. Biol. Med., 40, 173–181.
Che, M. Wang, R. Li, X. Wang, X. Y., and Zheng, X. F. S. (2016) Expanding roles of superoxide dismutases in cell regulation and cancer, Drug Discov. Today, 21, 143–149.
Scibior, D., and Czeczot, H. (2006) Catalase: structure properties, functions, Postepy Hig. Med. Dosw. (Online), 60, 170–180.
Radi, R. Turrens, J. F. Chang, L. Y. Bush, K. M. Crapo, J. D., and Freeman, B. A. (1991) Detection of catalase in rat heart mitochondria, J. Biol. Chem., 266, 22028–22034.
Cao, C. Leng, Y., and Kufe, D. (2003) Catalase activity is regulated by c-Abl and Arg in the oxidative stress response, J. Biol. Chem., 278, 29667–29675.
Dominguez, L. Sosa-Peinado, A., and Hansberg, W. (2010) Catalase evolved to concentrate H2O2 at its active site, Arch. Biochem. Biophys., 500, 82–91.
Dominguez, L. Sosa-Peinado, A., and Hansberg, W. (2014) How catalase recognizes H2O2 in a sea of water, Proteins, 82, 45–56.
Flohe, L. (2016) The impact of thiol peroxidases on redox regulation, Free Radic. Res., 50, 126–142.
Brigelius-Flohe, R., and Maiorino, M. (2013) Glutathione peroxidases, Biochim. Biophys. Acta, 1830 3289–3303.
Lubos, E. Loscalzo, J., and Handy, D. E. (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities, Antioxid. Redox Signal., 15, 1957–1997.
Wang, L. Zhang, L. Niu, Y. Sitia, R., and Wang, C. C. (2014) Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1a to promote oxidative protein folding, Antioxid. Redox Signal., 20, 545–556.
Espinoza, S. E. Guo, H. Fedarko, N. DeZern, A. Fried, L. P. Xue, Q. L. Leng, S. Beamer, B., and Walston, J. D. (2008) Glutathione peroxidase enzyme activity in aging, J. Gerontol. Ser. A Biol. Sci. Med. Sci., 63 505–509.
Ng, C. F. Schafer, F. Q. Buettner, G. R., and Rodgers, V. G. (2007) The rate of cellular hydrogen peroxide removal shows dependency on GSH: mathematical insight into in vivo H2O2 and GPx concentrations, Free Radic. Res., 41 1201–1211.
Flohe, L. (2013) The fairytale of the GSSG/GSH redox potential, Biochim. Biophys. Acta, 1830, 3139–3142.
Kalinina, E. V. Chernov, N. N., and Novichkova, M. D. (2014) Role of glutathione, glutathione transferase and glutaredoxin in regulation of redox-dependent processes, Biochemistry (Moscow), 79, 1562–1583.
Hopkins, B. L., and Neumann, C. A. (2019) Redoxins as gatekeepers of the transcriptional oxidative stress response, Redox Biol., 21, 101104.
Sharapov, M. G. Ravin, V. K., and Novoselov, V. I. (2014) Peroxiredoxins as multifunctional enzymes, Mol. Biol. (Moscow), 48, 600–628.
Rhee, S. G. (2014) Overview on peroxiredoxin, Mol. Cell, 39, 1–5.
Rhee, S. G., and Kil, I. S. (2017) Multiple functions and regulation of mammalian peroxiredoxins, Ann. Rev. Biochem., 86, 749–775.
Portillo-Ledesma, S. Randall, L. M. Parsonage, D. Rizza, J. D. Karplus, P. A. Poole, L. B. Denicola, A. and Ferrer-Sueta, G. (2018) Differential kinetics of twocysteine peroxiredoxin disulfide formation reveal a novel model for peroxide sensing, Biochemistry, 57, 3416–3424.
Rhee, S. G. Woo, H. A., and Kang, D. (2018) The role of peroxiredoxins in the transduction of H2O2 signals, Antioxid. Redox Signal., 28, 537–557.
Netto, L. E., and Antunes, F. (2016) The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction, Mol. Cell, 39, 65–71.
Lee, S. Kim, S. M., and Lee, R. T. (2013) Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance, Antioxid. Redox Signal., 18, 1165–1207.
Saccoccia, F. Angelucci, F. Boumis, G. Carotti, D. Desiato, G. Miele, A. E., and Bellelli, A. (2014) Thioredoxin reductase and its inhibitors, Curr. Protein Peptide Sci., 15, 621–646.
Yang, K. S. Kang, S. W. Woo, H. A. Hwang, S. C. Chae, H. Z. Kim, K., and Rhee, S. G. (2002) Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine sulfinic acid, J. Biol. Chem., 277, 38029–38036.
Noichri, Y. Palais, G. Ruby, V. D’Autreaux, B. Delaunay-Moisan, A. Nystrom, T. Molin, M., and Toledano, M. B. (2015) In vivo parameters influencing 2-Cys Prx oligomerization: the role of enzyme sulfinylation, Redox Biol., 6, 326–333.
Lowther, W. T., and Haynes, A. C. (2011) Reduction of cysteine sulfinic acid in eukaryotic, typical 2-Cys peroxiredoxins by sulfiredoxin, Antioxid. Redox Signal., 15, 99–109.
Rhee, S. G. Jeong, W. Chang, T. S., and Woo, H. A. (2007) Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action and biological significance, Kidney Int. Suppl., 106, S3–S8.
Rhee, S. G., and Kil, I. S. (2016) Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm, Free Radic. Biol. Med., 100, 73–80.
Nimse, S. B., and Pal, D. (2015) Free radicals, natural antioxidants, and their reaction mechanisms, RSC Advances, 5, 27986–28006.
Hacisevki, A. (2009) An overview of ascorbic acid biochemistry, J. Faculty Pharm. (Ankara), 38, 233–255.
Lee, Y. C. Huang, H. Y. Chang, C. J. Cheng, C. H., and Chen, Y. T. (2010) Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome, Human Mol. Genet., 19, 3721–3733.
May, J. M. Huang, J., and Qu, Z. C. (2005) Macrophage uptake and recycling of ascorbic acid: response to activation by lipopolysaccharides, Free Radic. Biol. Med., 39 1449–1459.
Oudemans-van Straaten, H. M. Spoelstra-de Man, A. M., and de Waard, M. C. (2014) Vitamin C revisited, Crit. Care, 18, 460.
Huang, J., and May, J. M. (2006) Ascorbic acid protects SH-SY5Y neuroblastoma cells from apoptosis and death induced by beta-amyloid, Brain Res., 1097, 52–58.
Chakraborthy, A. Ramani, P. Sherlin, H. J. Premkumar, P., and Natesan, A. (2014) Antioxidant and pro-oxidant activity of vitamin C in oral environment, Indian J. Dental Res., 25, 499–504.
Patel, V. S. Sampat, V. Espey, M. G. Sitapara, S. Wang, H. Yang, S. Asby, C. R., Jr. Thomas, D. D., and Mantel, L. L. (2016) Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection, Am. J. Respir. Cell Mol. Biol., 55, 511–520.
Rochette, L. Ghibu, S. Richard, C. Zeller, M. Cottin, Y., and Vergely, C. (2013) Direct and indirect antioxidant properties of a-lipoic acid and therapeutic potential, Mol. Nutr. Food Res., 57, 114–125.
Rochette, L. Ghibu, S. Muresan, A., and Vergely, C. (2015) Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes, Canad. J. Physiol. Pharmacol., 93, 1021–1027.
Maglione, E. Marrese, C. Migliaro, E. Marcuccio, F. Panico, C. Salvati, C. Citro, G. Quercio, M. Roncagliolo, F. Torello, C., and Brufani, M. (2015) Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain, Acta Biol. Med., 86, 226–233.
Traber, M. G., and Atkinson, J. (2007) Vitamin E, antioxidant and nothing more, Free Radic. Biol. Med., 43, 4–15.
Engin, K. N. (2009) Alpha-tocopherol: looking beyond an antioxidant, Mol. Vision, 15, 855–860.
Kwak, J. Y. Takeshige, K. Cheung, B. S., and Minakami, S. (1991) Bilirubin inhibits the activation of superoxideproducing NADPH-oxidase in a neutrophil cell-free system, Biochim. Biophys. Acta, 1076, 369–373.
Abraham, N. G., and Kappas, A. (2008) Pharmacological and clinical aspects of heme oxygenase, Pharmacol. Rev., 60, 79–127.
Chau, L. Y. (2015) Heme oxygenase-1: emerging target of cancer therapy, J. Biomed. Sci., 22, 22.
Xie, R. X. Li, D. W. Liu, X. C. Yang, M. F. Fang, J. Sun, B. L. Zhang, Z. Y., and Yang, X. Y. (2017) Carnosine attenuates brain oxidative stress and apoptosis after intracerebral hemorrhage in rats, Neurochem. Res., 42, 541–551.
Carrol, L. Karton, A. Radom, L. Davies, M. J., and Pattison, D. I. (2019) Carnosine and carcinine derivatives rapidly react with hypochlorous acid to form chloramines and dichloramines, Chem. Res. Toxicol., 32, 513–525.
Sarrami, F. Yu, L. J., and Karton, A. (2017) Computational design of bio-inspired carnosine-based HOBr antioxidants, J. Comput. Aided Mol. Des., 31, 905–913.
Valko, M. Rhodes, C. J. Moncol, J. Izakovic, M., and Mazur, M. (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer, Chem._Biol. Interact., 160, 1–40.
Berltt, B. S., and Stadtman, E. R. (1997) Protein oxidation in aging, disease, and oxidative stress, J. Biol. Chem., 272 20313–20316.
Bubb, K. J. Birgisdottir, A. B. Tang, O. Hansen, T., and Figtree, G. A. (2017) Redox modification of caveolar proteins in the cardiovascular system–role in cellular signaling and disease, Free Radic. Biol. Med., 109, 61–74.
Neavarro-Yepes, J. Burns, M. Anandhan, A. Khalimonchuk, O. del Razo, L. M. Qintanilla-Vega, B. Pappa, A. Panayiotidis, M. I., and Franco, R. (2014) Oxidative stress, redox signaling, and autophagy: cell death versus survival, Antioxid. Redox Signal., 21, 66–85.
Waris, G., and Ahsan, H. (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions, J. Carcinogen., 5, 14.
Dayem, A. A. Choi, H. Y. Kim, J. H., and Cho, S. G. (2010) Role of oxidative stress in stem, cancer, and cancer stem cells, Cancers (Basel), 2, 859–884.
Schmidt, H. H. Stocker, R. Vollbracht, C. Paulsen, G. Riley, D. Daiber, A., and Cuadrado, A. (2015) Antioxidants in translational medicine, Antioxid. Redox Signal., 23, 1130–1143.
Ahmad, K. A. Yuan Yuan, D. Nawaz, W. Ze, H. Zhuo, C. X. Talal, B. Taleb, A. Mais, E., and Qilong, D. (2017) Antioxidant therapy for management of oxidative stress induced hypertension, Free Radic. Res., 51, 428–438.
De Oliveira, M. R. (2015) Vitamin A and retinoids as mitochondrial toxicants, Oxid. Med. Cell. Longev., 2015 140267.
Czapski, G. Holcman, J., and Bielski, B. H. J. (1994) Reactivity of nitric oxide with simple short-lived radicals in aqueous solutions, J. Am. Chem. Soc., 116, 11465–11469.
Panasenko, O. M. Briviba, K., and Klotz, L. O. (1997) Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrate, Arch. Biochem. Biophys., 343, 254–259.
Pattison, D. I., and Davies, M. J. (2001) Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds, Chem. Res. Toxicol., 14, 1453–1464.
Peskin, A. V. Low, F. M. Paton, L. N. Maghzal, G. J. Hampton, M. B., and Winterbourn, C. C. (2007) The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents, J. Biol. Chem., 282, 11885–11892.
Manevich, Y., and Fisher, A. B. (2005) Peroxiredoxin 6, a Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism, Free Radic. Biol. Med., 38 1422–1432.
Furtmuller, P. G. Zederbauer, M. Jantschko, W. Helm, J. Bogner, M. Jakopitsch, C., and Obinger, C. (2006) Active site structure and catalytic mechanisms of human peroxidases, Arch. Biochem. Biophys., 445, 199–213.
Bielski, B. H. J. Arudi, R. L., and Sutherland, M. W. (1983) A study of the reactivity of HO2-./O2– with unsaturated fatty acids, J. Biol. Chem., 258, 4759–4761.
Bielski, B. H. J. (1978) Reevaluation of the spectral and kinetic properties of HO2 -and O2-free radicals, Photochem. Photobiol., 28, 645–649.
Kissner, R. Nauser, T. Bugnon, P. Lye, P. G., and Koppenol, W. H. (1997) Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis, Chem. Res. Toxicol., 10, 1285–1292.
Davies, M. J. (2005) The oxidative environment and protein damage, Biochim. Biophys. Acta, 1703, 93–109.
Repetto, M. G., and Boveris, A. (2012) Transition metals: bioinorganic and redox reactions in biological systems, in Transition Metals: Uses and Characteristics, Nova Science Publishers Inc., NY, USA, pp. 349–370.
Exner, M., and Herold, S. (2000) Kinetic and mechanistic studies of the peroxynitrite-mediated oxidation of oxymyoglobin and oxyhemoglobin, Chem. Res. Toxicol., 13, 287–293.
Madej, E. Folkes, L. K. Wardman, P. Czapski, G., and Goldstein, S. (2008) Thiyl radicals react with nitric oxide to form S-nitrosothiols with rate constants near the diffusioncontrolled limit, Free Radic. Biol. Med., 44, 2013–2018.
Trujillo, M. Ferrer-Sueta, G., and Radi, R. (2008) Kinetic studies on peroxynitrite reduction by peroxiredoxins, Methods Enzymol., 441, 173–196.
Furtmuller, P. G. Jantschko, W. Zederbauer, M. Schwanninger, M. Jakopitsch, C. Herold, S. Koppenol, W. H., and Obinger, C. (2005) Peroxynitrite efficiently mediates the interconversion of redox intermediates of myeloperoxidase, Biochem. Biophys. Res. Commun., 337 944–954.
Quijano, C. Hernandez-Saavedra, D. Castro, L. McCord, J. M. Freeman, B. A., and Radi, R. (2001) Reaction of peroxynitrite with Mn-superoxide dismutase, J. Biol. Chem., 276, 11631–11638.
Funding
Funding The work was supported by I. M. Sechenov First Moscow State Medical University Strategic Development Program under the Russian Academic Excellence 5100 Project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical norms This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Conflict of interest TThe authors declare no conflict of interest in financial or any other area
Russian Text © The Author(s), 2020, published in Uspekhi Biologicheskoi Khimii, 2020, Vol. 60, pp. 123–172.
Rights and permissions
About this article
Cite this article
Moldogazieva, N.T., Mokhosoev, I.M., Mel’nikova, T.I. et al. Dual Character of Reactive Oxygen, Nitrogen, and Halogen Species: Endogenous Sources, Interconversions and Neutralization. Biochemistry Moscow 85 (Suppl 1), 56–78 (2020). https://doi.org/10.1134/S0006297920140047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920140047