Abstract
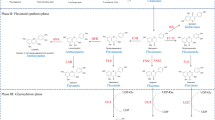
Peroxidases are widespread in animal and plant tissues, wherein they perform a variety of functions. Peroxidases have a broad specificity for substrates of various chemical structures. Along with hydrogen peroxide, phenolic compounds, and toxic compounds of aromatic nature, nitrogen-containing compounds are substrates for peroxidases. This work is devoted to the study of the role of wheat extracellular peroxidases in the metabolism of nitrogen-containing compounds. It has been shown that partially purified isozymes differing in peroxidase activity are involved in the metabolism of nitrogen-containing compounds. The formation of primary and secondary phenoxyl radicals during the combined oxidation of chlorogenic acid, nitrite, and H2O2 was demonstrated. With cooxidation with purified isoenzymes p˗coumaric acid and nitrite, the formation of 4˗hydroxy˗3˗nitrocinnamic acid was revealed. It is assumed that the same isoforms can participate both in the oxidation of nitrite with the formation of nitrophenol and in the reduction of nitrate. The participation of plant peroxidases in nitrogen metabolism can be represented as a set of reactions for the reduction and/or oxidation of nitrogen of different oxidation states with the formation of active intermediates.




Similar content being viewed by others
REFERENCES
Bolwell, G.P., Bindschedler, L.V., Blee, K.A., Butt, V.S., Davies, D.R., Gardner, S.L., Gerrish, C., and Minibayeva, F., The apoplastic oxidative burst in response to biotic stress in plants: a three˗component system, J. Exp. Bot., 2002, vol. 53, p. 1367.
Francoz, E., Ranocha, P., Nguyen-Kim, H., Jamet, E., Burlat, V., and Dunand, C., Roles of cell wall peroxidases in plant development, Phytochemistry, 2015, vol. 112, p. 15. https://doi.org/10.1016/j.phytochem.2014.07.020
Peive, Ya.V., Ivanova, N.N., Ovcharenko, G.A., and Shirinskaya, M.G., The possible participation of peroxidase in the reduction of nitrates in plants, Fiziol. Rast., 1975, vol. 22, p. 527.
Hewitt, E.J., Assimilatory nitrate˗nitrite reduction, Ann. Rev. Plant Physiol., 1975, vol. 26, p. 73.
Sakihama, Y., Tamaki, R., Shimoji, H., Ichiba, T., Fukushi, Y., Tahara, S., and Yamasaki, H., Enzymatic nitration of phytophenolics: evidence for peroxynitrite˗independent nitration of plant secondary metabolites, FEBS Lett., 2003, vol. 553, p. 377. https://doi.org/10.1016/s0014?5793(03)01059?7
Campbell, W.H., Structure and function of eukaryotic NAD(P)H:nitrate reductase, Cell. Mol. Life Sci., 2001, vol. 58, p. 194. https://doi.org/10.1007/PL00000847
van Der Vliet, A., Eiserich, J.P., Halliwell, B., and Cross, C.E., Formation of reactive nitrogen species during peroxidase˗catalyzed oxidation of nitrite: a potential additional mechanism of nitric oxide-dependent toxicity, J. Biol. Chem., 1997, vol. 272, p. 7617. https://doi.org/10.1074/jbc.272.12.7617
Palmerini, C.A., Marmottini, F., and Arienti, G., Production of nitric oxide by human salivary peroxidase and by bovine lactoperoxidase, J. Biochem. Mol. Toxicol., 2012, vol. 26, p. 87. https://doi.org/10.1002/jbt.21407
Adams, L., Franco, M.C., and Estevez, A.G., Reactive nitrogen species in cellular signaling, Exp. Biol. Med., 2015, vol. 240, p. 711. https://doi.org/10.1177/1535370215581314
Corpas, F.J., del Río, L.A., and Palma, J.M., Impact of nitric oxide (NO) on the ROS metabolism of peroxisomes, Plants, 2019, vol. 8, p. 37. https://doi.org/10.3390/plants8020037
Domingos, P., Prado, A.M., Wong, A., Gehring, C., and Feijo, J.A., Nitric oxide: a multitasked signaling gas in plants, Mol. Plant., 2015, vol. 8, p. 506. https://doi.org/10.1016/j.molp.2014.12.010
Santisree, P., Bhatnagar-Mathur, P., and Sharma, K.K., NO to drought˗multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci., 2015, vol. 239, p. 44. https://doi.org/10.1016/j.plantsci.2015.07.012
Abu-Soud, H.M. and Hazen, S.L., Nitric oxide is a physiological substrate for mammalian peroxidases, J. Biol. Chem., 2000, vol. 275, p. 37524. https://doi.org/10.1074/jbc.275.48.37524
Pintus, F., Spanò, D., Bellelli, A., Angelucci, F., Forte, E., Medda, R., and Floris, G., Nitric oxide, substrate of Euphorbia characias peroxidase, switches off the CN˗ inhibitory effect, FEBS Open Biol., 2012, vol. 2, p. 305. https://doi.org/10.1016/j.fob.2012.09.004
Huang, J., Sommers, E.M., Shapiro, D.B.K., and King, S.B., Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea, J. Am. Chem. Soc., 2002, vol. 124, p. 3473. https://doi.org/10.1021/ja012271v
Kim, Y.-H., Park, S.C., Yun, B.-W., and Kwak, S.-S., Overexpressing sweet potato peroxidase gene swpa4 affects nitric oxide production by activating the expression of reactive oxygen species˗ and nitric oxide˗related genes in tobacco, Plant Physiol. Biochem., 2017, vol. 120, p. 52. https://doi.org/10.1016/j.plaphy.2017.09.023
Kong, M., Zhang, Y., Li, Q., Dong, R., and Gao, H.J., Peroxidase˗catalyzed nitration of phenol in a biphasic system, Microbiol. Biotechnol., 2017, vol. 27, p. 297. https://doi.org/10.4014/jmb.1607.07039
Kochetov, G.A., Prakticheskoe rukovodstvo po enzimologii (Practical Manual on Enzymology), Moscow: Vysshaya Shkola, 1980.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding, Anal. Biochem., 1976, vol. 72, p. 248.
Ivanova, N.N. and Peive, Ya.V., Nitrate reduction by higher plant peroxidase, FEBS Lett., 1973, vol. 31, p. 229.
Yamasaki, H. and Grace, S.C., EPR detection of phytophenoxyl radicals stabilized by zinc ions: evidence for the redox coupling of plant phenolics with ascorbate in the H2O2˗peroxidase system, FEBS Lett., 1998, vol. 422, p. 377.
Kalyanaraman, B., Felix, C.C., and Sealy, R.C.S., Electron spin resonance˗spin stabilization of semiquinones produced during oxidation of epinephrine and its analogues, J. Biol. Chem., 1983, vol. 259, p. 354.
Freund, W., A new synthesis of arsonic acids. Part II. Coupling of αβ˗unsaturated carbonyl compounds with diazotised arsanilic acid and 4˗amino˗2˗nitrophenylarsonic acid, J. Chem. Soc., 1952, vol. 588, p. 3072.
Sakihama, Y., Cohen, M.F., Grace, S.C., and Yamasak, H., Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants, Toxicology, 2002, vol. 177, p. 67. https://doi.org/10.1016/s0300?483x
Hushpulian, D.M., Fechina, V.A., Kazakov, S.V., Sakharov, I.Yu., and Gazaryan, I.G., Non-enzymatic interaction of reaction products and substrates in peroxidase catalysis, Biochemistry (Moscow), 2003, vol. 68, p. 1006.
Abello, N., Kerstjens, H.A.M., Postma, D.S., and Bischoff, R., Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine˗nitrated, J. Proteome Res., 2009, vol. 8, p. 3222. https://doi.org/10.1021/pr900039c
Saito, S., Yamamoto-Katou, A., Yoshioka, H., Doke, N., and Kawakita, K., Peroxynitrite generation and tyrosine nitration in defense responses in tobacco BY˗2 cells, Plant Cell Physiol., 2006, vol. 47, p. 689. https://doi.org/10.1093/pcp/pcj038
Minibayeva, F. and Beckett, R.P., The roles of plant peroxidases in the metabolism of reactive nitrogen species and other nitrogenous compounds, in Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants, Signaling and Communication in Plants, Gupta, K.J. and Igamberdiev, A.U., Eds., Cham: Springer-Verlag, 2015, p. 43.
Galeeva, E.I., Trifonova, T.V., Ponomareva, A.A., Viktorova, L.V., and Minibayeva, F.V., Nitrate reductase from Triticum aestivum leaves: regulation of activity and possible role in production of nitric oxide, Biochemistry (Moscow), 2012, vol. 77, p. 404.
Araiso, T. and Dunford, H.B., Horseradish peroxidase. XLI. Complex formation with nitrate and its effect upon compound I formation, Biochem. Biophys. Res. Commun., 1980, vol. 94, p. 1177.
Funding
The work was carried out within the framework of the state assignment of the Federal Research Center Kazan Scientific Center of the Russian Academy of Sciences (analysis of enzyme activity) using the equipment of the Collective Spectro-Analytical Center for Physical and Chemical Research of the Structure, Composition and Properties of Substances and Materials at the Kazan Scientific Center of the Russian Academy of Sciences and partly has been supported by the Kazan Federal University Strategic Academic Leadership Program (obtaining a chemical standard).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Abbreviations: ROS—reactive oxygen species; RNS—reactive nitrogen species; POX—peroxidase; ECS—extracellular solution.
Rights and permissions
About this article
Cite this article
Galeeva, E.I., Viktorova, L.V., Guryanov, O.P. et al. Interaction of Apoplastic Peroxidases from Wheat Roots with Nitrite and Nitrate: Intermediates and Products. Russ J Plant Physiol 69, 8 (2022). https://doi.org/10.1134/S1021443722010046
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722010046