Abstract
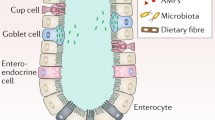
The animal gastrointestinal tract is a tube with two open ends; hence, from the microbial point of view it constitutes an open system, as opposed to the circulatory system that must be a tightly closed microbial-free environment. In particular, the human intestine spans ca. 200 m2 and represents a massive absorptive surface composed of a layer of epithelial cells as well as a paracellular barrier. The permeability of this paracellular barrier is regulated by transmembrane proteins known as claudins that play a critical role in tight junctions.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Lactic Acid Bacterium
- Pathogenic Bacterium
- Volatile Fatty Acid
- Intestinal Microbiota
- Probiotic Bacterium
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Intestinal Bacteria: Saprophytic Versus Pathogenic Organisms. A Historical Perspective
The animal gastrointestinal tract is a tube with two open ends; hence, from the microbial point of view it constitutes an open system, as opposed to the circulatory system that must be a tightly closed microbial-free environment. In particular, the human intestine spans ca. 200 m2 and represents a massive absorptive surface composed of a layer of epithelial cells as well as a paracellular barrier. The permeability of this paracellular barrier is regulated by transmembrane proteins known as claudins that play a critical role in tight junctions (Pruteanu and Shanahan 2013; Barmeyer et al. 2015). Breaches in the integrity of either the epithelial or claudin barriers have profound effect on human health, causing a variety of diseases. The intestine is not only normally full of nutrients, but also at a constant temperature making it an ideal environment for microorganisms to grow. As a result, intestinal invasion by pathogenic bacteria or viruses causes a disease known as “gastroenteritis”, a term believed to have been coined in ca. 1820. Until then this condition was often referred to as “typhoid fever,” although the most common etiological agents were not Salmonella typhi or even S. paratyphi. The first typhoid fever reported (often disseminated by asymptomatic carriers such as in the case of the infamous Mary Mallon, aka “Thyphoid Mary”) caused several disease outbreaks in the New York area from 1900 to 1910, while the second was an outbreak of paratyphoid fever (Bainbridge and Dudfield 1911). Gastroenteritis, nevertheless, can be caused by a variety of viral (norovirus, rotavirus and adenovirus), bacterial (Escherichia coli O157, Salmonella spp., Shigella spp., Campylobacter, or toxins produced by species such as Vibrio cholerae, Staphylococcus aureus, or Clostridium difficile), and parasitic pathogens (such as Giardia lamblia, Cryptosporidium spp., and Entamoeba histolytica). As pointed out by Schottmüller (1904), it is very difficult to assign the etiological agent for what we call “gastroenteritis”: “Bacillus paratyphosus B is capable of giving rise not only to paratyphoid fever, but also to acute gastroenteritis simulating “food-poisoning,” a fact not hitherto observed in this country (United Kingdom). Second, the distribution and dates of onset of the illness of the various cases were unlike those of ordinary “food-poisoning,” and pointed to a human source of infection.” It is currently estimated that there are three to five billion cases of gastroenteritis per year worldwide, and that they cause almost one and a half million deaths, with malnourished children as the population most under threat (Eckardt and Baumgart 2011).
The idea of using bacteria in antibiosis, i.e., to fight other microorganisms, originated with Vuillemin’s work (1890) in the nineteenth century. He coined the term “antibiosis” to epitomize the tremendous fight constantly taking place in the microbial world in the interaction between predator and prey. Pasteur and Joubert (1877) previously described the existence of antibiosis affecting the development of Bacillus anthracis, a disease-producing microorganism which pathogenic effect could be counteracted by “normal bacteria.” Some years later Cantani (1885) published an interesting article demonstrating the elimination of Mycobacterium tuberculosis from the lungs of a seriously infected patient by “insufflating” air containing a “normal nonpathogenic bacterium” By the beginning of the twentieth century, the actinomycetes bacterial group was seen as an important source of active principles that could be used against pathogenic bacteria. It was finally Greig–Smith who, in 1917, demonstrated that these bacteria play a relevant role in the control of other bacterial groups. Whipple et al. (1913) studied the origin of toxins in closed canine duodenal loops and suggested intestinal bacteria as the source of the toxins: “The preceding experiments show that the material which accumulates in a closed duodenal loop is highly toxic when introduced intravenously. This material in the loop may be thin and soup-like or thick and pasty, but in all cases the toxic material can be demonstrated. Only a few cubic centimeters of this material (dogs S-37and 32) may be diluted, incubated at 38 °C under toluol for days, then heated at 60 °C for thirty minutes, and filtered without removing or destroying the toxic substance. Intravenous injection of this broth-like substance will cause an initial drop in blood pressure followed by a rise to normal and a prolonged secondary fall to one half or one third of normal. The heart beat is slowed and may become irregular at this time” (Whipple et al. 1913).
Undoubtedly, intestinal microbiology is a complex matter in both animals and people. The intestinal flora includes hundreds, perhaps thousands, of microorganisms such as bacteria, protozoa, fungi, and viruses; in normal circumstances, these organisms digest complex food materials, synthesize vitamins, and allow the animal to grow and be healthy. Probably, the first person to actually see intestinal bacteria was Anton van Leeuwenhoek; in l675 he examined a variety of samples, including his own diarrheic discharges, and wrote: “With great astonishment I observed everywhere through the material which I was examining animalcules of the most minute size which moved themselves about very energetically” (In Robert Hooke: Collected Memoirs of Anton v. Leeuwenhoek. See also Anton v. Leeuwenhoek: Memoirs to Royal Society of London, 1675 and 1683). If a pathogenic microorganism enters the intestinal tract, it must be eliminated before it produces illness or even death.
The intestinal microbiota found in feces has been a source of problems since the beginning of human settlements, as a continuous source of epidemic outbreaks. Once science became aware of the problems posed by these microorganisms, the different human societies tried to prevent them from contaminating drinking water, or at least to reduce the microbial contamination of drinking water through the development of sewage systems. Although Romans were the sewage pioneers, it was not until modern times that the sewage treatment systems fully developed. Weston and Kendall (1901) were some of the first researchers to systematically study the sewage microbiotas part of their work in the Laboratory of the Sewerage and Water Board of New Orleans (Louisiana, USA), an area of research often ignored by microbiologists. They wrote in their article: “This paper, therefore, is an attempt to bring together scattered data, and to describe and tabulate into convenient form the more common bacteria of American streams.” The following year Kendall (1902) proposed a general bacterial classification that contained most of the currently known bacteria present in sewage and fecal waters. He later reported (1910) the isolation of Bacillus dysenteriae from human stools and its characterization as the agent causing bacterial diarrhea. Kendall (1916) was interested in food as a transmission vehicle for intestinal bacterial pathogens and, while at the Department of Bacteriology in Northwestern University Medical School (USA), he stated: “The public restaurant is a potential factor in the spread of certain types of disease because foods from many sources, manipulated by many hands, are dispensed to many patrons. The occasional dissemination of disease through food is well established; infected shellfish, milk, meat and vegetables have been shown to transmit typhoid bacilli and the viruses of other excrementitious diseases, botulism, and that large and somewhat poorly defined group of gastro-intestinal disturbances commonly classed as food poisoning to susceptible individuals who partake of them.”
Kendall published an interesting article in 1911, while at the Department of Preventive Medicine and Hygiene at Harvard Medical School (USA), on the possible use of bacteria against the establishment of potentially pathogenic intestinal bacteria: “The first logical attempt to modify the intestinal flora along definite lines was that of Metchnikoff. His work is too well known to need reviewing here, but inasmuch as he apparently failed to appreciate the full significance of his treatment, it will be well to go over the salient features in some detail. Metchnikoff was by no means the first to recognize the fact that the intestinal flora responds to dietary changes on the part of the host. Escherich as far back as 1886 noticed that there was a sudden, absolute and relative increase in the numbers of liquefying bacteria in the feces of dogs which were fed upon a purely protein diet” … “In other words, utilizable carbohydrate in artificial media shields protein from bacterial attack, or, expressed in another way, fermentation takes precedence over putrefaction. This hypothesis expresses a fundamental and important feature of bacterial activity. It plays a prominent part in nature, and it can be utilized to advantage in medicine” (our highlighting) … “His idea (Metchnikoff’s) is to combat these proteolytic bacteria (for example clostridia) in their own field of action by the introduction of an antagonistic flora, the lactic acid bacilli . These lactic acid bacilli are said to be inimical to the proteolytic organisms in consequence of the considerable amounts of lactic acid which they generate under suitable conditions. Metchnikoff believes, furthermore, that the lactic acid bacilli themselves are necessary, since his experiments indicate that the mere feeding of lactic acid will not accomplish the same result.” From these old publications it is clear that, even then, microbiologists were already setting the biological base for fighting pathogenic bacteria with “good” (saprophytic) microorganisms. The treatment of diarrheic syndromes in those days was particularly important, due to the lack of antibiotics or antimicrobials. This was expressed very well by Kendall (1911): “The routine treatment for this disease in the past was: starvation for several days until the stools became more normal in appearance, until the temperature dropped, or until it became apparent that the patient could not be permitted to go longer without food.” In that prolific year (1911) Kendall published yet another article on the principles concerning bacterial activity in the intestinal tract, in particular their use in therapeutics, his words constitute a prediction that microbiologists should be aware of: “… the fact remains that medicine is still uninformed concerning many of even the more general principles which underlie the modes of attack and action of these microbes. The most potent factor which underlies the incompleteness of our knowledge is not difficult to determine: bacteriology, “the handmaiden of medicine,” as it has been drolly expressed, besides contributing many of the most brilliant chapters of medicine, enters into so many fields of human activity and interest that it has been neglected as a pure science.”
Regarding the action exerted by saprophytic microorganisms on pathogenic organisms, Sugg and Neill published an article in 1929 on the immunological relationship between one Saccharomyces cerevisiae strain and Type II Streptococcus pneumoniae. In that early publication, these authors showed that horse anti-yeast antibodies caused a potent agglutination of the bacterium. In addition, these antibodies protected mice challenged with the capsulated pathogenic type II bacterial strain to the same extent as rabbit anti-type II S. pneumoniae antibodies. The anti-yeast serum was found to be specific against Type II S. pneumoniae s and, hence, inactive against either type I or type III. This report, as many others in those years, focused on “vaccine” development mainly against secondary bacteria (some of them pathogenic) that invaded the upper respiratory tract in humans after orthomyxovirus infection (as an example see Kneeland 1934); this topic however falls beyond the scope of this chapter.
Many pathogenic bacteria can alter the molecules on their surface during intestinal (or other organ) invasion, thus evading some host defenses. This process can either cause a long lasting disease or establish the host as a disease carrier, and these carriers became continuous microbial sources, capable of infecting other people (Saunders 1990). White described in 1929 three forms of antigenic variation in Salmonella, they included the “H” form (“O” variation described by Weil Felix; Wilson 1920), the smooth form (rough form variation of Arkwright 1920 and 1921) and the specific phase (non-specific variation of Andrewes 1922; see the introduction of Henning 1937) that appeared to be bacterial attempts to evade the host’s defense mechanisms. Concerning the “H” variation, the alternative expression of Salmonella genes H1 and H2, which specify different flagellar antigens (Silverman et al. 1979), results in the oscillation of phenotype known as “phase variation” or “Andrewes’s” variation, by which Salmonella successfully evades the host’s immune system. According to Silverman and co-workers this alternative antigen expression is controlled by the inversion of an 800-base-pair sequence of DNA adjacent to, or including part of, the H2 gene. But this interesting “evasion strategy” is also present in other bacteria. For instance, members of the Neisseriaceae family (i.e., Neisseria gonorrhoeae) express an opacity protein (Op, protein II), a major outer membrane antigen subjected to frequent phase transitions; this again represents a bacterial strategy to evade the host’s immune system (Black et al. 1984; Stern and Meyer 1987). The evasion mechanism in N. gonorhoeae may be reinforced if the bacterium undergoes pilus phase and antigenic variation, in which the pilin gene is turned on and off at quite high frequencies; in fact the pilin gene is expressed by two loci on the gonococcal chromosome (pilE1 and pilE2; see Segal et al. 1985s). Antigenic variation in the genus Shigella (identified by Kiyoshi Shiga in 1897; for a review see Yabuuchi 2002) was finally recognized by Weil and co-workers in 1946, while working with S. paradysenteriae. In fact, the original work was carried out with S. sonnei (or Sonne’s bacillus), a bacterial species isolated from human stools, possibly first isolated in the United States by Duval in 1904. Although the early history of this microorganism has indeed been confusing, as noted by Bojlén (1934): “Certainly no other pathogenic microbe has been ‘discovered’ so many times as Sonne’s bacillus” (see Baker et al. 1949). Wheeler and Mickle concluded in 1945 that the different variants of Sh. sonnei probably represented three culturally and antigenically distinct types of Sh. sonnei, namely Phase I (smooth), Phase II and Rough (Baker et al. 1949). Some Shigella species, such as S. flexneri (with 6 serotypes; Brenner 1976) could go even further to evade the host’s immune system; they modify the O antigen to resemble E. coli strains (Matsumoto 1964). S. boydii can also display antigen variations, but the origin and pathogenicity of this species in the human intestine is really complicated. In 1991 Albert and colleagues suggested an etiological role for Hafnia alvei in human diarrhea and that enteropathogenic E coli strains caused epithelial damage, these microorganisms were later reclassified as Escherichia albertii (Huys et al. 2003). Hyma et al. (2005) found that this species is closely related to strains of S. boydii serotype 13, a distant relative of E. coli representing a divergent lineage in the genus Escherichia. They concluded that the E. albertii-Shigella B13 lineage had split from an E. coli-like ancestor some 28 million years ago, and eventually constituted a novel evolutionary branch of enteric pathogens; as these organisms share antigens with the saprophytic E. coli strains, it can explain why some enteric pathogens are able to override the host’s immune system.
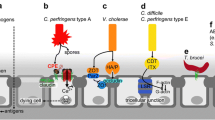
Vibrio cholerae is a non-sporulating Gram-negative enteropathogenic bacterium that moves using a single polar flagellum with a sheath. Filippo Pacini originally discovered this microorganism in 1854, which was again isolated by Robert Koch some 30 years later (see Howard-Jones 1984). V. cholerae O1 and O139 serogroups cause epidemic cholera and, while O1 causes the majority of outbreaks worldwide, O139 appears to be confined to Southeast Asia. V. cholerae O1 has two biotypes, classical and El Tor, and each biotype has two distinct serotypes, Inaba and Ogawa. The genes encoding cholera toxin are part of the genome of CTXϕ, a filamentous bacteriophage with a 6.9 kb ssDNA genome (McLeod et al. 2005). When this phage lysogenizes choleragenic strains of V. cholerae, the phage genome stably integrates into one of the host chromosomes, either chromosome 1 (with 2,961,149 base pairs and 2770 predicted open reading frames) or chromosome 2 (with 1,072,315 base pairs spanning 1115 open reading frames) (Fraser et al. 2000) and continually produces infectious viral progeny without lysing the bacterial cell wall. Viral progeny (including transducing particles) is discharged into the stool of infected people and released into the environment, thus amplifying the dispersion of the toxin genes. During interepidemic periods V. cholerae lives in various aquatic habitats, and recent findings (Joelsson et al. 2006) suggest that quorum sensing mechanisms control V. cholerae pathogenesis, biofilm formation, and protease production. These authors suggest that variations in quorum sensing systems are due to environmental selective pressures and could increase V. cholerae’s fitness in certain environments such as seawater. In contrast to other Vibrio species, V. cholerae does not require sodium chloride for isolation or growth but, according to V.P. Skulachev (Dibrov 2005), it must possess a Na+ cycle that plays a key role in the colonization of the small intestine (Bakeeva et al. 1986) and the cholera toxin-induced [Na+] increase in the intestinal lumen is in fact needed to maintain operative the sodium cycle in the relatively alkaline intestinal environment.
The huge genetic variation in the circulating V. cholerae strains (as an example see Tamplin et al. 1989 on the variation in epitopes of the B subunit of El Tor and classical biotype V. cholerae O1 cholera toxin) requires nearly 200 sera to biotype the bacterial strains. This problem was recently overcome using the simple sequence repeats (SSR), also termed VNTR (for variable number of tandem repeats), as a good and rapid means of bacterial typing (Danin-Poleg et al. 2007). In addition to the O1 and O139 serogroups, the non-O1 and non-139 strains can also cause acute diarrhea and, although they are normally non-toxigenic, some of them are becoming toxigenic (Chatterjee et al. 2009).
Stool transplant, from healthy people, could constitute an alternative, or even a complementary treatment to pseudomembranous colitis caused by C. difficile (Burke and Lamont 2013), but candidate vaccines are important, at least until stool transplant becomes a generalized medical practice. Sanofi Pasteur has developed a novel bivalent candidate vaccine, a formalin-inactivated highly purified preparation of toxoids A and B, that when injected intramuscularly elicits protective antibodies against this bacterium (Foglia et al. 2012).
From the moment that a pathogenic microorganism enters a person’s intestine, a frenzy battle begins, with the body synthesizing and secreting certain immunoglobulins (mostly A type). These abundant peptides with antimicrobial activity, produced by vertebrates (also known as AMPs), include the interferons (Isaacs and Lindenmann 1957) and the active calcium-binding protein (originally described in neutrophils as the L1 myelomonocytic antigen or the cystic fibrosis antigen) present in the cytoplasm of human neutrophils that is released when the neutrophils lyse (Sohnle et al. 1991), as well as the normal bacteria of the gut (including the vast and poorly known group of anaerobic bacteria).
Undoubtedly, an important mechanism regulating the complex relationships between the hundreds of different bacteria present in the animal intestine involves the quorum sensing signals. This quorum sensing depends on the production of one or more diffusible molecules, called “autoinducers” (N-acylhomoserine lactones in Gram-negative bacteria or “polypeptidic pheromones” in the Gram-positive world), which enable a microbial species to be aware of its population density (Hardman et al. 1998). As these authors propose “… Irrespective of the chemical ‘language’ employed, interference with either the synthesis or transmission of a quorum-sensing signal molecule in pathogenic bacteria offers an exciting new strategy for controlling infection…” The present book contains a specific chapter in which this quorum sensing mechanism is described in detail.
Antibiosis is a relationship between microorganisms common in nature, whether it involves prokaryotes suppressing the proliferation of eukaryotic microorganisms or vice versa. It occurs in many quarters, from animal intestines (Andremont et al. 1983) to the soil environment (Ayers and Papavizas 1963). We are still beginning to understand the complex microbial relationships taking part in the intestines of animals. The gut microbiota can even vary with the nature of the food intake, as the plant or animal origin of the ingesta could modify the microbial balance, which, in turn, could either block or facilitate the invasion by bacterial pathogens (Duncan et al. 1998). Even endoparasitic insects, such as the wasp Pimpla turionellae, have been reported to produce an anal hyaline secretion that strongly inhibits pathogenic bacteria and fungi (Willers et al. 1982).
2 Antibiosis in the Animal Intestinal Tract
2.1 Bacteriophage Activity Against Pathogenic Bacteria
This section is devoted to bacteriophage therapy in the intestines of warm-blooded animals, other phage therapies are described elsewhere in this book. The idea of using bacteriophages to fight intestinal pathogenic bacteria originated from the work of the French–Canadian Felix d’Herelle (April 25, 1873—February 22, 1949). This researcher even experimented with the possibility of phage therapy and, during World War I, produced over 12 million doses of medication for the Allied forces. d’Herelle also used phages to successfully treat dysentery, probably representing the first use of bacteriophages as therapeutic agents. This biological approach soon died out in Europe under pressure from chemotherapists; the antibiotic industry mainly followed Paul Ehrlich’s “magic bullet” concept, and the biological approach was forgotten under the powerful “chemical world.” Nevertheless, the idea survived in Russia and other East European countries. In fact, George Eliava founded in 1923 the Eliava Institute in Tbilisi (Georgia) devoted, even currently, to the development of phage therapy. As a result of the cold war, the advances in bacteriophage therapy taking place in Stalin’s Empire remained largely unknown in Western countries. Nevertheless, and despite this lack of communication, the West was also making slow advances in the field of intestinal bacterial infections, as epitomized by Klosterman and Small. In 1928, while studying a variety of stool samples in an attempt to control diphtheria, these authors isolated several bacteriophages from Corynebacterium diphtheriae. Much later Monsur et al. (1970) concluded that treatment of cholera with massive doses of the appropriate bacteriophage, although not as effective as tetracycline treatment, might selectively eliminate the majority of infecting vibrios without affecting the rest of the intestinal flora and without any apparent toxic effect on the patient. Years later, Smith and Huggins (1983) showed the effectiveness of phages B44/1 and B44/2 to control fatal diarrhea (caused by the enteropathogenic strain of E. coli O9:K30) in calves, piglets, and lambs. As it is well known, the most common pathogens associated with diarrhea in developing countries are Vibrio cholera (Chakraborty et al. 2001), the Salmonella/Shigella group, certain strains of enteropathogenic E. coli, and foodborne bacteria such as Campylobacter and Listeria (Mangen et al. 2007). Despite the fact that cholera is one of the oldest and most severe human diarrheal diseases, it is still widespread and little has been done recently on the use of vibriophages to control the disease, particularly in developing countries. In 2009, Bhowmick and co-workers reported the use of a mixture of five V. cholerae O1 biotype El Tor typing phages (ATCC 51352-B1, B2, B3, B4, and B5) as potential tools to control the disease; they achieved some success in a rabbit model of cholera. In 2010 Begum and colleagues described the isolation of one phage (IMM-001, with an isodiametric icosahedral head and long filamentous tail) that displayed a significant specificity toward CS7 fimbriae, with a high potential to control E. coli ETEC strains. More recently, three new V. cholera (O1 El Tor Inaba) DNA bacteriophages have been described, they are present in different water sources and represent good candidates for further bio-phage-control studies (Al-Fendi et al. 2014). In this species, RS1 satellite phage promotes the diversity of toxigenic strains by driving CTX prophage loss, and hence elimination of lysogenic immunity, thus contributing to the emergence of highly pathogenic strains (such as those associated with recent epidemic cholera outbursts in Asia and Haiti; Kamruzzaman et al. 2014). This means that it is now possible to develop effective bacteriophage therapy for cholera prophylaxis.
In the area of bacteriophage therapy against Salmonella, this approach was suggested, in the mid-1960s or early 1970s, to treat antibiotic-resistant recurrent gastroenteritis, as typhoid fever prophylaxis, to treat preschool children, and as a way to prevent secondary cases in typhoid affected areas (Courtieu et al. 1965; Nevskiĭ et al. 1965; Kiknadze et al. 1971, respectively). Years later Slopek et al. (1983) reported that phage therapy could be successfully applied in the treatment of septic infections by several bacteria, including Salmonella and Shigella. More recently Berchieri et al. (1991) reported that bacteriophages isolated from sewage, when concurrently inoculated into newly hatched chickens with any of three strains of S. typhimurium, resulted in a high reduction in bird mortality. Additionally, in 2005 Toro and colleagues demonstrated that the use of bacteriophages, in combination with competitive exclusion, indeed reduced the Salmonella bacterial load in infected chickens.
A serious drawback in bacteriophage therapy is the rapid clearance of the injected organisms from the fluids of warm blooded animals; but in 1996 Merril and co-workers managed to isolate long-circulating mutants of E. coli lambda phage and of S. typhimurium phage P22 that exhibited greater capability as antibacterial agents than the corresponding parental strain. In 2006 O’Flynn carried out a wide fecal screening program and isolated two lytic phages (st104a and st104b), these bacteriophages have the potential to be used in the control of S. enterica in pigs; additionally, st104a can be administered orally, as it is particularly resistant to porcine gastric juice. As is the case in pigs, poultry (mainly chickens) are the main reservoir for human transmission of Salmonella spp. and, although some progress has been made in lowering the Salmonella load by chemical treatment, it still remains a considerable health problem. Atterbury and co-workers made a further contribution to this field, in 2007 they developed a bacteriophage therapy for broiler chickens; out of the 232 bacteriophages tested, they found 3 capable of successfully controlling the cecal load of S. enterica serotypes (enteritidis, typhimurium, and hadar). Sillankorva and colleagues in 2010 investigated the task of controlling S. enteritidis in poultry, reaching the general conclusion that optimal host and growth conditions must be carefully studied and selected for the production of specific bacteriophages for animal therapy. The question of the amount of bacteriophage required to control the pathogenic Salmonella species is elusive, but there appears to be agreement in the utility of bacteriophages to biocontrol pathogens present in low numbers, given that a sufficiently high concentration of phages is used, and it appears that it is not even necessary to ascertain the concentration of pathogens (Bigwood et al. 2009). In addition, frequent treatment of the animals with bacteriophages, especially prior to colonization of the intestinal tract by Salmonella sp., is required to achieve effective bacterial reduction over time (Bardina et al. 2012). Problems such as (i) phages inducing neutralizing antibodies, (ii) phages being active only when administered shortly after bacterial infection, and (iii) the rapid emergence of phage-resistant bacteria during the course of therapy (Capparelli et al. 2010) have to be properly addressed before bacteriophage therapy can become of general use. Therefore, as the authors demonstrated, phage-resistant bacteria indeed constitute excellent vaccines, protecting against lethal doses of heterologous S. enterica serovars. The problem of the appearance of phage-resistant bacterial strains can be aggravated by the phase-variable glycosylation phenomenon present in the O-antigen of Salmonella, as reported by Kim and Ryu in 2012. Kang et al. (2013) described the isolation of a novel DNA-containing bacteriophage (wksl3, relative of SEPT3 phage) belonging to the Siphoviridae family that does not encode any phage lysogeny factors, toxins, pathogen-related genes, or foodborne allergens, but capable of controlling the growth of Salmonella enterica (serovars enteritidis) and typhimurium in food.
Waseh et al. (2010) described an alternative to the use of whole bacteriophages, they found that P22 phage tail spikes are sufficient to elicit a specific Salmonella agglutination response in the animal, thus mimicking antibody agglutination and facilitating bacterial elimination through intestinal movements, and perhaps more importantly inhibiting bacterial intestinal translocation. Another approach, reported by Oliveira et al. in 2014, involves the direct use of bacteriophage endolysins with a modicum of thermal stability, as well as resistance to gastrointestinal pHs. These endolysins are very active in Gram-positive bacteria, but not in Gram-negative due to the outer membrane present in these bacteria that is only permeable to bacteriophage endolysins at low pH.
Although currently bacteriophages cannot be used in humans or farm animals, until appropriate protocols are developed, they could be used to control the horizontal transmission of pathogenic Salmonella species from infected to noninfected animals (Lim et al. 2011). There are some bacteriophages that have lost their specificity for infecting particular bacterial species and display polyvalent activity on a variety of bacterial genera. This is the case for phage phiKP26 (reported by Amarillas and co-workers in 2013), proposed as a putative biocontrol agent for both Salmonella and E. coli. However, one should bear in mind that polyvalent bacteriophage activity could result in undesirable side effects, by destroying saprophytic bacteria. While bacteriophage therapy is being profusely used in the food industry, to reduce the effect of food born pathogenic Salmonella species, as well as in the poultry (to treat laying hens) and pig industries, its application in human health is still rare.
Bacteriophage therapy against Shigella dysenteriae is also mainly lacking, although the description of new viruses for this species (such as bacteriophage WZ1 isolated from waste water in 2015; Jamal et al. 2015) could pave the way for the use of this kind of complementary therapy in the treatment of bacillary dysentery. As it is the case for enteropathogenic Salmonella species, the use of bacteriophage therapy against enteropathogenic E. coli strains is currently restricted to farm animals, such as calves, piglets, and lambs (Smith and Huggins 1983; Smith et al. 1987), or to the treatment of milk and meat products (Tomat et al. 2013a, b). Reports on bacteriophage therapy against Campylobacter are even sparser. To the best of our knowledge, no human has ever been treated with Campylobacter jejuni phages, although this treatment is successful in broiler chickens, resulting in a drastic reduction in bacterial load (Loc Carrillo et al. 2005). This is also the case for Listeria monocytogenes; the fight against this bacterial pathogen is currently being experimentally applied in food science (Anany et al. 2011), but it has never been employed in human therapy. This situation may change once Romulus and Remus (two phages belonging to the Twortlikevirus genus described in 2013) are fully understood (Vandersteegen et al. 2013).
2.2 Bacterial Activity Against Intestinal Pathogenic Bacteria
This issue has always been elusive, even to the most seasoned microbiologists, due not only to the number of bacterial and fungal species involved, but also to bacteriophages and other viruses (Velasco et al. 1984). Even long-term confinement generates changes in the human microbiota, as reported by Shilov et al. (1971). The authors showed that the intestinal microbiota of astronauts that spent over 1 year of isolation in space underwent drastic changes; these involved a severe reduction in the number of aerobic bacterial species (less than 6 %), whereas anaerobic bacteria increased to almost 90 %. Gut microbiota is, therefore, a very complex microbial community in unstable equilibrium. When this equilibrium is broken, the body suffers from disorders that range from diseases caused by microbial pathogens to vitamin or essential amino acid deficiencies. Human intestines contain ca. 100 trillion microorganisms, about 10-fold the number of human cells present in the body (Guarner and Malagelada 2003), representing between 300 and 1000 different species (Sears 2005), although it is probable that 99 % of the bacteria belong to only 30 or 40 species (Beaugerie and Petit 2004).
Microbial antagonisms have long been observed in the intestinal tracts of animals, including humans, and can be due to a defined mixture of strains fighting colonization by a species either belonging to the same (Duval-Iflah et al. 1981) or a different genus (Ducluzeau et al. 1977), as well as to complex endogenous microflora against pathogenic microorganisms (Wilson et al. 1981). It is often proposed that intestinal anaerobic bacteria control the growth of members the Enterobacteriaceae family, through the production of volatile fatty acids and colicins, as well as by modification of bile acids and competition gut nutrients (Andremont et al. 1985). Borriello and Barclay (1986) studied the role of volatile fatty acids in preventing the establishment of C. difficile and found that this inhibition could not be linked to specific volatile fatty acids or enzymes. In addition, as the number of bacterial strains harboring antibiotic resistance plasmids is increasing steadily, the use of antibiotics can affect these microbial relationships and cause unexpected end results (Andremont et al. 1983, 1985).
The genus Lactobacillus has been long recognized as an efficient tool for controlling other intestinal bacteria, including enteropathogens. Watanabe et al. (1977) investigated the effects of three indigenous Lactobacillus groups (group I, including L. acidophilus and related strains; group II, represented by L. fermentum; and group III, consisting of L. murine and associated strains) on other bacterial populations in gnotobiotic rats. The authors found that the indigenous bacteria present in the wall of the nonglandular part of the stomach (including the stomach and upper part of the small intestine) were controlled by groups I and II, but not by III; this implies that the bacterial-controlling activity was linked to particular species (and not to a whole genus) and even, most likely, linked to particular bacterial strains. In addition, the species belonging to the Lactobacillus genus were reported to exhibit anticarcinogenic and hypocholesterolemic effects (Mital and Garg 1995). Other lactic acid bacteria, belonging to the Pedicoccus and Lactococcus genera, have also been reported to play antagonistic roles that control intestinal colonization by human enteropathogens in live poultry (Juven et al. 1991). In fact, Lactobacillus casei was shown to display in vivo and in vitro antagonistic activity against Salmonella typhimurium infections (Hudault et al. 1997). Coconnier et al. (1993) reported that even heat-killed human L. acidophilus inhibits the pathogenicity caused by diarrheagenic bacteria in cultured human intestinal cells. Blomberg and colleagues in 1993 studied the inhibition of E. coli K88 adhesion to piglet ileal mucus caused by Lactobacillus spp. strains, and concluded that L. fermentum 104R produced a soluble proteinaceous component (molecular mass above 250kD, as determined by gel filtration) that inhibited the adhesion of K88ab and K88ac fimbriae to ileal mucus by interacting with the mucus components. Jin and colleagues further confirmed this in 1996; they studied the antagonistic effects of intestinal Lactobacillus isolates on bacterial chicken pathogens. Their results showed that all of the 12 Lactobacillus tested could inhibit the growth of S. enteritidis 935/79, S. pullorum, S. typhimurium, S. blockley, and S. enteritidis 94/448, as well as that of three E.coli serotypes (O1:K1, O2:K1 and O78:K80).
More recently, Cleusix and co-workers reported that reuterin effectively controls the enteric microbiota. This compound is produced by Lactobacillus reuteri and present as 3-hydroxypropionaldehyde, its hydrate, or its dimer; it displays a broad-spectrum activity against enteropathogens, yeasts, fungi, protozoa, and viruses (Cleusix et al. 2007). However, Fetissov et al. suggested (2008) that the intestinal presence of a high number of Lactobacillus, or other probiotics, could produce oligopeptides resembling appetite-regulating peptide hormones, and alter the normal appetite/satiety equilibrium by generating autoantibodies.
In a classic paper, Barnes et al. (1979) reported several factors that influence the incidence and anti-Salmonella activity of the anaerobic caecal flora in young chicks (Bacteroides hypermegas and a Bifidobacterium sp.); these factor are mainly acidic conditions and the production of volatile fatty acids. Modulation of the bacterial microbiota can be achieved by selective elimination of the aerobic bacteria in the oropharyngeal cavity and intestinal tract, leaving the anaerobic microbiota intact to a large extent (van Furth and Guiot 1989); this treatment prevented colonization by resistant, but potentially pathogenic, bacteria or fungi, even in patients exhibiting severe granulocytopenia episodes. Hillman and co-workers in 1994 carried out in vitro experiments that demonstrated that the resident microflora of the porcine ileum (containing a balanced load of anaerobic and aerobic bacteria) actually inhibited the penetration of enterotoxigenic E. coli strains.
Indeed, dysbacteriosis, a series of illnesses that occur in the early postnatal period, can be avoided by simply instilling Lactobacillus acidophilus (with anti-klebsiella and anti S. aureus activity) into the mouth and nasal passages of neonates. Following this treatment, the babies were discharged from the maternity ward with a normal intestinal microflora (Moshchich et al. 1989).
Kieckens et al. (2015) showed that enterohemorrhagic Escherichia coli (EHEC) strains (of which E. coli O157:H7 is by far the best-studied serotype, as it constitutes an important foodborne pathogen worldwide) could be easily controlled by rectal administration of bovine lactoferrin in cattle. This pathogenic E. coli strain can live for long periods of time in the intestine of affected animals without showing any clinical symptoms. The same authors concluded that this abiotic way of fighting pathogenic bacteria by rectal treatment could represent a useful strategy to preclude transmission of EHEC infections from cattle to humans, which currently represents the most common way of transmission. These pathological serotypes of an otherwise commensal species, together with the labile toxin producing enterotoxic E. coli (ETEC), are the most common pathogens isolated from diarrheal stools of hospitalized children and adults, closely followed by Salmonella spp (Mendis et al. 1995).
Weinack and co-workers in 1982 reported a reciprocal competitive exclusion between either Salmonella thyphimurium or pathogenic E. coli strains and the native intestinal microflora of chickens and turkeys, with the result that the native intestinal microflora of both birds were protected against the pathogenic species. Hence, the chicken and turkey microflora appeared to be equally effective in protecting the two species from S. typhimurium, but protection against E. coli was somewhat greater in the chicken than in the turkey. This appears to define a pattern of microbial protection against pathogenic microbiota somewhat based on the evolutionary relationships between saprophytic and pathogenic microorganisms. In this sense, Ducluzeau and Bensaada reported in 1982 that Saccharomyces boulardii, when given to monoxenic mice, was active against Candida albicans, C. krusei and C. pseudotropicalis strains, but unfortunately ineffective against C. tropicalis. Interestingly, the antagonistic effect totally disappeared when S. boulardii cells were heat-killed. Rodrigues et al. (1996) investigated the effect of the yeast S. boulardii on oral infection of gnotobiotic mice with S. typhimurium and Shigella flexneri. They found that the yeast rapidly colonized the intestines of the germ-free animals, and this protected them from diarrheal disease when challenged with a high numbers of either pathogen.
A series of in vitro studies confirm the role played by the “normal” gut microbiota to control the growth of pathogenic bacteria that use the gastrointestinal tract as the main point of entry. Accordingly, Ushijima and Ozaki reported in 1986 the antagonism of E. coli, Bacteroides ovatus, Fusobacterium varium, and Enterococcus faecalis, either alone or together, against enteropathogens (i.e., Yersinia enterocolitica, Shigella flexneri, Salmonella typhimurium, Vibrio parahaemolyticus, V. cholerae serogroup non O1, S. aureus, and Clostridium perfringens). They found that, in anaerobic continuous flow cultures, it only took mixed cultures of the four resident bacteria a few days to eliminate Y. enterocolitica. On the other hand, E. coli alone was sufficient to eradicate Sh. flexneri, and E. coli together with B. ovatus could eliminate S. aureus, C. perfringens, V. parahaemolyticus, and V. cholerae serogroup non-O1. In addition, S. typhimurium was the species most resistant to elimination, and, depending on the nitrogen source available, C. perfringens (itself an anaerobic microorganisms) could even resist the action of the four resident bacteria together. It was Gorbach and co-workers who, in 1988, suggested that intestinal anaerobic bacteria represented an actual barrier against enteropathogens. These authors tested the intestinal microflora resistance to colonization, in human volunteers, and found that this resistance not only occurs but it is diminished by antibiotic administration, but is not dependent on the anaerobic microbiota. C. perfringens intestinal colonization has been extensively investigated in cesarean-delivered newborns, from birth to the two first weeks of life. Bezirtzoglou et al. (1989) found that breastfeeding directly modulates the numbers of C. perfringens present in the neonate’s gut, and that Bifidobacterium bifidum indeed plays a role in the control of C. perfringens. Interestingly, it was also found that saprophytic species could eradicate pathogenic microorganisms. In this regard, Kuroiwa and co-workers reported in 1990 that C. butyricum M588 exerted a preventive effect against the proliferation of C. difficile during antimicrobial therapy. Bernet and co-workers in 1993 investigated the role of bifidobacterial adhesion to cultured human intestinal epithelial cells on the inhibition of enteropathogen–cell interactions; they found that both B. breve and B. infantis were able to inhibit epithelial cell association of either enterotoxigenic or enteropathogenic E. coli, Yersinia pseudotuberculosis and S. typhimurium, in a concentration-dependent manner. Indeed, Tazume et al. reported in 1993 that, in the “abnormal” intestinal flora of patients with severe diarrhea, there is a close correlation between a decrease in the number of anaerobes and a reduction in the level of short-chain fatty acids and free bile acids; this, in turn, causes an increase in pH and water accumulation in the intestine that may facilitate enteric infections.
In newborn babies, the intestinal microbiota is the result of a specific selection process influenced by many factors (Ducluzeau 1993). In breast-fed infants, E. coli and streptococci are the first bacteria to colonize the gut, usually followed by Bifidobacterium species, which soon constitute the main microbiota. On the other hand, the gut of bottle-fed infants has a bigger bacterial variety, including other enterobacteria as well as different anaerobes. Breast milk is known to contain some “bifidus factors” that promote the growth of Bifidobacterium, as well as providing immunoglobulins, which prevent intestinal colonization by pathogenic enterobacteria.
After weaning, as the variety of the infant’s diet considerably increases, the gastrointestinal tract develops to harbor an enormous number of both aerobic and anaerobic bacteria, which exhibit a quasi-symbiotic relationship with the host. Regulation of the intestinal microbiota depends on a very complex network of interactions and factors that include immunoglobulins, gastric acid secretion and bacterial adherence to intestinal cells (Batt et al. 1996), which can exert either beneficial or detrimental effects on the host. The beneficial effects of the so-called “normal enteric microbiota” include the competitive exclusion of potentially pathogenic organisms, as well as the production of short-chain fatty acids and vitamins. The detrimental effects encompass competition for calories and essential nutrients, contribution to inflammatory bowel disease and colonization by transient pathogens that could, in turn, interfere with the mucosal barrier; the latter could give rise to bacterial translocation and cause bacteremia or even septicemia. Jackson et al. (1990) described that this bacterial translocation across intestinal walls can involve both Gram-negative and Gram-positive microorganisms.
It is well documented that intestinal bacteria can translocate from the intestinal tract to several parts of the body and cause serious illness or even death (Wells 1990). Only a few aerobic/facultative species appear to have the ability to translocate, and it was originally proposed that anaerobic bacteria prevented such translocation (van der Waaij et al. 1971). Although the mechanisms controlling bacterial translocation remain unclear, they are known to involve both microbial and host factors. Strictly anaerobic bacteria do not appear to translocate in healthy hosts; but other organisms, such as L. monocytogenes or Salmonella species, can either enter macrophages and reach the Peyer’s patches or distal organs or use host phagocytes to reach the draining mesenteric lymph node (Wells 1990). In some cases, the presence of fatal intestinal injuries, intense burns, or acute mesenteric ischemia can facilitate the translocation of both anaerobic and facultative anaerobic microorganisms.
Crohn’s disease constitutes another example of a detrimental health effect involving intestinal microbes. Although other factors cannot be excluded for this disease, such as chronic infection with a specific persistent pathogen (Balfour Sartor 2007), or an overly aggressive immune response to normal commensal enteric bacteria, as well as host genetic susceptibility resulting in defective mucosal barrier function or lack of bacterial killing ability. All these aspects, and probably others as yet not described, lead to an overly aggressive T-cell response to normal bacteria that finally causes the tissue damage characteristic of Crohn’s disease.
Colonic anaerobes, such as Bacteroides fragilis or Peptostreptococcus species, rarely cause infections as solitary pathogens; but, when accompanied by aerobic bacteria or in environments with an abundance of nitrogen source (dead tissue), they can cause quite severe infections in areas including the abdominal cavity. These mixed infections of aerobes and anaerobes must be treated by surgical drainage, in combination with antibiotic therapy (Fry and Schermer 2000). However, in general, anaerobic bacteria appear to play a key role in confining indigenous bacteria to the gut (Wells et al. 1987). Despite the fact that intestinal anaerobes include pathogenic species, they usually represent microorganisms beneficial to humans, since they are instrumental in restraining the growth of C. difficile in human carriers, as well as providing catabolic enzymes that allow digestion of organic compounds, which cannot be otherwise digested by eukaryotic enzymes. In addition, these microorganisms are essential for the catabolism of cholesterol, bile acids and steroid hormones, as well as for detoxification of certain carcinogens (Bokkenheuser 1993). These beneficial effects have also been reported by Shimizu and coworkers, who in 2006 noted altered gut microbiota in patients affected by severe systemic inflammatory response syndrome (i.e., significantly lower total anaerobic bacterial counts and two log higher “pathogenic” Staphylococcus and Pseudomonas bacterial counts than in healthy people).
Clostridium perfringens is another ubiquitous Gram-positive bacterium reported to include two types of strains, the necrotic enteritis-producing strains and the non-necrotic enteritis strains. Barbara et al. (2008) demonstrated that, at least in chicken intestines, the necrotic strains naturally displace the non-necrotic varieties. In addition to producing toxins, the bacterium proceeds to the digestion of epithelial tight junction proteins, thus contributing to bacterial translocation across the intestinal barrier and producing the necrotizing syndrome (Pruteanu and Shanahan 2013). This microorganism also produces many enzymes, including sialidases, which contribute to bacterial dispersion (Li and McClane 2014). One of the first, if not the first, defense mechanism in humans is linked to the mother’s colostrum, that normally exhibits high titers of specific secretory IgAs against C. perfringens (Liem et al. 1979); but after weaning, the child’s defense must rely on its own ability to produce those antibodies. As indicated above, the use of gnotobiotic animals has provided important insights into different research areas. These animals’ response to different bacterial pathogen challenges has been important to elucidate, on one hand, the pathogenic action of a given pathogen and, on the other hand, the protection provided by a particular saprophyte or “normal” bacterial flora (Adremont et al. 1983). Consequently, Yurdusev and colleages reported in 1987 that gnotobiotic mice harboring a Bacteroides thetaiotaomicron strain, a Fusobacterium necrogenes strain and a Clostridium sp. strain were protected against challenge by pathogenic C. perfringens B, C and D serotypes, although the authors were unable to isolate any diffusible substance. The same authors 2 years later (Yurdusev et al. 1989) confirmed again the antagonism exerted by B. thetaiotaomicron, in association with F. necrogenes, against C. perfringens, both in vivo (in gnotobiotic mice) and in vitro (in fecal suspensions). Ramare and colleagues published an interesting paper in 1993, demonstrating the trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats; this represented the first report of a potent antibacterial substances produced through a mechanism involving both intestinal bacteria and exocrine pancreatic secretions.
The in vivo role of intestinal bacteriocins as protective substances against pathogenic bacteria has not been clear, due to their narrow activity and their sensitivity to proteolytic degradation. Jennes et al. (2000) studied the intestinal bacteria in ostriches and found that Enterococcus gallinarum 012 synthesized a polypeptide (enterocin 012) active against E. faecalis, Lactobacillus acidophilus, L. sake, Listeria innocua, Propionibacterium acidipropionici, C. perfringens, Pseudomonas aeruginosa, and Salmonella typhimurium; but enterocin 012 was ineffective against Bacillus cereus, C. sporogenes, C. tyrobutyricum, Leuconostoc cremoris, Pediococcus pentosaceus, Staphylococcus carnosus, and Streptococcus thermophilus. Finally, Crost and colleagues produced an interesting publication in 2011 that constituted the first report confirming the in vivo protective action of the Ruminococcus gnavus E1 bacteriocin; the compound, originally isolated from human stools, exhibited a clear protective role against intestinal colonization (and hence intestinal necrosis) by C. perfringens. Activation of the bacteriocin is trypsin dependent and its activity is comparable to the protective effect exerted by metronidazole. Namkung and colleagues demonstrated in 2011 that the protective role of bacteriocins against this anaerobic pathogen (in particularly the strains producing intestinal necrosis) must be studied in combination with the intestinal production of n-butyric acid by other intestinal anaerobic bacteria. In addition, the inclusion of certain plants in the human diet could positively regulate the intestinal necrosis syndrome produced by this bacterium. This is the case for Artemisia annua, as its leaves can control necrotic enteritis in broiler chickens and compensate, to a certain extent, for the weight loss caused by the disease (Engberg et al. 2012).
The epsilon- (ε-) proteobacteria include one of the five classes in the Proteobacteria phylum (Vandamme et al. 1991) that can inhabit the gastrointestinal tracts of animals, as well as inhabiting water reservoirs or sewages, and cause serious illness (Bereswill and Kist 2003). Much of the interest in this bacterial group arises from the fact that some of its genera, such as Campylobacter, Helicobacter, or Wollinella, constitute human pathogens. While Helicobacter pylori is the causative agent of gastric and peptic ulcers (Ghose et al. 2005), and H. hepaticus could contribute to liver or gastric cancers (van Amsterdam et al. 2006), Campylobacter is usually a human pathogen; this Gram-negative microaerophilic bacteria is oxidase-positive and most of its strains are motile, with one or two polar flagella (Vandamme et al. 2006). Despite the undefined taxonomical situation of the bacterial genus, Holländer managed (1984) to characterize the main Campylobacter groups isolated from human stools. C. coli and C. jejuni are two very closely related species (sharing 28 proteins; Gupta 2006) and together represent the main source of bacterial foodborne disease in many developed countries (Ackerley and Jones 1985); in some cases, infection by these bacteria can cause sepsis or meningitis (Pequignot et al. 1973; Gubina et al. 1976). Escherich, in 1886, originally described the symptoms associated with “campilobacteriosis” (Kramer and Kanof 1960), but the genus itself was not described until 1963 (Debruyne et al. 2008). Hence, the microbiological history of this genus is short, in addition until 1971 Campylobacter was considered a vibrional species (Morris and Park 1971). The Campylobacter flagella (unusually composed of two types of flagellin) are involved not only in intestinal adherence, but also in translocation and cell internalization, thus playing an important role in bacterial virulence (Grant et al. 1993). Expression of flagella in Campylobacter spp. is subject to both phase and antigenic variations; in such a way that some strains can switch between flagellated and non-flagellated forms and other strains can even reversibly express flagella with different antigenic specificities (Caldwell et al. 1985; Alm et al. 1992). It appears that the central, surface-exposed region of the flagellar hook protein FlgE in C. jejuni, in fact displays hypervariability among strains (Lüneberg et al. 1998); this flagellar antigen variation is part of the defense mechanism of these pathogenic bacteria in the human intestine. Flagellar variations, however, could just represent one of the multiple variations displayed by epsilon bacteria, which include DNA uptake, DNA recombination, adhesion, and iron uptake; this is in contrast with more classical variations, such as those displayed by E. coli (Gilbreath et al. 2011). As it is the case for other mucosal pathogens (i.e., Neisseria or Haemophilus), Campylobacter lacks the O-polysaccharide repeating units in their outer core glycans, although they display structural diversity (Moran et al. 1996). These glycans are low-molecular weight lipopolysaccharide variants, known as lipooligosaccharides, which also play a role in evading the host’s defense mechanisms. Sugar N-formyltransferase is an important enzyme in C. jejuni that generates the LPS variations characterized by Thoden and co-workers in 2013.
Clostridium difficile is another bacterium that merits to be cited here, because of its ability to colonize the human large intestine and cause pseudomembranous colitis, a condition very hard to treat (Smith and King 1962; Larson et al. 1978). The bacterium was initially named Bacillus difficilis by Hall and O’Toole in 1935, because they found it difficult to isolate, but was later reclassified within the Clostridium genus as the soil constitutes its main habitat (for a comprehensive review of the phylogenetic status of this genus see Elsayed and Zhang 2004). While in the large intestine, pathogenic strains of C. difficile produce multiple toxins, such as enterotoxin (toxin A) and cytotoxin (toxin B). Both compounds are glucosyltransferases that target and inactivate the Rho family of GTPases, and this can produce diarrhea and inflammation of the large intestine, although the relative contributions of each of the toxins is not yet clear (Lyerly et al. 1986). In addition, toxin B induces actin depolymerization, by a mechanism that correlates with a decrease in the ADP-ribosylation of the low molecular mass GTP-binding Rho proteins (Just et al. 1995). Elimination of this bacterium from the large intestine is difficult, and is probably related to the unusual tetragonal structure exhibited by the outermost layer of the bacterial cell wall, formed by two proteins (Masuda et al. 1989). To the best of our knowledge, Wada et al. (1980) were the first to demonstrate, in the supernatants of cultured human colostral cells, a neutralizing activity against C. difficile toxin; this activity was mainly due to IgAs secreted by the macrophages found in the human colostrum. Neutralizing IgAs can also be found in stools of individuals infected with pathogenic C. difficile strains. In fact, Kyne and colleagues reported in 2000 that antibody titers against toxin A were higher in patients with mild C. difficile–associated disease than in individuals with prolonged or severe diarrhea.
Another clostridial species worth mentioning here is C. perfringens. This bacterium, formerly known as C. welchii, or even Bacillus welchii, is a Gram-positive anaerobe, ubiquitously distributed (including in human intestines), which can cause serious illness due to the production of several toxins, including an enterotoxin (synthesized in vivo during sporulation; Narayan 1982). Enterotoxin damages epithelial cells by binding to claudin family members, including claudin 3, 4, 6, 7, 8, and 14, but not 1, 2, 5, and 10 (van Itallie et al. 2008). Some circulating C. perfringens strains (such as that reported by Tilton and co-workers in 1981, isolated from the intestine of a dog which died from a parvoviral infection) secrete toxins similar to those produced by C. difficile. Since C. perfringens spores can survive the temperatures used for cooking food, this bacterium is a common cause of foodborne worldwide infections, producing a rarely fatal infection (necrotizing enteritis, known as pigbel). The pathogen can also contaminate surgical facilities, leading to postoperative infections (Parker 1969). Although rare, serious infections can cause gangrene of the entire large intestine during the seventh month of pregnancy (Jirán 1971).
C. welchii was originally described by Welch, who isolated it from the body of a 38-year-old male, in 1891 and 1892. He described the microorganism as Bacillus aerogenes capsulatus, and later on it was renamed C. welchii (Euzéby 1997).
The role of indigenous resident “normal” bacteria in the prevention of the “‘sudden infant death syndrome” is progressively gaining acceptance; as indicated above, bacterial colonization of human infant colon is influenced by many factors, including age and antibiotic exposure. As the intestinal microbiome is known to influence the development of the immune system, it must play an important role in protecting infants from the bacteria and/or their toxins involved in the pathogenesis of the “sudden infant death syndrome” (Highet et al. 2014). These authors analyzed the intestinal bacteria in infants affected by this syndrome, and compared it to the flora found in infants not suffering from the syndrome; they concluded that C. difficile and C. innocuum were significantly associated with the syndrome’s development.
It is generally accepted that probiotics exert beneficial effects in the gastrointestinal tract of warm blood animals, but Mangell and co-workers reported in 2012 that, at least in the case of Lactobacillus plantarum 299v, once the bacteria was established in the intestine of animals it had no effect on either enteric bacteria or bacterial translocation. The results were not consistent with others in the literature, such as the study by Cao et al. (2012) who reported the control of C. perfringes-induced necrosis enteritis by Lactobacillus fermentum 1.2029). In conclusion, the mechanisms behind the protective effects of probiotics in animals and humans, as well as the effects of bacteriocins, remain largely unknown and further research is required to ascertaining their utility in the fight against intestinal bacterial pathogens. Hence, it remains imperative to continue the research into antimicrobials as well as extend the available arsenal with new additions such as the semisynthetic antimicrobial thiopeptide LFF571 (Fig. 1). LFF571 was described as a translation inhibitor that binds bacterial elongation factor Tu (EF-Tu) and blocks the delivery of aminoacyl-tRNA (aa-tRNA) to the ribosome (Parmeggiani et al. 2006). In addition, essential oils, such as nerolidol, thymol, eugenol, and geraniol, could be exogenously added to the human diet, since they appear to effectively control the population of intestinal pathogenic bacteria, such as E. coli O157:H7, C. difficile DSM1296, C. perfringens DSM11780, S typhimurium 3530, and Salmonella enteritidis S1400 (Thapa et al. 2012).
Chemical structure of LFF571. LFF571, a semisynthetic thiopeptide (see Selva et al. 1991)
Gastroesophageal reflux disease is a common condition that, according to Del Piano et al. (2012), affects 175 million people just in Europe. The disease is currently treated with proton pump inhibitors, such as omeprazole, that increase the pH of the gastric fluids but can create unwanted side effects, such as proliferation of potentially pathogenic bacteria. These authors demonstrated that L. rhamnosus LR06, L. pentosus LPS01, L. plantarum LP01, and L. delbrueckii subsp. delbrueckii LDD01 successfully restored the “gastric barrier effect” in patients chronically treated with proton pump inhibitors. Microbiologists from all over the world are constantly striving to find new strains of Lactobacillus species, with the potential constitute better probiotics to be used in the fight against pathogenic bacteria. One such example is the novel JSA22 strain of Lactobacillus plantarum, isolated from traditional fermented soybean, active against S. enterica serovar typhimurium (Eom et al. 2015)
2.3 Bacterial Activity Against Protozoa and Other Intestinal Parasites
Only a few specific examples of the protection exerted by the “normal” microbiota against protozoan infections have been documented. As described in the previous section, it appears that species belonging to Lactobacillus genus can protect the human gastrointestinal area not only from pathogenic bacteria but also protozoa. Alak et al. (1997) reported that L. reuteri could induce intestinal resistance to Cryptosporidium parvum infection in mice suffering from immunodeficiency syndrome. It is not clear if this report is, in any way, related to Langer and Riggs’ findings (1999), concerning the apical complex glycoprotein CSL that contains a sporozoite ligand for intestinal epithelial cells. L. casei has also been reported to have anti Trichinella spiralis activity (possibly involving gamma interferon activity); Bautista-Garfias et al. (1999) demonstrated that treatment of mice with L. casei resulted in the elimination of adult worms from their intestines. Similarly, Singer and Nash (2000) reported that the “normal” flora in mice intestines protected them against G. lamblia infections. G. lamblia is a flagellated protozoan that causes watery diarrhea worldwide and can be treated in a variety of ways, including with plant extracts, as reported by Rahimi-Esboei and co-workers in 2013. The host defense against Giardia infection involves several different immunological and non-immunological mucosal processes (Roxström-Lindquist et al. 2006), including the production of cryptdins (small intestinal defensins produced by the Paneth cells; Aley et al. 1994). Immunological responses include the production of secretory IgAs both in milk and saliva (59 and 52 %, respectively), although the antibody titer in milk was 50 times higher than in saliva. The antibodies generated targeted the trophozoite membrane, flagella and cytoplasmic antigens (Téllez et al. 2005); this aspect, however, falls outside the scope of this chapter.
When G. lamblia, a microaerophilic organism lacking catalase activity, infects the proximal small intestine mucosa, it must overcome the adverse oxygen and nitric oxide concentrations in the mucosa; it does this with the help of two yet uncharacterized 2-cys peroxiredoxins, GiPrx1a and GiPrx1b, suggested to play a role in the antioxidant defense of Giardia and thus a factor contributing to its pathogenesis (Mastronicola et al. 2014). G. lamblia can be infected with a dsRNA virus, although it is currently unknown if this virus could be eventually used to combat G. lamblia infections. This phage is a reovirus-like viral particle discovered by Wang and Wang in 1986, these authors described the phage as spherical virus-like particles (VLP) with a diameter of 33–35 nm and a dsRNA genome encompassing a region of 7 kilobase pairs. The capsid contains a major, highly antigenic, 100 kDa protein (Wang et al. 1988; Miller et al. 1988). Furfine and Wang (1990) used the dsRNA virus to infect other virus-free strains of the intestinal parasitic bacteria, and found that Giardia could even be infected by electroporation with purified viral ssRNA (for a comprehensive review see Wang and Wang 1991). More recently, Humen et al. (2005) used Lactobacillus johnsonii La1 as a probiotic, in a Meriones unguiculatus model infected with Giardia intestinalis, and found that the lactic acid bacterium antagonized the intestinal parasite to such extent that it protected the membrane integrity of the microvillus. The authors additionally concluded that the cellular response to Giardia antigens was stimulated in spleen cells. It appears that Lactobacillus species capable of producing bacteriocins, such as L.acidophilus (P106) and L.plantarum (P164), are able to affect G. lamblia. As a matter of fact, an ultrastructural examination proved that the bacteriocines produced marked changes in the cellular architecture of the trophozoites, with evident disorganization of the cell membrane, adhesive disk and cytoplasmic components (Amer et al. 2014).
Oral administration of recombinant acid lactic bacteria, such as Lactococcus lactis and Streptococcus gordonii, can stimulate the intestinal immune responses against G. lamblia cyst wall protein-2, and significantly increase the number of CD4(+) T-helper and B-cells in the mesenteric lymph nodes and Peyer’s patches of treated animals (Lee et al. 2009). This indicates that probiotics could indeed constitute a good approach to control parasitic diseases.
Another successful strategy focuses on immunizing the animals with recombinant E.coli strains harboring the Eimeria acervulina trophozoite antigen (Kim et al. 1989), this approach resulted in the immunization conferring the animals with partial resistance to coccidiosis.
Mucin degrading bacterial glycosidases from “normal” colon microbiota in warm-blooded animals, in combination with colonic luminal proteases can degrade the key adherence lectin present on E. histolytica trophozoites, and this effectively decreases the pathogen’s epithelial cell adherence and prevents E. histolytica from invading the intestinal mucosa (Variyam 1996). The use of bacterial proteins, known to be harmless to humans while maintaining high activity against worms (i.e., crytal proteins isolated from the parasporal crystal of Bacillus thuringiensis), is a new strategy worth mentioning here. Accordingly, Urban and colleagues demonstrated in 2013 that Cry5B protein could intoxicate Ascaris suum, thus triggering the activation of the p38 mitogen-activated protein kinase pathway and resulting in a near complete elimination of the intestinal parasite infection in pigs.
Not only intestinal bacteria can play a role in controlling parasites, Buske et al. (2013) recently suggested that certain fungi could share this ability. These authors reported that the fungus Duddingtonia flagrans systematically reduces Haemonchus contortus larval population.
Blastocystis hominis is a common human intestinal parasite that causes 4.3 % of diarrheal cases in humans (Mendis et al. 1995). It belongs to the Stramenopiles (Silberman et al. 1996; Tan 2008) and, although it was described over a century ago, little is still known about its pathogenicity, genetic diversity, or treatment (Roberts et al. 2014). In addition, some researchers claim that despite its high prevalence, it does not cause a diarrheic syndrome, at least not in Nepalese people (Shlim et al. 1995). The organism was originally classified as the cyst of a flagellate, as a vegetable, or even as a yeast, but it was subsequently reclassified as a protist (Tan 2008). Chandramathi et al. (2014) demonstrated that stress exacerbates the infectivity and pathogenicity of B. hominis, both in vivo and in vitro. B. hominis and G. lamblia have been found to preferentially infect people carrying the A > T transversional mutation (36 and 28 %, respectively; Mahdi and Ali 2002) that replaces the sixth amino acid of the β-globin chain and causes sickle-cell anemia. Although metronidazole is the most frequently prescribed antimicrobial for these infections (Gupta and Parsi 2006), one study showed the potential benefits of using S. boulardii to treat Blastocystis infection (Dinleyici et al. 2010). Blastocystis infections stimulate immunoglobulin G (IgG) and IgA production (Hussain et al. 1997; Mahmoud and Saleh 2003), apparently involved in the elimination of the parasite. Antibodies, or at least the cytotoxic monoclonal antibody 1D5, can trigger programmed cell death in the parasite B. hominis, and this cell death is independent from the caspase and mitochondrial pathways (Nasirudeen and Tan 2005). If this could be demonstrated as a general mechanism for Blastocystis eradication from the human gut, it would preclude intestinal bacteria participation, unless the presence of these microorganisms could induce heterophilic antibodies capable of initiating apoptosis.
Production of IgA against certain intestinal parasites (including bacteria) could in turn cause nephropathy, due to acute glomerulonephritis caused by deposition of IgA-containing immune complexes in the glomerular mesangium. The discovery that the distribution of the six new risk loci in humans associated with acute glomerulonephritis can vary in different ethnic groups (it is most prevalent in East Asians, less frequent in Europeans, and relatively rare in individuals of African ancestry; Kiryluk et al. 2014) advocates that great care must be taken in the administration of probiotic bacteria that could result in hyperproduction of secretory IgA.
Microsporidia are opportunistic agents that infect immunocompromised patients, such as those suffering from AIDS (Atías 1995). Five strains of this pathogen are currently known: Encephalitozoon, Enterocytozoon, Nosema, Pleistophora, and Septata (van Gool and Dankert 1995) and the clinical symptoms they cause can range from hepatic necrosis, ocular infections affecting not only the cornea but also the eye surroundings (even including the paranasal sinuses), to multisystemic infection affecting the central nervous system. The diagnosis of microsporidiosis currently depends on morphological demonstration of the organisms themselves, either in scrapings or tissues and, unfortunately, there is yet no evidence on the efficacy of probiotic treatment with either bacteria or yeasts.
As mentioned above, until more is known about the effect of “normal” intestinal bacteria on the protozoa that colonize the human intestine, it would be wise to use probiotics in combination with other approaches, such as the novel antiparasitic compounds known as heterocyclic N-oxides (Fig. 2) (Mfuh and Larionov 2015), or the triazolyl-quinolone-based chalcone derivatives that are active against G. intestinalis (Bahadur et al. 2015).
2.4 Bacterial Activity Against Intestinal Viruses
One of the first reports on the use of bacteria to combat intestinal viruses comes from Loria et al. (1976); they studied the intestinal tract of mice and discovered that the group B coxsackievirus offers natural protection against peroral infection. They concluded that this protective effect consists of at least two separate components: (i) a barrier function that prevents virus from passing through the gut mucosa into the circulation, and (ii) a clearance mechanism that eliminates the virus from the enteric tract after the infection. The authors however did not reveal the involvement of any microbial entity responsible for the clearance. In 1989 Ogra and colleagues studied the effect of oral immunization of the gastrointestinal tract, with bacterial and viral antigens, on mucosal immunity, but could reach a definite conclusion on the cross-protection provided. Koopman et al. (1989) reported that fusiform anaerobic bacteria caused the elimination of murine viral pathogens from the caecum of mice, and this resulted in the normalization of the intestinal microbiota content and function. Indeed, after the treatment the murine duodenal extracts exhibited high activity against the lactate dehydrogenase-elevating virus (LDV), but once again the authors could not associate the virucidal effect of a 10–100 kDa intestinal protein to any particular bacterial species (Broen et al. 1992). Finally, Duffy and co-workers were able to demonstrate in 1993 the protective effect of a human strain of B. bifidum against murine Group A rotavirus. Saavedra et al. (1994) further these studies by successfully using the combination of B. bifidum and Streptococcus thermophiles against human rotavirus, while Majamaa et al. (1995) demonstrated that lactic acid bacteria are effective in the treatment of acute rotavirus gastroenteritis. Worldwide, approximately 90 % of people have antibodies against Herpes Simplex Virus type 1 (HSV-1), and around 30 % of them will develop symptoms. An et al. (2012) showed that Bifidobacterium adolescentis SPM 0214 is active against HSV-1. This finding is fortunate, since not only the percentage of HSV-1 infected people is increasing, but also the virus resistance to antiviral drugs, such as acyclovir, is constantly on the rise.
Another function that the intestinal bacteria could carry out is the transformation of bioactive molecules into even more active metabolites with the potential to control the proliferation of rotavirus and even H. pylori. This is the case of Eubacterium L-8 and Streptococcus LJ-22 that can convert glycyrrhizin into two metabolites (18β-glycyrrhetinic acid-3-O-β-d-glucuronopyranosyl-(1→2)-β-d-glucuronide and 18β-glycyrrhetinic acid-3-O-β-d-glucuronide) with increased antiviral and antibacterial activities (Kim et al. 2000). Human breast milk contains all the elements necessary for normal growth and development of infants. It also has large amounts of sialic acid-containing, or sialo-conjugated, molecules that favor the growth of bifidobacteria and lactobacilli, while inhibiting bacterial toxins and enterovirus adhesion to intestinal epithelial cells (Nakano et al. 2001). Human breast milk additionally contains large quantities of fucosylated oligosaccharides, produced by enzymes encoded by the genes associated with the expression of the Lewis blood group system, that play a clearly protective role against both intestinal bacterial or viral enteropathogens (Newburg et al. 2005). Soon after the discovery that intestinal lactic acid bacteria possess antiviral activity, novel recombinant species harboring particular enteroviral genes were developed and used to elicit quick antiviral immunological responses (Ho et al. 2005). Commensal bacteria have also been used to cheaply express HIV antigens and efficiently control early stage HIV infection. Accordingly, Rao and colleagues described in 2005 the development of a highly colonizing probiotic recombinant strain of E. coli Nissle 1917 that secreted the HIV-gp41-hemolysin A hybrid antigen, and blocked HIV internalization. The authors reported that the bacterial strain could colonize mice for periods ranging from weeks to months; the recombinant E. coli strain was predominantly found in the colon and cecum, with lower bacterial concentrations present in the rectum, vagina, and small intestine. Zhao et al. (2006) found that recombinant Listeria monocitogenes expressing the gag protein from HIV could offer mice vaginal protection against human immunodeficiency virus type 1. In a similar manner, Yang et al. (2005) orally used an attenuated strain of S. flexneri carrying the human papillomavirus (HPV) L1 capsid protein to protect the animals against papillomavirus infection. Shiga toxin is a toxin belonging to a large family of ribosome-inactivating proteins with N-glycosidase activity that causes rRNA depurination and is found in many plants and in some bacteria (Endo et al. 1987). Ferens and colleagues reported in 2006 that this toxin displays a protective role against leukemia-producing virus; they experimentally infected sheep with bovine leukemia virus and found that an intestinal Shiga toxin-producing E. coli strain mitigated the infection.
Pan et al. (2009) successfully used recombinant S. thyphimurium attenuated strains as a vehicle for DNA vaccines against avian influenza viruses.
As described above for other biological control purposes, Bifidobacterium or Lactobacillus probiotic bacteria have the ability to lessen the effect of vesicular stomatitis virus infection, by establishing an antiviral state in macrophages that results in the production of NO and inflammatory cytokines, such as interleukin 6 and interferon-gamma (Ivec et al. 2007). Similarly, Pant et al. reported that treatment with Lactobacillus rhamnosus strain GG, in combination with anti-rotavirus antibodies significantly reduced the diarrheal disease caused by rotavirus (Pant et al. 2007). Indeed, Pregliasco et al. (2008) carried out a 3-stage randomized study that showed the positive effect of a combination of 3 probiotics (L. plantarum, L. rhamnosus and Bifidobacterium lactis), lactoferrin and prebiotics (such as short-chain fructooligosaccharides or galactooligosaccharides), on lowering the impact (number of disease cases) of winter-associated diseases. Maragkoudakis et al. (2010) obtained similar results, although using cell monolayers and only probiotic bacteria (L. rhamnosus GG, L. casei, Enterococcus faecium PCK38, L. fermentum ACA-DC179, L. pentosus PCA227 and L. plantarum PCA236 and PCS22). They concluded that the bacteria protected the cells from invasion by rotaviruses and gastroenteritis viruses. Accordingly, Kawase et al. (2010) protected mice against influenza viruses by supplemented their diet with lactobacilli from the human intestinal tract. In fact, E faecium (strain NCIMB 10415) has been approved as a probiotic feed supplement for use in animals in the European Union; this strain is effective against the enteropathogenic coronavirus transmissible gastroenteritis virus (TGEV; Chai et al. 2013).
Vlasova et al. (2013) found that, in gnotobiotic pigs infected with human rotavirus, both lactobacilli and bifidobacteria could promote immune homeostasis by modulating the innate immune response against the virus. Muñoz et al. (2011) described that different Bifidobacterium species (such as B. longum subsp. infantis CECT 7210) could also display a probiotical effect against intestinal rotavirus, while maintaining the main properties required from a probiotic; these properties include resistance to gastrointestinal juices, biliary salts, NaCl, and low pH, as well as adhesion to intestinal mucus and sensitivity to antibiotics. More recently, Liu et al. (2013) described that Lactobacillus rhamnosus GG on its own is moderately effective against rotavirus diarrhea, lessening the disease by partially preventing injuries to the intestinal epithelium. In fact, in a human gut microbiota transplanted neonatal gnotobiotic pig model, this microorganism enhances Th1 cellular immunity, without affecting the antibody responses (Wen et al. 2014). Some Lactobacillus strains (such as L. plantarum AYA) can induce pleiotropic responses when orally administered; they induce the production of high amounts of IgAs, which protects both the lungs and intestines from influenza viruses (Kikuchi et al. 2014). Marranzino et al. (2012) described that certain L. rhamnosus strains (such as Lr1505 and Lr431), when used as probiotics, can induce additionally effects; in fact, it was reported that regular consumption of this probiotic improves resistance to infections, even in body areas away from the gut, by increasing the macrophages activity at those sites.
Probiotic bacteria also play a role in the control of HIV infections in humans. According to Cunningham-Rundles et al. (2011), the alterations in the gut microbiota that occur early in HIV-1 infection lead to dominance of potential pathogens and reduce the levels of Bifidobacterium and Lactobacillus species, which consequently increase mucosal inflammation. The authors carried out pilot studies and concluded that probiotic bacteria, given as a supplement, protect against the loss of CD4+ T cells. According to Lehtoranta et al. (2014), probiotics also increase the general resistance against the picornaviradae that cause the common cold in winter.
Other lactic acid bacteria, such as recombinant L. lactis, have been successfully used as vehicles for coronavirus antigen delivery (as it is the case for the 70 kDa fragment of the N-terminal globular domain of the spike of coronavirus TGEV; Tang and Li 2009), and constitute an effective protection against this diarrhea-causing virus.
In conclusion, the information currently available indicates that either wild type or recombinant strains of lactic acid bacteria constitute a good means of controlling intestinal colonization by certain pathogenic viruses and that they represent a future means of designing more effective vaccines. Additionally, the unspecific response described above for lactic acid bacteria, such as the increased IgA production, merits further investigation and opens the door to further novel uses for these bacteria.
2.5 Bacterial Activity Against Pathogenic Yeasts
Gillot (1958) originally suggested the use of active substances produced by L. acidophilus against Candida albicans. Several years later, Balish and Phillips (1966) demonstrated, in germ-free chicks, the protective role of normal bacteria (such as E. coli) against the pathogenic yeast. Controlling the intestinal population of C. albicans is important since this yeast can promote food antigen sensitization in mice, by affecting the mucosal barrier (Yamaguchi et al. 2006). The anti-Candida activity of the enteric bacterial microbiota is important to control yeast growth, additionally preventing their adherence to epithelial surfaces; but unfortunately little is known on the probiotic and biotherapeutic effects of the intestinal “normobacteria” against candidiasis (Balish and Wagner 1998). A number of bacterial species have the ability to inhibit C. albicans in vitro, these include E. coli, Salmonella spp and mainly Lactobacillus species (Isenberg et al. 1960; Caves et al. 1973; Hummel et al. 1975; Balish and Wagner 1998). The control of the intestinal growth of microorganisms such as C. albicans, M. avium subsp paratuberculosis and adherent-invasive E. coli strains is important, since they are suspected to play a role in the infectious etiology of Crohn’s disease. This suspicion is based on the genetic susceptibility, which relates to impaired function in the defense against intracellular microorganisms (Pineton de Chambrun et al. 2008). In fact, Sendid et al. (2009) found that a high number of Crohn’s disease biomarkers (mainly anti-cell wall glycan antibodies) were induced during C. albicans development in the intestine.
But the relationship between intestinal bacteria and yeasts can also work in reverse, this means that a yeast strain can also act as a probiotic that protects the animal against pathogenic bacteria. In this way, the ascomycetous yeast S. boulardii can grow at 37 °C (McFarland and Bernasconi 1993) and ameliorate different types of diarrheal diseases (Bartlett 1992; McFarland et al. 1995). This yeast was reported to modulate the immune system (Buts et al. 1990), degrade C. difficile toxins A and B as well as their respective receptors on the colonic mucosa (Castagliuolo et al. 1996), inhibit cholera toxin action (Brandão et al. 1998), and stimulate digestive enzymatic activities (Buts et al. 1986; Jahn et al. 1996). It can also inhibit the production of pro-inflammatory cytokines by inhibiting main regulators of inflammation (including nuclear factor κB (NF-κB), mitogen-activated protein kinases (MAP kinases), and ERK1/2 and p38), but it stimulates the production of anti-inflammatory molecules such as peroxisome proliferator-activated receptor-gamma (Im and Pothoulakis 2010), among others (for a more comprehensive review see Martins et al. 2005; Pothoulakis 2009).
S. boulardii can also interfere with C. albicans; in fact, both S. boulardii and its extracts significantly inhibit C. albicans adhesion to epithelial cell lines, as well as reducing cytokine-mediated inflammatory host response (Murzyn et al. 2010). In addition, Martins et al. (2005) isolated twelve different S. cerevisiae strains from a variety of sources and found that strain 905 was the best equipped to become a successful probiotic. This strain displayed a good ability to grow in the gastrointestinal tract of germ-free mice and it displayed a protective effect against experimental infection with S. tithymurium and C. difficile. Strain 905 was also shown to reduce the intestinal translocation of S. enterica serotype typhimurium and to stimulate the immune system in both gnotobiotic and wild type mice (Martins et al. 2007). S. cerevisiae has additional probiotic capabilities, such as the ability to modulate transcription of proteins involved in inflammation, and the capacity to inhibit the ETEC-induced expression of pro-inflammatory proteins IL-6, IL-8, CCL20, CXCL2, and CXCL10, as well as IL-6, and IL-8 (Zanello et al. 2011). In a more recent study, Martins et al. (2009) described a comparative study on the suitability of using four different microbial species (Bifidobacterium animalis, E. coli, L. casei, and S. boulardii) as probiotics, with the conclusion that the yeast displayed better immunomodulation characteristics, whereas B. animalis and L. casei constituted better antagonistic microorganisms.
2.6 Bacterial Activity Against Protozoan Insect Vectors
The relationship between the bacteria found in the midgut of insects, intestinal protozoa and host arthropods has always been, to say the least, peculiar. This association has become so close that often the organisms are dependent on each other for survival, making it difficult to study them individually (Dillon and Dillon 2004). Learning how these gut bacteria interact with their insect host could give an insight into how to control the insect’s life cycle and, perhaps, contribute to the biological control of vector-transmitted protozoan diseases (Chanbusarakum and Ullman 2008). Additional aspects could include: (i) the effect of the Rickettsia-like maternally inherited Wolbachia, known to alter the arthropod’s sexual differentiation, resulting in female-biased sex ratios or parthenogenesis (Rousset et al. 1992), or (ii) the novel insecticidal toxins produced by the nematode-symbiotic bacteria Photorhabdus luminescens and Xenorhabdus nematophilus (Ffrench-Constant and Bowen 2000). Species of Photorhabus such as P. temperate are entomopathogenic bacteria that have a negative, although indirect, effect on the insect’s microbiota. This is the case for the sugarcane stalk borer Diatraea saccharalis (Lepidoptera); soon after infection of the insect with P. temperate 90 % of its intestinal microbiota is killed, eventually resulting in the animal’s death (Carneiro et al. 2008) The study of the intestinal bacterial population of hematophagous insects is gaining importance, as it could represent a biological way to control classic protozoal diseases of difficult eradication worldwide, particularly in third world countries. One of the insects targeted is the sand fly Phlebotomus papatasi, the vector for leishmaniasis, Carrión’s disease, bartonellosis, and a variety of arboviral diseases (Depaquit et al. 2010). This emphasizes the fact that knowledge of the vector’s intestinal microbiota is important in order to advance the fight against these diseases. In 2012 Mukhopadhyay and colleagues reported a wide range of variation in the aerobic flora inhabiting the sand fly gut, which possibly reflected the different breeding habitats; but there are common bacteria (such as Bacillus pumilus and Bacillus flexus), possibly involved in oviposition and therefore with the potential to controll the insect’s life cycle. Maleki-Ravasan et al. (2013) continued the study of the sand fly microbiota, with a focus on the bacterial population found in P. papatasi midgut, and their findings disagreed with previous reports for Phlebotomus duboseqi. The authors found that, in addition to Enterobacteriaceae (with Proteus mirabilis and P. vulgaris as the most prevalent isolates) and non-enterobacteria (Bacillus, Staphylococcus, and Pseudomonas) species, the main bacterial strain for females was Ochrobactrum sp., a species acquired by transstadial passage (Volf et al. 2002). The Malpighian tubes of P. papatasi are often carriers of Aspergillus sclerotiorum and S. cerevisiae strains, and that makes the insect refractory to artificial infections with the protozoan parasite Leishmania and even with bacteria such as E. coli, S. aureus, Shigella sonnei, Streptococcus group A, and Pseudomonas aeruginosa (Schlein et al. 1985). In addition, this particular sand fly, when challenged with bacteria such as Erwinia carotovora subsp. carotovora, synthesizes a novel defensin (with a molecular mass of 4095.5 MH+) displaying a strong antiparasitic activity against the promastigote forms of Leishmania major (Boulanger et al. 2004). Chanbusarakum and Ullman (2008) found insect bacterial endosymbionts phylogenetically related to Erwinia in the western flower thrips (Frankliniella occidentalis).
Chaga’s disease is caused by the protozoan Trapanosoma cruzi and uses triatomine insects, such as Rhodnius prolixus, as vectors. This protozoan interferes with the insect’s immunity system, lowering its gut’s microbiota and allowing the protist to permanently establish itself inside the insect (Castro et al. 2012a). The bacterial microbiota of R. prolixus can be increased by simply treating the insect with physalin B (a natural secosteroid, extracted from the plant Physalis angulata), causing a reduction in the number of T. cruzi parasite cells (Castro et al. 2012b). Current work involves the in vitro manipulation of one of the insect’s gut bacterium (Rhodococcus rhodnii) to express antiparasitic agents in the gut, where the trypanosomes are also located as a means of controlling the parasite’s population. According to Dotson et al. (2003) stable integrons are desirable to ensure that the bacterium constantly produces the antiparasitic drug. The surface of T. cruzi is covered by a thick mucin-like substance, involved in binding this protozoon to the insect’s intestinal membrane; hence, disruption of these glycoconjugates by 1,3-β-d-glucanase-producing R. rhodnii strains could stop the parasite’s development (Jose et al. 2013). In addition, R. rhodnii intestinal microbiota could be modified to deliver other molecules with antiparasitic activity. Accordingly, Taracena et al. (2015) successfully used a systemic RNAi strategy to control Chaga’s disease; they fed R. prolixus nymphs and adults with transgenic E. coli HT115 (DE3) strains expressing dsRNA for the Rhodnius heme-binding protein and for catalase.
Despite all the research carried out, malaria still remains a health problem in African countries, where it still causes more than 1 million deaths annually (Boissière et al. 2012). The disease in these countries is caused by Plasmodium falciparum, transmitted by the mosquito Anopheles gambiae. The ability of the mosquitoes to transmit Plasmodium parasites is highly variable between individuals and this individual susceptibility appears to have a genetic basis (Blandin et al. 2009), but is also influenced by the bacteria colonizing the insect’s midgut, which can provide a protective role against the malarial parasite (Dong et al. 2009); indeed, the use of antibiotics to disrupt the insect’s gut microbiota can enhance Plasmodium infection. A low number of the bacterial species constituting the insect’s gut microbiota have been isolated and identified by classical microbiological methods (Favia et al. 2007). On the other hand, Boissière et al. (2012) used pyrosequencing techniques to study the microbiota present in one mosquito. They found that the midgut microbiota in that adult mosquito included five dominant Phyla (Proteobacteria, Bacteroidetes, Actinobacteria, Fusobacteria, and Firmicutes), but the vast majority of sequence tags (>90 %) corresponded to just a few taxa and only 21 bacterial families, while 28 genera only represented >1 %. Their conclusion was, therefore, that A. gambiae is colonized by a few dominant bacterial species (Boissière et al. 2012). Sharma and co-workers described similar results in 2013, working in Asian malaria transmitted by Anopheles stephensi. Akhouayri et al. (2013) suggested the use of the endosymbiont Elizabethkingia meningoseptica, either alone or in combination with other approaches, in the biocontrol of A. gambiae.
Often the relationship between bacteria and protozoa within the insect’s gut is entirely different, as is the case for protozoal endosymbiotic bacteria. Recent studies revealed that facultative bacterial symbionts could substantially affect various ecological traits in herbivorous insects (Hosokawa et al. 2007), such as resistance to pathogenic fungi (Scarborough et al. 2005), and even their ability to broaden the plant food range (Tsuchida et al. 2004).
In 2006, Kämpfer and colleagues reported the isolation of a new Gram-negative, rod-shaped bacterium (CCUG 49520T), from the midgut of the mosquito Anopheles arabiensis, which exhibited <93 % similarity to species of the families Enterobacteriaceae and Vibrionaceae, and contained phosphatidylethanolamine and phosphatidylglycerol as their major lipids. Kämpfer et al. (2006) proposed the creation of a new genus and species (Thorsellia anophelis) for this microorganism and its classification as a new member of the Gammaproteobacteria. Duguma et al. (2013) described Thorsellia anophelis as, by far, the most predominant bacterium in the midgut of not only Anopheles mosquitoes, but also in Culex tarsalis. Four major Thorsellia lineages had been thus far identified, all closely affiliated with an insect endosymbiont known as Arsenophonus (Briones et al. 2008). These authors suggest that: “aquatically derived bacteria such as T. anophelis can persist through mosquito metamorphosis and become well-established in the adult mosquito midgut.” The isolation and identification of culturable bacteria from different wild Anopheles species must be considered as a first step in the paratransgenic approach to fight malarial diseases worldwide (Chavshin et al. 2014).
2.7 Protozoan Activity Against Pathogenic Bacteria
To the best of our knowledge, very little research has been published on this topic; but perhaps one of the first well-documented studies, as mentioned above, refers to an insect anal secretion with strong antibacterial activity. Willers et al. (1982) reported a hyaline anal secretion from P. turionellae (a lepidopteran endoparasitic species) that exhibited antibacterial activity against microorganisms such as E. coli, Micrococcus luteus, and Pseudomonas phaseolicola, and was additionally able to control the entomopathogenic fungus Beauveria bassiana. However, the authors could not establish whether the active principle was synthesized by the parasitic insect or by a biological entity residing in its midgut.
Cystatins are cysteine protease inhibitors that play a great number of roles in insects and other organisms, such as pathogenic protozoa (Kang et al. 2012); most of these roles relate to the differentiation of the insect’s immune system of (Liu et al. 2010) and the completion of the protozoan’s life cycle (Lee et al. 2013). The parasitic wasp Cortesia congregate injects its eggs into the caterpillar Manduca sexta, but a bracovirus is needed to ensure the success of the parasite; indeed, a virally encoded cystatin modulates the immune system of the wasp. This represented the first report demonstrating the bracoviral origin of a cystatin (Espagne et al. 2005). Once again, neither the wasp, and nor the proptozoan or bacteria were reported to be affected by the potent viral cystatin.
Kotsyfakis et al. (2010), while working on a murine model of Lyme disease, caused by Borrelia burgdorferi with the tick Ixodes scapularis as its vector, discovered two salivary cystains (sialostatins L and L2). Their results revealed that the structure of the two tick salivary cysteine protease inhibitors not only facilitated the vector blood feeding, but also that the compounds (in particular L2) were involved in the transmission of B. burgdorferi to the vertebrate host (Kotsyfakis et al. 2010).
Kang and colleagues in 2012 identified and characterized a new cystatin from C. parvum, with a low sequence homology to other cystatins belonging to the chagasin-family, capable of inhibiting papain, human cathepsin B, human cathepsin L, and cryptopain-1, with Ki values in the picomolar range; and, therefore, involved in the establishment of the disease (Kang et al. 2012). These authors, however, do not mention anything in their report concerning the action of this molecule on pathogenic bacteria; and this was also the case for the report published by Schwarz et al. (2012). In summary, although there are hints indicating that the protozoan parasite could be involved in the development of the insect’s gut microbiota, little information is still available, and further research is required to reach a conclusion in this matter.
2.8 Bacterial Activity Against Uropathogenic Bacteria
Urinary tract infection (UTI) is the most common infection in patients with spinal cord injury (SCI) and it constitutes a major cause of morbidity and mortality in this population. SCI patients almost permanently need antimicrobial treatment and hence constitute a natural reservoir for the emergence of antibiotic-resistant bacteria. This is due, in part, to the fact that most of them need urinary catheters, which can be used by bacteria to penetrate the bladder. Some of the bacteria that colonize these neuropathic bladders do not produce symptoms, and they are classified as benign colonizers, and, theoretically, bladder colonization by these bacteria could prevent infection by pathogenic microorganisms. In 1999 Richard Hull and his group studied the urinary tract colonization mechanisms used by bacteria associated with asymptomatic bacteriuria (ABU). They examined the virulence properties of E. coli 83972, a strain isolated from a clinical ABU episode (Hull et al. 1999). They showed that this strain maintained the genetic potential to express both type P and type 1 pili, but it did not express either d -mannose-resistant or d -mannose-sensitive hemagglutinins (the main cause of uropathogenicity). They proposed that ABU strains could represent a microbiological approach to prevent urinary tract infections in spinal cord injured patients, and reached the conclusion that E. coli 83972 can be safely used as a long-term asymptomatic bladder colonizer that could successfully prevent the establishment of uropathogenic bacteria. This means that bladder colonization by E. coli 83972 could reduce the incidence of urinary tract infection in patients with neurogenic bladder secondary to spinal cord injury (Hull et al. 2000; Darouiche and Hull 2000). Clinical trials demonstrated the feasibility of this technique (Darouiche et al. 2001), additionally demonstrating that the artificial colonization of urinary catheters by a benign E. coli completely prevented the adhesion and further colonization by not only E. faecalis (Trautner et al. 2002) but also many other uropathogenic species (Trautner et al. 2003, 2005a, b). The method is currently being improved; by combining it with additional strategies, such as bacteriocin impregnation (Trautner et al. 2007), increased expression of certain fimbriae (Trautner et al. 2008), and the addition of agents that prevent biofilm formation (Mansouri et al. 2013). This method is expected to be approved in the near future, and will probably represent a powerful weapon to treat UTI in people with spinal cord injuries.
References
Ackerley LM, Jones A (1985) Food poisoning–fact or fiction? An observation of the current interpretation of the term ‘food-poisoning’. J Int Med Res 13:241–244
Akhouayri IG, Habtewold T, Christophides GK (2013) Melanotic pathology and vertical transmission of the gut commensal Elizabethkingia meningoseptica in the major malaria vector Anopheles gambiae. PLoS ONE 8:e77619
Alak JI, Wolf BW, Mdurvwa EG, Pimentel-Smith GE, Adeyemo O (1997) Effect of Lactobacillus reuteri on intestinal resistance to Cryptosporidium parvum infection in a murine model of acquired immunodeficiency syndrome. J Infect Dis 175:218–221
Albert MJ, Alam K, Islam M, Montanaro J, Rahaman AS, Haider K, Hossain MA, Kibriya AK, Tzipori S (1991) Hafnia alvei, a probable cause of diarrhea in humans. Infect Immun 59:1507–1513
Aley SB, Zimmerman M, Hetsko M, Selsted ME, Gillin FD (1994) Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun 62:5397–5403
Al-Fendi A, Shueb RH, Ravichandran M, Yean CY (2014) Isolation and characterization of lytic vibriophage against Vibrio cholerae O1 from environmental water samples in Kelantan, Malaysia. J Basic Microbiol 54:1036–1043
Alm RA, Guerry P, Power ME, Trust TJ (1992) Variation in antigenicity and molecular weight of Campylobacter coli VC167 flagellin in different genetic backgrounds. J Bacteriol 174:4230–4238
Amarillas L, Cháidez-Quiroz C, Sañudo-Barajas A, León-Félix J (2013) Complete genome sequence of a polyvalent bacteriophage, phiKP26, active on Salmonella and Escherichia coli. Arch Virol 158:2395–2398
Amer EI, Mossallam SF, Mahrous H (2014) Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp Parasitol 146:52–63
An HM, Lee DK, Kim JR, Lee SW, Cha MK, Lee KO, Ha NJ (2012) Antiviral activity of Bifidobacterium adolescentis SPM 0214 against herpes simplex virus type 1. Arch Pharm Res 35:1665–1671
Anany H, Chen W, Pelton R, Griffiths MW (2011) Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Appl Environ Microbiol 77:6379–6387
Andremont A, Raibaud P, Tancrède C (1983) Effect of erythromycin on microbial antagonisms: a study in gnotobiotic mice associated with a human fecal flora. J Infect Dis 148:579–587
Andremont A, Gerbaud G, Tancrede C, Courvalin P (1985) Plasmid-mediated susceptibility to intestinal microbial antagonisms in Escherichia coli. Infect Immun 49:751–755
Andrewes FW (1922) Studies in group agglutination. I. Salmonella group and its antigenic structure. J Path Bact 25:505–515
Arkwright JA (1920) Remarks on the virus of typhus fever and the means by which it is conveyed. Proc R Soc Med 13:87–95
Arkwright JA (1921) Variation in bacteria in relation to agglutination both by salts and by specific serum. J Pathol Bacteriol 24:36–60
Atías A (1995) Update on microsporidiosis in humans. Rev Med Chil 123:762–772
Atterbury RJ, Van Bergen MA, Ortiz F, Lovell MA, Harris JA, De Boer A, Wagenaar JA, Allen VM, Barrow PA (2007) Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl Environ Microbiol 73:4543–4549
Ayers WA, Papavizas GC (1963) Violet-pigmented pseudomonads with antifungal activity from the rhizosphere of beans. Appl Microbiol 11:533–538
Bahadur V, Mastronicola D, Singh AK, Tiwari HK, Pucillo LP, Sarti P, Singh BK, Giuffrè A (2015) Antigiardial activity of novel triazolyl-quinolone-based chalcone derivatives: when oxygen makes the difference. Front Microbiol 6:256
Bainbridge FA, Dudfield R (1911) An outbreak of acute gastroenteritis caused by B. paratyphosus (B.). J Hyg (Lond) 11:24–29
Bakeeva LE, Chumakov KM, Drachev AL, Metlina AL, Skulachev VP (1986) The sodium cycle. III. Vibrio alginolyticus resembles Vibrio cholerae and some other vibriones by flagellar motor and ribosomal 5S-RNA structures. Biochim Biophys Acta 850:466–472
Baker EE, Walther FG, Perlman E (1949) The specific antigens of variants of Shigella sonnei. J Exp Med 89:325–338
Balfour Sartor R (2007) Bacteria in Crohn’s disease: mechanisms of inflammation and therapeutic implications. J Clin Gastroenterol 41(Suppl 1):S37–S43
Balish E, Phillips AW (1966) Growth and virulence of Candida albicans after oral inoculation in the chick with a monoflora of either Escherichia coli or Streptococcus faecalis. J Bacteriol 91:1744–1749
Balish E, Wagner RD (1998) Probiotic bacteria for prophylaxis and therapy of candidiasis. Rev Iberoam Micol 15:261–264
Barbara AJ, Trinh HT, Glock RD, Glenn Songer J (2008) Necrotic enteritis-producing strains of Clostridium perfringens displace non-necrotic enteritis strains from the gut of chicks. Vet Microbiol 126:377–382
Bardina C, Spricigo DA, Cortés P, Llagostera M (2012) Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl Environ Microbiol 78:6600–6607
Barmeyer C, Schulzke JD, Fromm M (2015) Claudin-related intestinal diseases. Semin Cell Dev Biol. pii:S1084-9521(15)00103-2
Barnes EM, Impey CS, Stevens BJ (1979) Factors affecting the incidence and anti-salmonella activity of the anaerobic caecal flora of the young chick. J Hyg (Lond) 82:263–283
Bartlett JG (1992) Antibiotic-associated diarrhea. Clin Infect Dis 15:573–581
Batt RM, Rutgers HC, Sancak AA (1996) Enteric bacteria: friend or foe? J Small Anim Pract 37:261–267
Bautista-Garfias CR, Ixta O, Orduña M, Martínez F, Aguilar B, Cortés A (1999) Enhancement of resistance in mice treated with Lactobacillus casei: effect on Trichinella spiralis infection. Vet Parasitol 80:251–260
Beaugerie L, Petit J-C (2004) Antibiotic-associated diarrhoea. Best Pract Res Clin Gastroenterol 18:337–352
Begum YA, Chakraborty S, Chowdhury A, Ghosh AN, Nair GB, Sack RB, Svennerholm AM, Qadri F (2010) Isolation of a bacteriophage specific for CS7-expressing strains of enterotoxigenic Escherichia coli. J Med Microbiol 59:266–272
Berchieri A Jr, Lovell MA, Barrow PA (1991) The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res Microbiol 142:541–549
Bereswill S, Kist M (2003) Recent developments in Campylobacter pathogenesis. Curr Opin Infect Dis 16:487–491
Bernet MF, Brassart D, Neeser JR, Servin AL (1993) Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl Environ Microbiol 59:4121–4128
Bezirtzoglou E, Romond MB, Romond C (1989) Modulation of Clostridium perfringens intestinal colonization in infants delivered by caesarean section. Infection 17:232–236
Bhowmick TS, Koley H, Das M, Saha DR, Sarkar BL (2009) Pathogenic potential of vibriophages against an experimental infection with Vibrio cholerae O1 in the RITARD model. Int J Antimicrob Agents 33:569–573
Bigwood T, Hudson JA, Billington C (2009) Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol Lett 291:59–64
Black WJ, Schwalbe RS, Nachamkin I, Cannon JG (1984) Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun 45:453–457
Blandin SA, Wang-Sattler R, Lamacchia M, Gagneur J, Lycett G, Ning Y, Levashina EA, Steinmetz LM (2009) Dissecting the genetic basis of resistance to malaria parasites in Anopheles gambiae. Science 326:147–150
Blomberg L, Henriksson A, Conway PL (1993) Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol 59:34–39
Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, Morlais I (2012) Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8:e1002742
Bojlén K (1934) The specific antigens of variants of Shigella. Comm Inst Serotherap Danois, 24
Bokkenheuser V (1993) The friendly anaerobes. Clin Infect Dis 4:S427–S434
Borriello SP, Barclay FE (1986) An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol 21:299–309
Boulanger N, Lowenberger C, Volf P, Ursic R, Sigutova L, Sabatier L, Svobodova M, Beverley SM, Späth G, Brun R, Pesson B, Bulet P (2004) Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect Immun 72:7140–7146
Brandão RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJM, Neves MJ, Santos RG, Gomes NCM, Nicoli JR (1998) Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol 64:564–568
Brenner DJ (1976) Shigella flexneri 6 biotypes: a review. Health Lab Sci 13:218–222
Briones AM, Shililu J, Githure J, Novak R, Raskin L (2008) Thorsellia anophelis is the dominant bacterium in a Kenyan population of adult Anopheles gambiae mosquitoes. ISME J 2:74–82
Broen JJ, DesJarlais SE, Duman RG, Anderson SN, Mueller RA, Cafruny WA (1992) Virucidal effect of murine duodenal extracts: studies with lactate dehydrogenase-elevating virus. Antiviral Res 18:327–340
Burke KE, Lamont JT (2013) Fecal transplantation for recurrent clostridium difficile infection in older adults: a review. J Am Geriatr Soc 61:1394–1398
Buske R, Santurio JM, de Oliveira CV, Bianchini LA, da Silva JH, de la Rue ML (2013) In vitro influence of temperature on the biological control activity of the fungus Duddingtonia flagrans against Haemonchus contortus in sheep. Parasitol Res 112:473–478
Buts JP, Bernasconi P, Van Craynest MP, Maldague P, De Meyer R (1986) Response of human and rat small intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res 20:192–196
Buts JP, Bernasconi P, Vaerman JP, Dive C (1990) Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci 35:251–256
Caldwell MB, Guerry P, Lee EC, Burans JP, Walker RI (1985) Reversible expression of flagella in Campylobacter jejuni. Infect Immun 50:941–943
Cantani A (1885) Un tentativo di batterioterapia. Gior Int Sci Med 7:493
Cao L, Yang XJ, Li ZJ, Sun FF, Wu XH, Yao JH (2012) Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.20291. Poult Sci 91:3065–3071
Capparelli R, Nocerino N, Iannaccone M, Ercolini D, Parlato M, Chiara M, Iannelli D (2010) Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J Infect Dis 201:52–61
Carneiro CN, DaMatta RA, Samuels RI, Silva CP (2008) Effects of entomopathogenic bacterium Photorhabdus temperata infection on the intestinal microbiota of the sugarcane stalk borer Diatraea saccharalis (Lepidoptera: Crambidae). J Invertebr Pathol 99:87–91
Castagliuolo I, Lamont JT, Nikulasson ST, Pothoulakis C (1996) Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun 64:5225–5232
Castro DP, Moraes CS, Gonzalez MS, Ratcliffe NA, Azambuja P, Garcia ES (2012a) Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS ONE 7:e36591
Castro DP, Moraes CS, Gonzalez MS, Ribeiro IM, Tomassini TC, Azambuja P, Garcia ES (2012b) Physalin B inhibits Trypanosoma cruzi infection in the gut of Rhodnius prolixus by affecting the immune system and microbiota. J Insect Physiol 58:1620–1625
Caves JM, Carpenter JA, Hamdy MK (1973) Interaction between Salmonella enteritidis and Candida albicans. Proc Soc Exp Biol Med 143:433–439
Chai W, Burwinkel M, Wang Z, Palissa C, Esch B, Twardziok S, Rieger J, Wrede P, Schmidt MF (2013) Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol 158:799–807
Chakraborty S, Deokule JS, Garg P, Bhattacharya SK, Nandy RK, Nair GB, Yamasaki S, Takeda Y, Ramamurthy T (2001) Concomitant infection of enterotoxigenic Escherichia coli in an outbreak of cholera caused by Vibrio cholerae O1 and O139 in Ahmedabad, India. J Clin Microbiol 39:3241–3246
Chanbusarakum L, Ullman D (2008) Characterization of bacterial symbionts in Frankliniella occidentalis (Pergande), Western flower thrips. J Invertebr Pathol 99:318–325
Chandramathi S, Suresh K, Sivanandam S, Kuppusamy UR (2014) Stress exacerbates infectivity and pathogenicity of Blastocystis hominis: in vitro and in vivo evidences. PLoS ONE 9(5):e94567
Chatterjee S, Ghosh K, Raychoudhuri A, Chowdhury G, Bhattacharya MK, Mukhopadhyay AK, Ramamurthy T, Bhattacharya SK, Klose KE, Nandy RK (2009) Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J Clin Microbiol 47:1087–1095
Chavshin AR, Oshaghi MA, Vatandoost H, Pourmand MR, Raeisi A, Terenius O (2014) Isolation and identification of culturable bacteria from wild Anopheles culicifacies, a first step in a paratransgenesis approach. Parasit Vectors 7:419
Cleusix V, Lacroix C, Vollenweider S, Duboux M, Le Blay G (2007) Inhibitory activity spectrum of reuterin produced by Lactobacillus reuteri against intestinal bacteria. BMC Microbiol 7:101
Coconnier MH, Bernet MF, Chauvière G, Servin AL (1993) Adhering heat-killed human Lactobacillus acidophilus, strain LB, inhibits the process of pathogenicity of diarrhoeagenic bacteria in cultured human intestinal cells. J Diarrhoeal Dis Res 11:235–242
Courtieu AL, Duboeuf C, Bogenmann J, Maka G, Longeray C (1965) Recurrent gastroenteritis, due to Salmonella panama, becoming resistant to the antibiotics used. Sterilization by bacteriophages. J Med Lyon 46:1481–1486
Crost EH, Ajandouz EH, Villard C, Geraert PA, Puigserver A, Fons M (2011) Ruminococcin C, a new anti-Clostridium perfringens bacteriocin produced in the gut by the commensal bacterium Ruminococcus gnavus E1. Biochimie 93:1487–1494
Cunningham-Rundles S, Ahrné S, Johann-Liang R, Abuav R, Dunn-Navarra AM, Grassey C, Bengmark S, Cervia JS (2011) Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection. Nutrients 3:1042–1070
Danin-Poleg Y, Cohen LA, Gancz H, Broza YY, Goldshmidt H, Malul E, Valinsky L, Lerner L, Broza M, Kashi Y (2007) Vibrio cholerae strain typing and phylogeny study based on simple sequence repeats. J Clin Microbiol 45:736–746
Darouiche RO, Hull RA (2000) Bacterial interference for prevention of urinary tract infection: an overview. J Spinal Cord Med 23:136–141
Darouiche RO, Donovan WH, Del Terzo M, Thornby JI, Rudy DC, Hull RA (2001) Pilot trial of bacterial interference for preventing urinary tract infection. Urology 58:339–344
Debruyne L, Gevers D, Vandamme P (2008) Taxonomy of the family campylobacteraceae. In: Nachamkin I, Szymanski CM, Blaser MJ (eds) Campylobacter, 3rd edn. ASM Press, Washington
Del Piano M, Anderloni A, Balzarini M, Ballarè M, Carmagnola S, Montino F, Orsello M, Pagliarulo M, Tari R, Soattini L, Sforza F, Mogna L, Mogna G (2012) The innovative potential of Lactobacillus rhamnosus LR06, Lactobacillus pentosus LPS01, Lactobacillus plantarum LP01, and Lactobacillus delbrueckii Subsp. delbrueckii LDD01 to restore the “gastric barrier effect” in patients chronically treated with PPI: a pilot study. J Clin Gastroenterol 46(Suppl):S18–S26
Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C (2010) Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: a review. Euro Surveill 15:19507
Dibrov P (2005) The sodium cycle in Vibrio cholerae: riddles in the dark. Biochemistry (Mosc) 70:150–153
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92
Dinleyici EC, Eren M, Dogan N, Reyhanioglu S, Yargic ZA, Vandenplas Y (2010) Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol Res 108:541–545
Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5:e1000423
Dotson EM, Plikaytis B, Shinnick TM, Durvasula RV, Beard CB (2003) Transformation of Rhodococcus rhodnii, a symbiont of the Chagas disease vector Rhodnius prolixus, with integrative elements of the L1 mycobacteriophage. Infect Genet Evol 3:103–109
Ducluzeau R (1993) Development, equilibrium and role of microbial flora in the newborn. Ann Pediatr (Paris) 40:13–22
Ducluzeau R, Bensaada M (1982) Comparative effect of a single or continuous administration of Saccharomyces boulardii on the establishment of various strains of Candida in the digestive tract of gnotobiotic mice. Ann Microbiol (Paris) 133:491–501
Ducluzeau R, Ladire M, Callut C, Raibaud P, Abrams GD (1977) Antagonistic effect of extremely oxygen sensitive clostridia from the microflora of conventional mice and of Escherichia coli against Shigella flexneri in the digestive tract of gnotobiotic mice. Infect Immun 17:415–424
Duffy LC, Zielezny MA, Riepenhoff-Talty M, Dryja D, Sayahtaheri-Altaie S, Griffiths E, Ruffin D, Barrett H, Rossman J, Ogra PL (1993) Effectiveness of Bifidobacterium bifidum in experimentally induced MRV infection: dietary implications in formulas for newborns. Endocr Regul 27:223–229
Duguma D, Rugman-Jones P, Kaufman MG, Hall MW, Neufeld JD, Stouthamer R, Walton WE (2013) Bacterial communities associated with culex mosquito larvae and two emergent aquatic plants of bioremediation importance. PLoS ONE 8:e72522
Duncan SH, Flint HJ, Stewart CS (1998) Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol Lett 164:283–288
Duval CW (1904) Another member of the dysentery group. J Am Med Assn 43:381–383
Duval-Iflah Y, Raibaud P, Rousseau M (1981) Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun 34:957–969
Eckardt AJ, Baumgart DC (2011) Viral gastroenteritis in adult. Recent Pat Anti-Infect Drug Discovery 6:54–63
Elsayed S, Zhang K (2004) Human infection caused by Clostridium hathewayi. Emerg Infect Dis 10:1950–1952
Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 262:5908–5912
Engberg RM, Grevsen K, Ivarsen E, Fretté X, Christensen LP, Højberg O, Jensen BB, Canibe N (2012) The effect of Artemisia annua on broiler performance, on intestinal microbiota and on the course of a Clostridium perfringens infection applying a necrotic enteritis disease model. Avian Pathol 41:369–376
Eom JS, Song J, Choi HS (2015) Protective effects of a novel probiotic strain of Lactobacillus plantarum JSA22 from traditional fermented soybean food against infection by Salmonella enterica Serovar typhimurium. J Microbiol Biotechnol 25:479–491
Espagne E, Douris V, Lalmanach G, Provost B, Cattolico L, Lesobre J, Kurata S, Iatrou K, Drezen JM, Huguet E (2005) A virus essential for insect host-parasite interactions encodes cystatins. J Virol 79:9765–9776
Euzéby JP (1997) List of bacterial names with standing in nomenclature: a folder available on the Internet Int. J Syst Bacteriol 47:590–592
Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, Rizzi A, Urso R, Brusetti L, Borin S, Mora D, Scuppa P, Pasqualini L, Clementi E, Genchi M, Corona S, Negri I, Grandi G, Alma A, Kramer L, Esposito F, Bandi C, Sacchi L, Daffonchio D (2007) Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Natl Acad Sci USA 104:9047–9051
Ferens WA, Cobbold R, Hovde CJ (2006) Intestinal Shiga toxin-producing Escherichia coli bacteria mitigate bovine leukemia virus infection in experimentally infected sheep. Infect Immun 74:2906–2916
Fetissov SO, Hamze Sinno M, Coëffier M, Bole-Feysot C, Ducrotté P, Hökfelt T, Déchelotte P (2008) Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition 24:348–359
Ffrench-Constant RH, Bowen DJ (2000) Novel insecticidal toxins from nematode-symbiotic bacteria. Cell Mol Life Sci 57:828–833
Foglia G, Shah S, Luxemburger C, Pietrobon PJ (2012) Clostridium difficile: development of a novel candidate vaccine. Vaccine 30:4307–4309
Fraser CM, Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH et al (2000) DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483
Fry DE, Schermer CR (2000) The consequences of suppression of anaerobic bacteria. Surg Infect (Larchmt) 1:49–56
Furfine ES, Wang CC (1990) Transfection of the Giardia lamblia double-stranded RNA virus into Giardia lamblia by electroporation of a single-stranded RNA copy of the viral genome. Mol Cell Biol 10:3659–3662
Ghose C, Perez-Perez GI, van Doorn LJ, Dominguez-Bello MG, Blaser MJ (2005) High frequency of gastric colonization with multiple Helicobacter pylori strains in Venezuelan subjects. J Clin Microbiol 43:2635–2641
Gilbreath JJ, Cody WL, Merrell DS, Hendrixson DR (2011) Change is good: variations in common biological mechanisms in the epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol Mol Biol Rev 75:84–132
Gorbach SL, Barza M, Giuliano M, Jacobus NV (1988) Colonization resistance of the human intestinal microflora: testing the hypothesis in normal volunteers. Eur J Clin Microbiol Infect Dis 7:98–102
Grant CCR, Konkel ME, Cieplak W Jr, Tompkins LS (1993) Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun 61:1764–1771
Greig-Smith R (1917) Contributions to our knowledge of soil fertility, XV. Proc Linn Soc NSW 42:162–166
Guarner F, Malagelada J (2003) Gut flora in health and disease. The Lancet 361:512–519
Gubina M, Zajc-Satler J, Mehle J, Drinovec B, Pikelj F, Radsel-Medvescek A, Suhac M (1976) Septicaemia and meningitis with Campylobacter fetus subspecies intestinalis. Infection 4:115–118
Guillot N (1958) Production by Lactobacillus acidophilus of a substance active against Candida albicans. Ann Inst Pasteur (Paris) 95:194–207
Gupta R (2006) Molecular signatures (unique proteins and conserved indels) that are specific for the epsilon proteobacteria (Campylobacterales). BMC Genom 7:167
Gupta R, Parsi K (2006) Chronic urticaria due to Blastocystis hominis. Australas J Dermatol 47:117–119
Hall IC, O’Toole E (1935) Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 49:390–402
Hardman AM, Stewart GS, Williams P (1998) Quorum sensing and the cell-cell communication dependent regulation of gene expression in pathogenic and non-pathogenic bacteria. Antonie Van Leeuwenhoek 74:199–210
Henning MW (1937) On the variation of the specific phase of Salmonella amersfoort (n.sp.). J Hyg (Lond) 37:561–570
Highet AR, Berry AM, Bettelheim KA, Goldwater PN (2014) Gut microbiome in sudden infant death syndrome (SIDS) differs from that in healthy comparison babies and offers an explanation for the risk factor of prone position. Int J Med Microbiol 304:735–741
Hillman K, Murdoch TA, Spencer RJ, Stewart CS (1994) Inhibition of enterotoxigenic Escherichia coli by the microflora of the porcine ileum, in an in vitro semicontinuous culture system. J Appl Bacteriol 76:294–300
Ho PS, Kwang J, Lee YK (2005) Intragastric administration of Lactobacillus casei expressing transmissible gastroentritis coronavirus spike glycoprotein induced specific antibody production. Vaccine 23:1335–1342
Holländer R (1984) Characterization of Campylobacter jejuni/coli-isolates from human faeces. Zentralbl Bakteriol Mikrobiol Hyg A 258(1):128–134
Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T (2007) Obligate symbiont involved in pest status of host insect. Proc Biol Sci 274:1979–1984
Howard-Jones N (1984) Robert Koch and the cholera vibrio: a centenary. BMJ 288(6414):379–381
Hudault S, Liévin V, Bernet-Camard MF, Servin AL (1997) Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol 63:513–518
Hull RA, Rudy DC, Donovan WH, Wieser IE, Stewart C, Darouiche RO (1999) Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect Immun 67:429–432
Hull R, Rudy D, Donovan W, Svanborg C, Wieser I, Stewart C, Darouiche R (2000) Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J Urol 163:872–877
Humen MA, De Antoni GL, Benyacoub J, Costas ME, Cardozo MI, Kozubsky L, Saudan KY, Boenzli-Bruand A, Blum S, Schiffrin EJ, Pérez PF (2005) Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infect Immun 73:1265–1269
Hummel RP, Maley MP, Miskell PW, Altemeier WA (1975) Suppression of Candida albicans by Escherichia coli. J Trauma 15:413–418
Hussain R, Jaferi W, Zuberi S, Baqai R, Abrar N, Ahmed A, Zaman V (1997) Significantly increased IgG2 subclass antibody levels to Blastocystis hominis in patients with irritable bowel syndrome. Am J Trop Med Hyg 56:301–306
Huys G, Cnockaert M, Janda JM, Swings J (2003) Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimens of Bangladeshi children. Int J Syst Evol Microbiol 53:807–810
Hyma KE, Lacher DW, Nelson AM, Bumbaugh AC, Janda JM, Strockbine NA, Young VB, Whittam TS (2005) Evolutionary genetics of a new pathogenic Escherichia species: Escherichia albertii and related Shigella boydii strains. J Bacteriol 187:619–628
Im E, Pothoulakis C (2010) Recent advances in Saccharomyces boulardii research. Gastroenterol Clin Biol Suppl 1:S62–S70
Isaacs A, Lindenmann J (1957) Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147:258–267
Isenberg HD, Pisano MA, Carito SL, Beckman JI (1960) Factors leading to overt monilial disease. I. Preliminary studies of the ecological relationship between Candida albicans and intestinal bacteria. Antibiot Chemother 10:353–363
Ivec M, Botić T, Koren S, Jakobsen M, Weingartl H, Cencic A (2007) Interactions of macrophages with probiotic bacteria lead to increased antiviral response against vesicular stomatitis virus. Antiviral Res 75:266–274
Jackson RJ, Smith SD, Rowe MI (1990) Selective bowel decontamination results in gram-positive translocation. J Surg Res 48:444–447
Jamal M, Chaudhry WN, Hussain T, Das CR, Andleeb S (2015) Characterization of new Myoviridae bacteriophage WZ1 against multi-drug resistant (MDR) Shigella dysenteriae. J Basic Microbiol 55:420–431
Jahn HU, Ullrich R, Schneider T, Liehr RM, Schieferdecker HL, Holst H, Zeitz M (1996) Immunological and trophical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion 57:95–104
Jennes W, Dicks LM, Verwoerd DJ (2000) Enterocin 012, a bacteriocin produced by Enterococcus gallinarum isolated from the intestinal tract of ostrich. J Appl Microbiol 88:349–357
Jin LZ, Ho YW, Abdullah N, Ali MA, Jalaludin S (1996) Antagonistic effects of intestinal Lactobacillus isolates on pathogens of chicken. Lett Appl Microbiol 23:67–71
Jirán B (1971) Gangrene of the entire large intestine in the seventh month of pregnancy without occlusion of the intestinal vessels. Int Surg 55:361–366
Joelsson A, Liu Z, Zhu J (2006) Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun 74:1141–1147
Jose C, Klein N, Wyss S, Fieck A, Hurwitz I, Durvasula R (2013) Recombinant Arthrobacter β-1, 3-glucanase as a potential effector molecule for paratransgenic control of Chagas disease. Parasit Vectors 6:65
Just I, Selzer J, von Eichel-Streiber C, Aktories K (1995) The low molecular mass GTP-binding protein Rh is affected by toxin a from Clostridium difficile. J Clin Investig 95:1026–1031
Juven BJ, Meinersmann RJ, Stern NJ (1991) Antagonistic effects of lactobacilli and pediococci to control intestinal colonization by human enteropathogens in live poultry. J Appl Bacteriol 70:95–103
Kämpfer P, Lindh JM, Terenius O, Haghdoost S, Falsen E, Busse HJ, Faye I (2006) Thorsellia anophelis gen. nov., sp. nov., a new member of the Gammaproteobacteria. Int J Syst Evol Microbiol 56:335–338
Kamruzzaman M, Robins WP, Bari SM, Nahar S, Mekalanos JJ, Faruque SM (2014) RS1 satellite phage promotes diversity of toxigenic Vibrio cholerae by driving CTX prophage loss and elimination of lysogenic immunity. Infect Immun 82:3636–3643
Kang M, Ju HL, Yu JR, Sohn WM, Na BK (2012) Cryptostatin, a chagasin-family cysteine protease inhibitor of Cryptosporidium parvum. Parasitology 139:1029–1037
Kang HW, Kim JW, Jung TS, Woo GJ (2013) wksl3, a New biocontrol agent for Salmonella enterica serovars enteritidis and typhimurium in foods: characterization, application, sequence analysis, and oral acute toxicity study. Appl Environ Microbiol 79:1956–1968
Kawase M, He F, Kubota A, Harata G, Hiramatsu M (2010) Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett Appl Microbiol 51:6–10
Kendall AI (1902) A proposed classification and method of graphical tabulation of the characters of bacteria. Public Health Pap Rep 28:481–493
Kendall AI (1911) The biology and biochemistry of bacteria and their relation to therapeutics. J Med Res 24:411–424
Kendall AI (1916) The Bacillus carrier and the restaurant. Am J Public Health (NY) 6:726–729
Kendall AI, Walker AW (1910) The isolation of Bacillus dysenteriae from stools. J Med Res 23:481–485
Kieckens E, Rybarczyk J, De Zutter L, Duchateau L, Vanrompay D, Cox E (2015) Clearance of Escherichia coli O157:H7 infection in calves by rectal administration of bovine lactoferrin. Appl Environ Microbiol 81:1644–1651
Kiknadze GP, Siradze TI, Badashvili VA, Menteshashvili NI, Popkhadze TD (1971) Prevention by phage treatment of secondary cases in typhoid foci. Zh Mikrobiol Epidemiol Immunobiol 48:127–130
Kikuchi Y, Kunitoh-Asari A, Hayakawa K, Imai S, Kasuya K, Abe K, Adachi Y, Fukudome S, Takahashi Y, Hachimura S (2014) Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS ONE 9:e86416
Kim M, Ryu S (2012) Spontaneous and transient defence against bacteriophage by phase-variable glucosylation of O-antigen in Salmonella enterica serovar typhimurium. Mol Microbiol 86:411–425
Kim KS, Jenkins MC, Lillehoj HS (1989) Immunization of chickens with live Escherichia coli expressing Eimeria acervulina merozoite recombinant antigen induces partial protection against coccidiosis. Infect Immun 57:2434–2440
Kim DH, Hong SW, Kim BT, Bae EA, Park HY, Han MJ (2000) Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch Pharm Res 23:172–177
Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M et al (2014) Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet 46:1187–1196
Klosterman JA, Small KW (1928) Recent studies on methods of isolating a bacteriophage for Bacillus diphtheriae. J Exp Med 47:121–130
Kneeland Y (1934) The protection afforded by vaccination against secondary invaders during colds in infancy. J Exp Med 60:655–660
Koopman JP, van der Logt JT, Heessen FW, van den Brink ME, Scholten PM, Hectors MP, Nagengast FM (1989) Elimination of murine viral pathogens from the caecal contents of mice by anaerobic preparation. Lab Anim 23:76–80
Kotsyfakis M, Horka H, Salat J, Andersen JF (2010) The crystal structures of two salivary cystatins from the tick and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol Microbiol 77:456–470
Kramer B, Kanof A (1960) Diarrhea in children: a historical review. J Pediatr 57:769–783
Kuroiwa T, Iwanaga M, Kobari K, Higashionna A, Kinjyo F, Saito A (1990) Preventive effect of Clostridium butyricum M588 against the proliferation of Clostridium difficile during antimicrobial therapy. Kansenshogaku Zasshi 64:1425–1432
Kyne L, Warny M, Qamar A, Kelly CP (2000) Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 342:390–397
Langer RC, Riggs MW (1999) Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells. Infect Immun 67:5282–5291
Larson HE, Price AB, Honour P, Borriello SP (1978) Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet 311(8073):1063–1066
Lee P, Abdul-Wahid A, Faubert GM (2009) Comparison of the local immune response against Giardia lamblia cyst wall protein 2 induced by recombinant Lactococcus lactis and Streptococcus gordonii. Microbes Infect 11:20–28
Lee JY, Song SM, Moon EK, Lee YR, Jha BK, Danne DB, Cha HJ, Yu HS, Kong HH, Chung DI, Hong Y (2013) Cysteine protease inhibitor (AcStefin) is required for complete cyst formation of Acanthamoeba. Eukaryot Cell 12:567–574
Lehtoranta L, Kalima K, He L, Lappalainen M, Roivainen M, Närkiö M, Mäkelä M, Siitonen S, Korpela R, Pitkäranta A (2014) Specific probiotics and virological findings in symptomatic conscripts attending military service in Finland. J Clin Virol 60:276–281
Li J, McClane BA (2014) The Sialidases of Clostridium perfringens type D strain CN3718 differ in their properties and sensitivities to inhibitors. Appl Environ Microbiol 80:1701–1709
Liem ND, Pillot J, Lebrun L (1979) Presence of antibodies in human colostral secretory IgA against enteric commensal bacteria: biological implications. Ann Microbiol (Paris) 130:441–448
Lim TH, Lee DH, Lee YN, Park JK, Youn HN, Kim MS, Lee HJ, Yang SY, Cho YW, Lee JB, Park SY, Choi IS, Song CS (2011) Efficacy of bacteriophage therapy on horizontal transmission of Salmonella gallinarum on commercial layer chickens. Avian Dis 55:435–438
Liu YH, Han YP, Li ZY, Wei J, He HJ, Xu CZ, Zheng HQ, Zhan XM, Wu ZD, Lv ZY (2010) Molecular cloning and characterization of cystatin, a cysteine protease inhibitor, from Angiostrongylus cantonensis. Parasitol Res 107:915–922
Liu F, Li G, Wen K, Wu S, Zhang Y, Bui T, Yang X, Kocher J, Sun J, Jortner B, Yuan L (2013) Lactobacillus rhamnosus GG on rotavirus-induced injury of ileal epithelium in gnotobiotic pigs. J Pediatr Gastroenterol Nutr 57:750–758
Loc Carrillo C, Atterbury RJ, El-Shibiny A, Connerton PL, Dillon E, Scott A, Connerton IF (2005) Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl Environ Microbiol 71:6554–6563
Loria RM, Shadoff N, Kibrick S, Broitman S (1976) Maturation of intestinal defenses against peroral infection with group B coxsackievirus in mice. Infect Immun 13:1397–1401
Lüneberg E, Glenn-Calvo E, Hartmann M, Bär W, Frosch M (1998) The central, surface-exposed region of the flagellar hook protein FlgE of Campylobacter jejuni shows hypervariability among strains. J Bacteriol 180:3711–3714
Lyerly DM, Phelps CJ, Toth J, Wilkins TD (1986) Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun 54:70–76
Mahdi NK, Ali NH (2002) Intestinal parasites, including Cryptosporidium species, in Iraqi patients with sickle-cell anaemia. East Mediterr Health J 8:345–349
Mahmoud MS, Saleh WA (2003) Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J Egypt Soc Parasitol 33:13–30
Majamaa H, Isolauri E, Saxelin M, Vesikari T (1995) Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr 20:333–338
Maleki-Ravasan N, Oshaghi MA, Hajikhani S, Saeidi Z, Akhavan AA, Gerami-Shoar M, Shirazi MH, Yakhchali B, Rassi Y, Afshar D (2013) Aerobic microbial community of insectary population of Phlebotomus papatasi. J Arthropod Borne Dis 8:69–81
Mangell P, Thorlacius H, Syk I, Ahrné S, Molin G, Olsson C, Jeppsson B (2012) Lactobacillus plantarum 299v does not reduce enteric bacteria or bacterial translocation in patients undergoing colon resection. Dig Dis Sci 57:1915–1924
Mangen MJ, Havelaar AH, Poppe KP, de Wit GA (2007) Cost-utility analysis to control Campylobacter on chicken meat: dealing with data limitations. Risk Anal 27:815–830
Mansouri MD, Hull RA, Stager CE, Cadle RM, Darouiche RO (2013) In vitro activity and durability of a combination of an antibiofilm and an antibiotic against vascular catheter colonization. Antimicrob Agents Chemother 57:621–625
Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A (2010) Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol 141(Suppl 1):S91–S97
Marranzino G, Villena J, Salva S, Alvarez S (2012) Stimulation of macrophages by immunobiotic Lactobacillus strains: influence beyond the intestinal tract. Microbiol Immunol 56:771–781
Martins FS, Nardi RM, Arantes RM, Rosa CA, Neves MJ, Nicoli JR (2005) Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J Gen Appl Microbiol 51:83–92
Martins FS, Rodrigues AC, Tiago FC, Penna FJ, Rosa CA, Arantes RM, Nardi RM, Neves MJ, Nicoli JR (2007) Saccharomyces cerevisiae strain 905 reduces the translocation of Salmonella enterica serotype Typhimurium and stimulates the immune system in gnotobiotic and conventional mice. J Med Microbiol 56:352–359
Martins FS, Silva AA, Vieira AT, Barbosa FH, Arantes RM, Teixeira MM, Nicoli JR (2009) Comparative study of Bifidobacterium animalis, Escherichia coli, Lactobacillus casei and Saccharomyces boulardii probiotic properties. Arch Microbiol 191:623–630
Mastronicola D, Falabella M, Testa F, Pucillo LP, Teixeira M, Sarti P, Saraiva LM, Giuffrè A (2014) Functional characterization of peroxiredoxins from the human protozoan parasite Giardia intestinalis. PLoS Negl Trop Dis 8(1):e2631
Masuda K, Itoh M, Kawata T (1989) Characterization and reassembly of a regular array in the cell wall of Clostridium difficile GAI 4131. Microbiol Immunol 33:287–298
Matsumoto H (1964) A new serotype of Escherichia coli possessing identical O antigens as Shigella flexneri 2b and variant x, and its serological variation. Jpn J Microbiol 8:143–148
McFarland LV, Bernasconi P (1993) Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis 6:157–171
McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, Bowen KE, Cox JL (1995) Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 90:439–448
McLeod SM, Kimsey HH, Davis BM, Waldor MK (2005) CTXphi and Vibrio cholerae: exploring a newly recognized type of phage-host cell relationship. Mol Microbiol 57:347–356
Mendis L, Kumarasinghe G, Chow C, Liew HY, Ramachandran NP, Jayawardene K, Thong KT, Howe JL, Lim EW, Zaman V (1995) Bacteria, viruses, yeasts and protozoans associated with diarrheal disease in Singapore. Pathology 27:48–52
Merril CR, Biswas B, Carlton R, Jensen NC, Creed GJ, Zullo S, Adhya S (1996) Long-circulating bacteriophage as antibacterial agents. Proc Natl Acad Sci USA 93:3188–3192
Mfuh AM, Larionov OV (2015) Heterocyclic N-oxides—an emerging class of therapeutic agents. Curr Med Chem 22(24):2819
Miller RL, Wang AL, Wang CC (1988) Identification of Giardia lamblia isolates susceptible and resistant to infection by the double-stranded RNA virus. Exp Parasitol 66:118–123
Mital BK, Garg SK (1995) Anticarcinogenic, hypocholesterolemic, and antagonistic activities of Lactobacillus acidophilus. Crit Rev Microbiol 21:175–214
Monsur KA, Rahman MA, Huq F, Islam MN, Northrup RS, Hirschhorn N (1970) Effect of massive doses of bacteriophage on excretion of vibrios, duration of diarrhoea and output of stools in acute cases of cholera. Bull World Health Organ 42:723–732
Montllor CB, Maxmen A, Purcell AH (2002) Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol Entomol 27:189–195
Moran AP, Prendergast MM, Appelmelk BJ (1996) Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol 16:105–115
Morris JA, Park RW (1971) Metabolism of Campylobacter spp. (Vibrio spp.) in connexion with pathogenicity and the use of neuraminidase to suppress pregnancy. Nature 232:132–133
Moshchich PS, Chernyshova LI, Bernasovskaia EP, Sel’nikova OP, Sargsian VP (1989) Prevention of dysbacteriosis in the early neonatal period using a pure culture of acidophilic bacteria. Pediatriia 3:25–30
Mukhopadhyay J, Braig HR, Rowton ED, Ghosh K (2012) Naturally occurring culturable aerobic gut flora of adult Phlebotomus papatasi, vector of Leishmania major in the old world. PLoS ONE 7:e35748
Muñoz JA, Chenoll E, Casinos B, Bataller E, Ramón D, Genovés S, Montava R, Ribes JM, Buesa J, Fàbrega J, Rivero M (2011) Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl Environ Microbiol 77:8775–8783
Murzyn A, Krasowska A, Augustyniak D, Majkowska-Skrobek G, Łukaszewicz M, Dziadkowiec D (2010) The effect of Saccharomyces boulardii on Candida albicans-infected human intestinal cell lines Caco-2 and Intestin 407. FEMS Microbiol Lett 310:17–23
Nakano T, Sugawara M, Kawakami H (2001) Sialic acid in human milk: composition and functions. Acta Paediatr Taiwan 42:11–17
Namkung H, Yu H, Gong J, Leeson S (2011) Antimicrobial activity of butyrate glycerides toward Salmonella typhimurium and Clostridium perfringens. Poult Sci 90:2217–2222
Narayan KG (1982) Food borne infection with Clostridium perfringens type A. Int J Zoonoses 9:12–32
Nasirudeen AM, Tan KS (2005) Programmed cell death in Blastocystis hominis occurs independently of caspase and mitochondrial pathways. Biochimie 87:489–497
Nevskiĭ MV, Potatueva ON, Rakhimov AR, Bgasheva VS, Karpova AN, Ganiev MG (1965) Bacteriophage prophylaxis of typhoid fever in preschool children. Zh Mikrobiol Epidemiol Immunobiol 42:62–63
Newburg DS, Ruiz-Palacios GM, Morrow AL (2005) Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 25:37–58
O’Flynn G, Coffey A, Fitzgerald GF, Ross RP (2006) The newly isolated lytic bacteriophages st104a and st104b are highly virulent against Salmonella enterica. J Appl Microbiol 101:251–259
Ogra PL, Okamoto Y, Freihorst J, LaScolea LJ Jr, Merrick JM (1989) Immunization of the gastrointestinal tract with bacterial and viral antigens: implications in mucosal immunity. Immunol Invest 18:559–570
Oliveira H, Thiagarajan V, Walmagh M, Sillankorva S, Lavigne R, Neves-Petersen MT, Kluskens LD, Azeredo J (2014) A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 9:e108376
Pacini F (1854) Osservazioni microscopiche e deduzioni patologiche sul cholera asiatico (Microscopic observations and pathological deductions on Asiatic cholera). Gazzetta Medica Italiana: Toscana, 2nd series 4:397–401
Pan Z, Zhang X, Geng S, Cheng N, Sun L, Liu B, Huang J, Jiao X (2009) Priming with a DNA vaccine delivered by attenuated Salmonella typhimurium and boosting with a killed vaccine confers protection of chickens against infection with the H9 subtype of avian influenza virus. Vaccine 27:1018–1023
Pant N, Marcotte H, Brüssow H, Svensson L, Hammarström L (2007) Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol 7:86
Parker MT (1969) Postoperative clostridial infections in Britain. Br Med J 3:671–676
Parmeggiani A, Krab IM, Okamura S, Nielsen RC, Nyborg J, Nissen P (2006) Structural basis of the action of pulvomycin and GE2270 A on elongation factor Tu. Biochemistry 45:6846–6857
Pasteur L, Joubert JF (1877) Charbon et septicémie. C r Séanc Acad Sci 85:101–115
Pequignot H, Audebert A, Christoforov B, Duflo B, Feydit P, Guerre J, Millet-Giacon M, Nevot P, Schaeffer A (1973) 2 new cases of septicemia due to Campylobacter fetus. Sem Hop 49:985–990
Pineton de Chambrun G, Colombel JF, Poulain D, Darfeuille-Michaud A (2008) Pathogenic agents in inflammatory bowel diseases. Curr Opin Gastroenterol 24:440–447
Pothoulakis C (2009) Review article: anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment Pharmacol Ther 30:826–833
Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L (2008) A new chance of preventing winter diseases by the administration of synbiotic formulations. J Clin Gastroenterol 42(Suppl 3 Pt 2):S224–S233
Pruteanu M, Shanahan F (2013) Digestion of epithelial tight junction proteins by the commensal Clostridium perfringens. Am J Physiol Gastrointest Liver Physiol 305:G740–G748
Rahimi-Esboei B, Ebrahimzadeh MA, Gholami Sh, Falah-Omrani V (2013) Anti-giardial activity of Sambucus ebulus. Eur Rev Med Pharmacol Sci 17:2047–2050
Ramare F, Nicoli J, Dabard J, Corring T, Ladire M, Gueugneau AM, Raibaud P (1993) Trypsin-dependent production of an antibacterial substance by a human Peptostreptococcus strain in gnotobiotic rats and in vitro. Appl Environ Microbiol 59:2876–2883
Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, Fekete RA, Kar S, Adhya S, Hamer DH (2005) Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci USA 102:11993–11998
Roberts T, Stark D, Harkness J, Ellis J (2014) Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog 6:17
Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR (1996) Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol 81:251–256
Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M (1992) Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc Biol Sci 250:91–98
Roxström-Lindquist K, Palm D, Reiner D, Ringqvist E, Svärd SG (2006) Giardia immunity: an update. Trends Parasitol 22:26–31
Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH (1994) Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049
Saunders JR (1990) Genetic mechanisms for modulating virulence determinants on the bacterial surface. Sci Prog 74:279–290
Scarborough CL, Ferrari J, Godfray HC (2005) Aphid protected from pathogen by endosymbiont. Science 310:1781
Schlein Y, Polacheck I, Yuval B (1985) Mycoses, bacterial infections and antibacterial activity in sandflies (Psychodidae) and their possible role in the transmission of leishmaniasis. Parasitology 90:57–66
Schottmüller (1904) Zur Aetiologie der acuteni Gastroenteritis (Cholera nostras). Afinchener medizinische Wiochenschrift 294–349
Schwarz A, Valdés JJ, Kotsyfakis M (2012) The role of cystatins in tick physiology and blood feeding. Ticks Tick Borne Dis 3:117–127
Sears CL (2005) A dynamic partnership: celebrating our gut flora. Anaerobe 11:247–251
Segal E, Billyard E, So M, Storzbach S, Meyer TF (1985) Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293–300
Selva E, Beretta G, Montanini N, Saddler GS, Gastaldo L, Ferrari P, Lorenzetti R, Landini P, Ripamonti F, Goldstein BP (1991) Antibiotic GE2270 a: a novel inhibitor of bacterial protein synthesis. I. Isolation and characterization. J Antibiot (Tokyo) 44:693–701
Sendid B, Jouault T, Vitse A, Fradin C, Colombel JF, Poulain D (2009) Anti-glycan antibodies establish an unexpected link between C. albicans and Crohn’s disease. Med Sci (Paris) 25:473–481
Sharma A, Dhayal D, Singh OP, Adak T, Bhatnagar RK (2013) Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop 128:41–47
Shilov VM, Lizko NN, Borisova OK, Prokhorov VY (1971) Changes in the microflora of man during long-term confinement. Life Sci Space Res 9:43–49
Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H (2006) Altered gut flora and environment in patients with severe SIRS. J Trauma 60:126–133
Shlim DR, Hoge CW, Rajah R, Rabold JG, Echeverria P (1995) Is Blastocystis hominis a cause of diarrhea in travelers? A prospective controlled study in Nepal. Clin Infect Dis 21:97–101
Silberman JD, Sogin ML, Leipe DD, Clark CG (1996) Human parasite finds taxonomic home. Nature 380:398
Sillankorva S, Pleteneva E, Shaburova O, Santos S, Carvalho C, Azeredo J, Krylov V (2010) Salmonella enteritidis bacteriophage candidates for phage therapy of poultry. J Appl Microbiol 108:1175–1186
Silverman M, Zieg J, Hilmen M, Simon M (1979) Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci USA 76:391–395
Singer SM, Nash TE (2000) The role of normal flora in Giardia lamblia infections in mice. J Infect Dis 181:1510–1512
Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R (1983) Results of bacteriophage treatment of suppurative bacterial infections. II. Detailed evaluation of the results. Arch Immunol Ther Exp (Warsz) 31:293–327
Smith HW, Huggins MB (1983) Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol 129:2659–2675
Smith LD, King EO (1962) Occurrence of Clostridium difficile in infections of man. J Bacteriol 84:65–67
Smith HW, Huggins MB, Shaw KM (1987) The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol 133:1111–1126
Sohnle PG, Collins-Lech C, Wiessner JH (1991) Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis 163:187–192
Stern A, Meyer TF (1987) Common mechanism controlling phase and antigenic variation in pathogenic neisseriae. Mol Microbiol 1:5–12
Sugg JY, Neill JM (1929) Studies on immunological relationships among the pneumococci. III. Relationship between a variety of Saccharomyces cerevisiae and the type II variety of Diplococcus pneumoniae (Pneumococcus). J Exp Med 49:183–193
Tamplin ML, Ahmed MK, Jalali R, Colwell RR (1989) Variation in epitopes of the B subunit of El Tor and classical biotype Vibrio cholerae O1 cholera toxin. J Gen Microbiol 135:1195–1200
Tan KS (2008) New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin Microbiol Rev 21:639–665
Tang L, Li Y (2009) Oral immunization of mice with recombinant Lactococcus lactis expressing porcine transmissible gastroenteritis virus spike glycoprotein. Virus Genes 39:238–245
Taracena ML, Oliveira PL, Almendares O, Umaña C, Lowenberger C, Dotson EM, Paiva-Silva GO, Pennington PM (2015) Genetically modifying the insect gut microbiota to control Chagas disease vectors through systemic RNAi. PLoS Negl Trop Dis 9:e0003358
Tazume S, Ozawa A, Yamamoto T, Takahashi Y, Takeshi K, Saidi SM, Ichoroh CG, Waiyaki PG (1993) Ecological study on the intestinal bacteria flora of patients with diarrhea. Clin Infect Dis Suppl 2:S77–S82
Téllez A, Palm D, Weiland M, Alemán J, Winiecka-Krusnell J, Linder E, Svärd S (2005) Secretory antibodies against Giardia intestinalis in lactating Nicaraguan women. Parasite Immunol 27:163–169
Thapa D, Losa R, Zweifel B, Wallace RJ (2012) Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 158:2870–2877
Thoden JB, Goneau MF, Gilbert M, Holden HM (2013) Structure of a sugar N-formyltransferase from Campylobacter jejuni. Biochemistry 52:6114–6126
Tilton RC, Van Kruiningen HJ, Kwasnik I, Ryan RW (1981) Toxigenic Clostridium perfringens from a parvovirus-infected dog. J Clin Microbiol 14:697–698
Tomat D, Mercanti D, Balagué C, Quiberoni A (2013a) Phage biocontrol of enteropathogenic and Shiga toxin-producing Escherichia coli during milk fermentation. Lett Appl Microbiol 57:3–10
Tomat D, Migliore L, Aquili V, Quiberoni A, Balagué C (2013b) Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli in meat products. Front Cell Infect Microbiol 3:20
Toro H, Price SB, McKee AS, Hoerr FJ, Krehling J, Perdue M, Bauermeister L (2005) Use of bacteriophages in combination with competitive exclusion to reduce Salmonella from infected chickens. Avian Dis 49:118–124
Trautner BW, Darouiche RO, Hull RA, Hull S, Thornby JI (2002) Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J Urol 167:375–379
Trautner BW, Hull RA, Darouiche RO (2003) Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology 61:1059–1062
Trautner BW, Hull RA, Darouiche RO (2005a) Colicins prevent colonization of urinary catheters. J Antimicrob Chemother 56:413–415
Trautner BW, Hull RA, Darouiche RO (2005b) Prevention of catheter-associated urinary tract infection. Curr Opin Infect Dis 18:37–41
Trautner BW, Hull RA, Thornby JI, Darouiche RO (2007) Coating urinary catheters with an avirulent strain of Escherichia coli as a means to establish asymptomatic colonization. Infect Control Hosp Epidemiol 28:92–94
Trautner BW, Cevallos ME, Li H, Riosa S, Hull RA, Hull SI, Tweardy DJ, Darouiche RO (2008) Increased expression of type-1 fimbriae by nonpathogenic Escherichia coli 83972 results in an increased capacity for catheter adherence and bacterial interference. J Infect Dis 198:899–906
Tsuchida T, Koga R, Fukatsu T (2004) Host plant specialization governed by facultative symbiont. Science 303:1989
Urban JF Jr, Hu Y, Miller MM, Scheib U, Yiu YY, Aroian RV (2013) Bacillus thuringiensis-derived Cry5B has potent anthelmintic activity against Ascaris suum. PLoS Negl Trop Dis 7(6):e2263
Ushijima T, Ozaki Y (1986) Potent antagonism of Escherichia coli, Bacteroides ovatus, Fusobacterium varium, and Enterococcus faecalis, alone or in combination, for enteropathogens in anaerobic continuous flow cultures. J Med Microbiol 22:157–163
van Amsterdam K, van Vliet AH, Kusters JG, van der A Ende (2006) Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol Rev 30:131–156
van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-v Lekkerkerk (1971) Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 69:405–411
van Furth R, Guiot HF (1989) Modulation of the host flora. Eur J Clin Microbiol Infect Dis 8:1–7
van Gool T, Dankert J (1995) Human microsporidiosis: clinical, diagnostic and therapeutic aspects of an increasing infection. Clin Microbiol Infect 1:75–85
Van Itallie CM, Betts L, Smedley JG, McClane BA, Anderson JM (2008) Structure of the claudin-binding domain of Clostridium perfringens enterotoxin. J Biol Chem 283:268–274
Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Ley J (1991) Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol 41:88–103
Vandamme P, Dewhirst FE, Paster BJ, On SLW (2006) In: Garrity G, Brenner DJ, Staley JT, Krieg NR, Boone DR, De Vos P, Goodfellow M, Rainey FA, Schleifer K-H (eds) Bergey’s manual of systematic bacteriology: the proteobacteria (Part C), vol 2, 2nd edn. Springer, Berlin, pp 1147–1160
Vandersteegen K, Kropinski AM, Nash JH, Noben JP, Hermans K, Lavigne R (2013) Romulus and Remus, two phage isolates representing a distinct clade within the Twortlikevirus genus, display suitable properties for phage therapy applications. J Virol 87:3237–3247
Variyam EP (1996) Luminal bacteria and proteases together decrease adherence of Entamoeba histolytica trophozoites to Chinese hamster ovary epithelial cells: a novel host defence against an enteric pathogen. Gut 39:521–527
Velasco AC, Mateos ML, Más G, Pedraza A, Díez M, Gutiérrez A (1984) Three-year prospective study of intestinal pathogens in Madrid, Spain. J Clin Microbiol 20:290–292
Vlasova AN, Chattha KS, Kandasamy S, Liu Z, Esseili M, Shao L, Rajashekara G, Saif LJ (2013) Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLoS ONE 8:e76962
Volf P, Kiewegová A, Nemec A (2002) Bacterial colonisation in the gut of Phlebotomus duboseqi (Diptera: Psychodidae): transtadial passage and the role of female diet. Folia Parasitol (Praha) 49:73–77
Vuillemin JP (1890) Antibiosis. Compte Rendu de l’Assoc Française pour l’Avancem des Sci ii, 526 (OED)
Wada N, Nishida N, Iwaki S, Ohi H, Miyawaki T, Taniguchi N, Migita S (1980) Neutralizing activity against Clostridium difficile toxin in the supernatants of cultured colostral cells. Infect Immun 29:545–550
Wang AL, Wang CC (1986) Discovery of a specific double-stranded RNA virus in Giardia lamblia. Mol Biochem Parasitol 21:269–276
Wang AL, Wang CC (1991) Viruses of the protozoa. Annu Rev Microbiol 45:251–263
Wang AL, Miller RL, Wang CC (1988) Antibodies to the Giardia lamblia double-stranded RNA virus major protein can block the viral infection. Mol Biochem Parasitol 30:225–232
Waseh S, Hanifi-Moghaddam P, Coleman R, Masotti M, Ryan S, Foss M, MacKenzie R, Henry M, Szymanski CM, Tanha J (2010) Orally administered P22 phage tailspike protein reduces Salmonella colonization in chickens: prospects of a novel therapy against bacterial infections. PLoS ONE 5:e13904
Watanabe T, Morotomi M, Kawai Y, Mutai M (1977) Reduction of population levels of some indigenous bacteria by lactobacilli in the gastrointestinal tract of gnotobiotic rats. Microbiol Immunol 21:495–503
Weil AJ, Farsetta K, Knaub V (1946) The phase variation of Shigella paradysenteriae (Flexner) type IV. J Immunol 52:221–229
Weinack OM, Snoeyenbos GH, Smyser CF, Soerjadi AS (1982) Reciprocal competitive exclusion of Salmonella and Escherichia coli by native intestinal microflora of the chicken and turkey. Avian Dis 26:585–595
Welch WH (1891) Aneurism with demonstration of bacilli causing air in the tissues, and description of the bacillus. Case report presented at (Nov 2). The Johns Hopkins Hospital Medical Society, Baltimore, MD
Welch WHG, Nuttall GHF (1892) A gas-producing bacillus (Bacillus aerogenes capsulatus, Nov. Spec.) capable of rapid development in the body after death. Bull Johns Hopkins Hosp Baltimore 3:81–91
Wells CL (1990) Relationship between intestinal microecology and the translocation of intestinal bacteria. Antonie Van Leeuwenhoek 58:87–93
Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL (1987) Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun 55:2689–2694
Wen K, Tin C, Wang H, Yang X, Li G, Giri-Rachman E, Kocher J, Bui T, Clark-Deener S, Yuan L (2014) Probiotic Lactobacillus rhamnosus GG enhanced Th1 cellular immunity but did not affect antibody responses in a human gut microbiota transplanted neonatal gnotobiotic pig model. PLoS ONE 9:e94504
Weston RS, Kendall AI (1901) Some common bacteria in American streams, including some new species isolated at New Orleans, Louisiana. Public Health Pap Rep 27:402–407
Wheeler KM, Mickle FL (1945) Antigens of Shigella sonnei. J Immunol 51:257–267
Whipple GH, Stone HB, Bernheim BM (1913) Intestinal obstruction: I. A study of a toxic substance produced in closed duodenal loops. J Exp Med 17:286–306
White BP (1929) A system of bacteriology. Med Res Council (London) 4:86–158
Willers D, Lehmann-Danzinger H, Führer E (1982) Antibacterial and antimycotic effect of a newly discovered secretion from larvae of an endoparasitic insect, Pimpla turionellae L. (hym.). Arch Microbiol 133:225–229
Wilson WJ (1920) The Wilson–Weil–Felix reaction in typhus fever. J Hyg (Lond) 19:115–130
Wilson KH, Silva J, Fekety FR (1981) Suppression of Clostridium difficile by normal hamster cecal flora and prevention of antibiotic-associated cecitis. Infect Immun 34:626–628
Yabuuchi E (2002) Bacillus dysentericus (sic) 1897 was the first rather than Bacillus dysenteriae 1898. Int J Syst Evol Microbiol 52:1041
Yamaguchi N, Sugita R, Miki A, Takemura N, Kawabata J, Watanabe J, Sonoyama K (2006) Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut 55:954–960
Yang XF, Qu XZ, Wang K, Zheng J, Si LS, Dong XP, Wang YL (2005) Construction of prophylactic human papillomavirus type 16 L1 capsid protein vaccine delivered by live attenuated Shigella flexneri strain sh42. Acta Biochim Biophys Sin (Shanghai) 37:743–750
Yurdusev N, Nicolas JL, Ladire M, Ducluzeau R, Raibaud P (1987) Antagonistic effect exerted by three strictly anaerobic strains against various strains of Clostridium perfringens in gnotobiotic rodent intestines. Can J Microbiol 33:226–231
Yurdusev N, Ladire M, Ducluzeau R, Raibaud P (1989) Antagonism exerted by an association of a Bacteroides thetaiotaomicron strain and a Fusobacterium necrogenes strain against Clostridium perfringens in gnotobiotic mice and in fecal suspensions incubated in vitro. Infect Immun 57:724–731
Zanello G, Berri M, Dupont J, Sizaret PY, D’Inca R, Salmon H, Meurens F (2011) Saccharomyces cerevisiae modulates immune gene expressions and inhibits ETEC-mediated ERK1/2 and p38 signaling pathways in intestinal epithelial cells. PLoS ONE 4(6):e18573
Zhao X, Zhang M, Li Z, Frankel FR (2006) Vaginal protection and immunity after oral immunization of mice with a novel vaccine strain of Listeria monocytogenes expressing human immunodeficiency virus type 1 gag. J Virol 80:8880–8890
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Villa, T.G., Sánchez-Pérez, A., Viñas, M. (2016). The Biological Fight Against Pathogenic Bacteria and Protozoa. In: Villa, T., Vinas, M. (eds) New Weapons to Control Bacterial Growth. Springer, Cham. https://doi.org/10.1007/978-3-319-28368-5_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-28368-5_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28366-1
Online ISBN: 978-3-319-28368-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)