Abstract
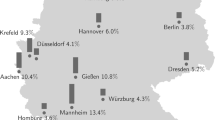
The Omicron variant is associated with increased transmissibility, but evidence about the impact of Omicron in seropositivity of children is limited. This study aims to evaluate SARS-CoV-2 seroprevalence in children during the different variants’ subperiods. A prospective multicenter seroprevalence study was conducted in 7 University public hospitals in Greece from November 2021 to August 2022 (3 subperiods: November 2021-February 2022, March 2022-May 2022, June 2022-August 2022). Children from different age groups, admitted to the hospital or examined in outpatient clinics for reasons other than COVID-19 were enrolled. Neutralizing antibodies (Nabs), anti-Spike (anti-S) and anti-nucleocapsid (anti-N) SARS-CoV-2 IgG in serum were evaluated. A total of 2127 children (males:57,2%; median age:4,8years) were enrolled. Anti-N IgG seropositivity increased from 17,8% in the first sub-period to 40,7% in the second sub-period and then decreased in the third sub-period (36,7%). Anti-S IgG seropositivity appeared to have an increasing trend over the study period, starting from 34,8% and reaching 80,7%. Children aged 1–4 years old have significantly higher anti-N IgG titers compared to children aged 0–1 years old (p < 0,001). Infants have significantly lower anti-S IgG titers compared to all other age groups (p < 0,001). Immunocompromised children and infants have the lowest seropositivity for NAbs.
Conclusions During the Omicron period, seropositivity significantly increased, as a result of higher transmissibility. Neonates and infants have lower antibody titers compared to other age groups, while young children aged 1–4 years old present higher antibody titers, suggesting that this age group may mount a higher antibody response. Continuous surveillance seroprevalence studies are needed in children, in order to identify the true extent of SARS-CoV-2 and guide the planning of adequate public health measures.
What is Known: • Seroprevalence surveys among children may be extremely useful, in order to properly monitor the immunity, either natural or acquired, through the quantification of IgG antibodies and to plan further immunization policies. • There are variations in the seroprevalence of COVID-19 between the different periods, according to the vaccination rates, the type of circulating variant and the transmissibility of the virus. • The Omicron variant is associated with increased transmissibility, but evidence about the impact of Omicron in seropositivity of children is limited. |
|
What is New: • In this large multicenter seroepidemiological study, SARS-CoV-2 seroprevalence rate in children is higher during the Omicron period in comparison to the previous pandemic waves, due to the high transmissibility of the virus and the increased rates of reinfection. • Neonates and infants have lower antibody titers compared to other age groups, while young children aged 1–4 years old present higher antibody titers, indicating that the children of this age group mount a higher antibody response. • This study provides essential information about immunity and the level of protection in the pediatric population, as neutralizing antibodies were evaluated, in addition to the anti-N and anti-S IgG antibodies. |

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- anti-N:
-
anti-Nucleocapsid protein
- anti-S:
-
anti-Spike protein
- CMIA:
-
Chemiluminescent microparticle immunoassay
- COVID-19:
-
Coronavirus disease 2019
- ELISA:
-
Enzyme linked immunoassay
- ER:
-
Emergency room
- GMT:
-
Geometric mean titers
- IgG:
-
Immunoglobulin G
- IQR:
-
Interquartile range
- Nabs:
-
Neutralizing antibodies
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
WHO Coronavirus Disease (COVID-19) Dashboard. A https://covid19.who.int/. Accessed 30 May 2023
Elliott P, Eales O, Steyn N et al (2022) Twin peaks: the Omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science 376:eabq4411
Elliott P, Bodinier B, Eales O et al (2022) Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science 375:1406–1411
Schonfeld D, Fernández H, Ramírez J et al (2022) SARS-CoV-2 seroprevalence in the city of Puerto Madryn: underdiagnosis and relevance of children in the pandemic. PLoS ONE 17:e0263679
Cui X, Zhao Z, Zhang T et al (2021) A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol 93:1057–1069
Williams PCM, Howard-Jones AR, Hsu P et al (2020) SARS-CoV-2 in children: spectrum of disease, transmission and immunopathological underpinnings. Pathology 52:801–808
Peeling RW, Wedderburn CJ, Garcia PJ et al (2020) Serology testing in the COVID-19 pandemic response. Lancet Infect Dis 20:e245–e249
Metcalf CJ, Farrar J, Cutts FT et al (2016) Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 388:728–730
Mayne ES, Scott L, Semete B et al (2020) The role of serological testing in the SARS-CoV-2 outbreak. S Afr Med J 110:842–845
Fenwick C, Croxatto A, Coste AT et al (2021) Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 95:e01828–e01820
Dimopoulou D, Kyritsi M, Dadouli K et al (2023) Seroprevalence of anti-SARS-CoV-2 antibodies among children and their parents in Greece. Eur J Pediatr 182:439–449
Filippatos F, Tatsi EB, Dellis C et al (2022) Seroepidemiology of SARS-CoV-2 in pediatric population during a 16-month period prior to vaccination. J Med Virol 94:2174–2180
Filippatos F, Tatsi EB, Dellis C et al (2022) SARS-CoV-2 seroepidemiology in paediatric population during Delta and Omicron predominance. Epidemiol Infect 150:e177
Naeimi R, Sepidarkish M, Mollalo A et al (2023) SARS-CoV-2 seroprevalence in children worldwide: a systematic review and meta-analysis. EClinicalMedicine 56:101786
Clarke KEN, Jones JM, Deng Y et al (2022) Seroprevalence of infection-induced SARS-CoV-2 antibodies - United States, September 2021-February 2022. MMWR Morb Mortal Wkly Rep 71:606–608
Ahava MJ, Jarva H, Jääskeläinen AJ, Lappalainen M, Vapalahti O, Kurkela S (2022) Rapid increase in SARS-CoV-2 seroprevalence during the emergence of Omicron variant, Finland. Eur J Clin Microbiol Infect Dis 41:997–999
Doucette EJ, Gray J, Fonseca K et al (2023) A longitudinal seroepidemiology study to evaluate antibody response to SARS-CoV-2 virus infection and vaccination in children in Calgary, Canada from July 2020 to April 2022: Alberta COVID-19 childhood cohort (AB3C) study. PLoS ONE 18:e0284046
Sieber J, Mayer M, Schmidthaler K et al (2022) Long-lived immunity in SARS-CoV-2-recovered children and its neutralizing capacity against Omicron. Front Immunol 13:882456
Vahidy FS, Nicolas JC, Meeks JR et al (2020) Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 10:e039849
Saatci D, Ranger TA, Garriga C et al (2021) Association between race and cOVID-19 outcomes among 2.6 million children in England. JAMA Pediatr 175:928–938
Bambra C, Riordan R, Ford J, Matthews F (2020) The COVID-19 pandemic and health inequalities. J Epidemiol Community Health 74:964–968
Tani H, Inasaki N, Yazawa S et al (2023) Neutralizing antibody levels and epidemiological information of patients with breakthrough COVID-19 infection in Toyama, Japan. Jpn J Infect Dis.
Rescigno M, Agrati C, Salvarani C et al (2023) Neutralizing antibodies to Omicron after the fourth SARS-CoV-2 mRNA vaccine dose in immunocompromised patients highlight the need of additional boosters. Front Immunol 14:1104124
Han MS, Um J, Lee EJ et al (2022) Antibody responses to SARS-CoV-2 in children with COVID-19. J Pediatr Infect Dis Soc 11:267–273
Di Chiara C, Cantarutti A, Costenaro P et al (2022) Long-term immune response to SARS-CoV-2 infection among children and adults after mild infection. JAMA Netw Open 5:e2221616
Dunay GA, Barroso M, Woidy M et al (2023) Long-term antibody response to SARS-CoV-2 in children. J Clin Immunol 43:46–56
Yang HS, Costa V, Racine-Brzostek SE et al (2021) Association of age with SARS-CoV-2 antibody response. JAMA Netw Open 4:e214302
Weisberg SP, Connors TJ, Zhu Y et al (2021) Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 22:25–31
Pierce CA, Preston-Hurlburt P, Dai Y et al (2020) Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 12(564):eabd5487
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DD, MT, DS, DK, GL, KR, AN, FD, MT, AK, EE, EV, EP, IZ, AM, KM, ES, MS and PCV. The first draft of the manuscript was written by DD and MT. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institution’s Ethical and Research Committee (approval number:507/20.10.2020).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dimopoulou, D., Sotiri, D., Kousi, D. et al. SARS-CoV-2 seroprevalence among children in Greece during Omicron variant period. Eur J Pediatr 183, 2491–2499 (2024). https://doi.org/10.1007/s00431-024-05486-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05486-7