Abstract
Effective treatment of waste streams such as municipal waste-activated sludge (WAS) presents an opportunity for energy and nutrient recovery, water reclamation, and mitigation of climate change. WAS is a waste product of the activated sludge treatment (AST) process widely used for municipal wastewater. Currently, WAS treatment and disposal account for up to 50% of the total operation cost and 40% of the total greenhouse gas emissions from wastewater treatment plants. Anaerobic digestion (AD) is usually preferred for WAS treatment since it is more economical compared to other existing technologies. The decomposition of sludge during AD releases nutrients, which are then discharged in the anaerobic effluent, polluting recipient water bodies and increasing the nutrient burden. The nutrients, mainly nitrogen (N), phosphorus (P), and potassium (K), can be crystallised into struvite (magnesium ammonium phosphate, NH4MgPO4.6H2O) with numerous agricultural applications as fertilisers. The present review focusses on struvite recovery from anaerobically digested WAS and its potential application for crop production.
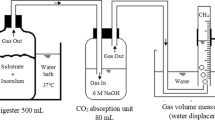
Graphical Abstract

Similar content being viewed by others
Introduction
Vast amounts of water, valuable energy, and agricultural nutrients could be recovered from the ever-growing volume of municipal wastewater produced globally. About 380 billion cubic metres of wastewater is produced annually worldwide with projected increases of 24% and 51%, respectively, by 2030 and 2050 [1]. The major nutrients in the wastewater streams include phosphorus (3.0 Tg), nitrogen (16.6 Tg), and potassium (6.3 Tg) annually (Tg = million metric tons) [2]. Up to around 370 k tons of P are contained in the sludge generated from wastewater treatment plants (WWTP) in Europe [3]. The full energy and nutrient recovery from wastewater could offset up to 13% of the global demand for nutrients in agriculture, reduce overreliance on fossil fuels, and minimise eutrophication [4]. Wastewater is no longer considered waste to be treated and disposed of but is now seen as a resource [5, 6].
There is a high demand for agricultural nutrients such as phosphorus, nitrogen, and potassium [7]. Phosphorus is an important ingredient for life and is a non-renewable resource. Currently, phosphorus is mainly obtained through extractive activities from natural reserves [2]. The majority of phosphorus mineral rock concentrates are found only in a few regions worldwide, including Iraq, Morocco, Algeria, Syria, and China, thus posing a direct threat to global food security [8]. The phosphatic rock extractive peak will be reached in the next 100 years resulting in a decrease in natural reserves [2, 9, 10]. When phosphate reserves decline there will be an increase in the extraction and market prices, and at this point, the industry will be forced to look for alternative sources of the mineral with the already increased demand. Moreover, the alternative nutrient source through nitrogen conversion to fertiliser using the Haber–Bosch process is energy intensive and thus high cost [3, 11,12,13].
Phosphates and other ions present in the wastewater are absorbed and stored in the form of polyphosphates inside the bacterial cells in waste-activated sludge (WAS) during the activated sludge treatment (AST) process. Most wastewater treatment plants utilise anaerobic digestion (AD) for WAS stabilisation, solids reduction, and biogas production [8]. The majority of the phosphorus held in polyphosphates including some of the P contained in the organic matter is released to the supernatant (liquid phase) during the anaerobic digestion process, significantly increasing the phosphate concentration in the final effluent [14]. As a result, the anaerobic supernatant effluent is an ideal stream for phosphorus recovery via struvite precipitation [8]. The continued nutrient loss from wastewater streams poses several environmental threats including the eutrophication of water bodies [3, 11,12,13].
Nutrient recovery from wastewater treatment effluent has attracted considerable research interest [15]. To date recovery treatments such as nitrification–denitrification processes [16], anaerobic ammonium oxidation, ammonium stripping [17], breakpoint chlorination, adsorption, ion exchange, and reverse osmosis [18] have been employed. These methods, however, still come short in terms of sustainability, reduction of pollution load, and the potential to create a circular economy. Struvite crystallisation from wastewater is sustainable and promising for the reclamation of nutrient-rich wastewater [1]. The chemical approach of crystallisation is widely considered since it uses a simple procedure of modifying the physiochemical properties of the solution, transforming the nutrient from soluble to particulate composition [19]. Struvite, which contains nitrogen (N) and phosphorus (P), has piqued the interest of environmentalists and agriculturalists. Struvite has the potential to be used as a plant fertiliser for crops due to its solubility [20]. The current study reviews the potential for struvite recovery from anaerobically digested WAS and its application in crop production. Of special interest is the emphasis on the crystallisation mechanism and the factors affecting the process.
Municipal wastewater and waste-activated sludge
Municipal wastewater (MWW), also known as sewage, includes liquid waste generated from institutions and residences, usually discharged through pipes or sewer systems. The MWW contains a mixture of wastewater generated from different domestic sources including sinks, showers, toilets, and kitchens. MWW is majorly composed of water with a small concentration of dissolved and suspended inorganic and organic matter. Organic matter is majorly constituted by lignin, carbohydrates, fat, proteins, and detergents. In addition to the organic and inorganic matter, bacteria, helminths, protozoa, and pathogenic viruses are likely to be present in raw MWW [21]. To prevent environmental pollution and overcome the increasing pressure on water resources, the reclamation of MWW has been considered. This can be achieved through the widely used conventional municipal wastewater treatment system. There are several stages of treatment for MWW, which are, preliminary, primary, secondary, and tertiary as depicted in Fig. 1 [22]. The preliminary stage includes screening and grit removal where big solids such as rags that would interfere with mechanical equipment are removed. Grit removal ensures the separation of sand-like heavy solids that usually settle in channels, thereby interfering with treatment processes (Fig. 1).
The primary stage of treatment involves sedimentation of the screened and de-gritted wastewater stream to remove settleable solids (particulate pollutants). Up to half of the suspended solids are removed in the primary stage. The residue resulting from the primary treatment is the primary sludge, which is a concentration of suspended particles in water. The biodegradability of primary sludge is high and thus can be easily treated through biodegradation [23]. Although the goal of the primary stage is to separate the readily removable suspended solids, dissolved and colloidal wastes are not sufficiently removed, thus calling for a secondary treatment [22]. The secondary MWW treatment stage follows a biological treatment approach. Microorganisms attached to media (in a “trickling filter” or one of its variations), in suspension (in the activated sludge process) or in ponds or other processes, are used to digest biodegradable organic material/pollutants [24]. Given its high efficiency, low cost, and simple operation, the activated sludge treatment (AST) process is nowadays the most widely used biological secondary treatment method [22].
During the biological treatment in the AST process, microorganisms oxidise part of the organic material leading to the formation of carbon dioxide and other end products, while the remainder provides the materials and energy required by the microorganism community [24, 25]. After the biological treatment, the microorganisms biologically flocculate forming settleable particles (excess biomass), which are separated in the secondary clarifiers as waste-activated sludge (a concentrated suspension also known as trickling filter humus or biological sludge). The generated waste-activated sludge represents about 1–2% of the total treated wastewater volume, a major drawback of the AST system [25,26,27,28,29]. The WAS consists of easily decomposable organic matter, pathogens, heavy metals, and toxic chemicals, thus posing the risk of secondary environmental pollution [30]. The remediation of excess WAS is a major challenge legally, environmentally, and economically [28, 31]. WAS treatment and disposal account for up to 60% of the total operation cost and 40% of the total greenhouse gas (GHG) emissions from wastewater treatment processes [27, 32,33,34].
Waste-activated sludge as a source of nitrogen and phosphorus
Municipal WAS contains a high concentration of organic matter in the form of COD, as shown in Table 1 [35]. Also present in WAS are nutrients such as nitrogen and phosphorus (P) and heavy metals, including zinc, copper, nickel, lead, mercury, and chromium [32]. More than 90% of P in the municipal wastewater influent is usually transferred to the sludge. In modern facilities, off-gases such as N2 are removed during denitrification but may still be present in the sludge in conventional WWTP [12, 36]. After biological treatment, the effluent is directed to the settling tanks where the excess biomass is separated as waste-activated sludge (WAS), which makes up to 2% of the volume of the treated wastewater [37]. The phosphorus-rich WAS is linked to the major issues of high cost, the difficulty of sludge treatment, and limited sludge disposal options [38]. Table 1 shows some of the characteristics (chemical and physical) of WAS which is always seen as a nuisance even though the sludge granules contain nutrients and organic matter in large amounts [37, 39]. It is thus important to develop an efficient, energy-sensitive, cost-effective, environmentally friendly, and sustainable WAS treatment system that would ensure maximum resource recovery with zero-waste generation [10, 37, 40].
Waste-activated sludge treatment through anaerobic digestion
Anaerobic digestion (AD) is widely used for sludge stabilisation, solids reduction, and energy recovery through biogas production [8, 9, 12, 42, 44]. The AD process is multi-step consisting of parallel and series reactions, proceeding in the four successive stages of (i) hydrolysis, (ii) acidogenesis, (iii) acetogenesis, and (iv) methanogenesis as depicted in Fig. 2 [45]. The overall bioconversion process involves direct and indirect interaction between varying groups of bacteria, where the product of one group is the substrate for another group. The AD process not only reduces the amount of sludge that must be disposed of but also generates valuable biomethane gas, improves the dewatering properties of the digested sludge, generates high-quality biosolids for land application, and serves as a carbon source for denitrification [16]. The multiple breakdown steps of the AD process are enabled by the intricate interactions of various bacteria [46]. During the four phases of AD, bacteria feed on the organic contents to produce carbon dioxide and methane in the absence of oxygen [47]. The rate-limiting stage in anaerobic digestion has been determined to be biological hydrolysis [46, 48].
Nutrients release during the anaerobic digestion of waste-activated sludge
The disintegration of the sludge during AD releases nutrients in excessive amounts. The nutrients can easily pollute receiving water bodies leading to algal bloom [41]. Several studies (Table 2) have reported a significant increase in the concentration of phosphorus and nitrogen in the liquid phase during the anaerobic digestion of WAS [9, 10, 40, 49]. Effluents from anaerobically digested sludge have become severe sources of pollution given the high concentration of nutrients, including phosphorus [4]. Phosphorus is not transformed into any gaseous compound, unlike nitrogen which is removed easily as N2 through anammox and denitrification processes [9]. As a result, removing phosphorus from the effluents should be a top focus to preserve water quality and reduce environmental pressure. Moreover, for sustainable development and food security, it is crucial to enhance the recovery of the nutrients contained in WAS [50].
Struvite recovery from anaerobically digested waste-activated sludge
Phosphorus removal techniques have been created using various chemical and biological methods, including metal precipitation, built-in wetland systems, natural nutrient removal techniques, improved biological phosphorus removal techniques, and the struvite crystallisation process [51]. The struvite crystallisation process is the best method since it can simultaneously recover and remove phosphorus from wastewater [12]. Struvite is a crystalline material made up of magnesium (Mg), ammonium (NH4), and phosphate (MgNH4PO4·6H2O) in equimolar concentrations. It is a high-quality fertiliser because of its slow rate of nutrient release, low frequency of application, and low heavy metal concentration, which helps make it environmentally benign [52].
Nucleation and crystal development are the two primary mechanisms that cause struvite to form. To begin, important ions in soluble form in wastewater, such as PO43−, NH4+, and Mg2+, interact with one another to form the seed or nucleus of struvite in the supersaturated solution [53]. After the struvite nuclei have formed, the next step is to keep growing the nuclei until the solution attains a chemical equilibrium, indicating the complete formation of struvite [54, 55]. Equation 1 shows the struvite precipitation reaction in wastewater solution by the Mg:P:N molar ratio of 1:1:1.
Crystallisation is a process of transforming a liquid solution into a solid, by a chemical equilibrium-controlled process, where a supersaturated solution nucleates the solute [56]. Despite its intricacy, crystallisation is frequently used in industrial applications to separate a desired solid phase. There are two stages to this chemical engineering process: nucleation and crystal growth [57]. Crystals development takes place during the stage of nucleation [53]. When ions combine to form the early state of crystals, crystal embryos are generated [58]. In the crystal growth stage, the growth of crystals continues until equilibrium is attained. To crystallise a substance like struvite, the driving force of supersaturation must be met to cause the initial formation of crystals [58].
The initial crystal states of the compounds, anomalies in matter transfer between the aqueous and solid phases, thermodynamics, and reaction kinetics all have an impact on the struvite recovery process. The prediction and control of nucleation and crystal formation are affected by several physical and chemical parameters, including ion concentration, pH, supersaturation level, blending energy, heating rate, and the presence of foreign ions in the solution [59]. In crystallisation processes, supersaturation is a critical parameter [60]. The ratio of ionic activity to the solubility constant determines the saturation. Ionic species activity is determined by the valence of the ions as well as the total ionic strength of the solution [40, 61].
Effect of pH on struvite precipitation
One of the primary factors affecting the struvite crystallisation process is the pH (both NH4+ and PO43− activities are substantially pH dependent); with increasing pH values, PO43− activity increases while NH4+ activity decreases [59, 62]. The pH range between 7.0 and 11.5 is where struvite precipitation is most likely to take place. However, 8 to 9.5 is the ideal pH range for struvite formation. The pH range for the precipitation of struvite and the removal of nutrients is also impacted by interfering ions in the solution [19, 63]. The ideal pH for struvite precipitation was examined based on a batch experiment. The removal efficiency for ammonia N and phosphate P in synthetic and actual wastewaters, respectively, at different pHs are shown in Figs 3(a) and (b). The highest ammonia N and phosphate P removal occurred at pH 9.0 and 11.0, respectively, for synthetic wastewater [Fig 3(a)] [63]. Both ammonia N and phosphate P removal efficiencies relied on the reaction pH. According to Fig. 3(b), the ideal pH range for phosphate P removal was between a pH of 8 and a pH of 10. In both types of wastewater, the highest phosphate P removal efficiency was over 95%, while the maximum ammonia N removal efficiency was much lower in real wastewater because of the high initial concentration of ammonia N. Thus, the pH range of 8.0 to 10.0 can be thought of as ideal for the removal of both ammonia N and phosphate P [20, 63].

(Adapted from Kim et al. [63]).
Ammonia N and P phosphate removal at different pHs for (a) synthetic and (b) real wastewater
The morphology of struvite can also be affected by pH (the Zeta potential can interfere with agglomeration development); therefore, a change in pH can result in a considerable difference in crystal size [64]. Siciliano and De Rosa [65] using anaerobic digestion effluent for struvite recovery found positive results attributed to alkaline conditions with a pH not greater than 9. With a degree of purity above 90% of recovered precipitate, Hao et al. [66] stated a range of pH 9 to 9.5 as the most favourable. As pH continues to increase beyond level 9, the struvite precipitation may be hindered due to the decreased availability of ammonium ions, which are converted into ammonia gas. Also, phosphate ions in the solution notably increase [2]. The optical microscope photographs of the struvite crystals at varied reaction pH levels are shown in Fig. 4 [63]. Larger struvite crystals are formed at higher pH levels leading to higher yields [62, 67, 68].

(Adapted from Kim et al. [63].
Struvite crystals (1000X) formed at (a) pH 8, (b) pH 8.5, and (c) pH 9
The formation of struvite crystals is also influenced by the type of ionic species in the wastewater. The presence of competitive ions (Ca2+, Na+, K+, Al3+, Fe3+, and more), regarding the pH value, has a significant influence on the process of precipitation. For instance, at high calcium (Ca2+) concentrations, the formation of a metastable form of hydroxyapatite could occur at a pH value higher than 10 leading to a low amount of struvite precipitates [69]. In a study by Xu et al. [70], the XRD patterns of struvite recovered from WAS at pHs 8 and 9 matched that of pure struvite, while at pH 10 the pattern had peak deviation indicating the emergence of impurities (Fig. 5). Thus, a specific pH value for struvite formation cannot be easily prescribed, and literature may only be used as a guide. As a result, direct investigations are key in determining the optimal pH value for the removal and recovery of nutrients in the form of struvite.

(Adapted from Xu et al. [70]).
XRD patterns of struvite (pure and precipitates) obtained at different pHs
Effects of molar ratios on struvite formation
Struvite forms when NH4+, PO43, and Mg2+ are present in equimolar concentrations in the waste stream [59]. The struvite precipitation process aimed at the removal and recovery of phosphorus, with an excess of ammonia present, requires the addition of magnesium only making it sustainable. On the other hand, struvite processes aimed at the removal and recovery of ammonia and phosphorus require the addition of phosphorus and magnesium to achieve the stoichiometric molar ratios and are thus more expensive. Despite the requirement for equal molar amounts for struvite formation, reagents ought to be overdosed due to the presence of competitive ions in waste streams. These competitive ions can react with Mg2+ and PO43− ions, reducing their availability for struvite formation [71]. Previous studies have shown that larger crystals take a long time to develop, in days or weeks [72]. Other studies have discovered an ideal mixing intensity when crystal development is rapid and massive crystal shearing is low. As a result, mixing strength influences the formation of crystals and the efficient removal of phosphorus by crystal sedimentation [57].
Adding magnesium ions has little to do with phosphorus elimination. Therefore, it is important to regulate the external addition of phosphate and magnesium to ensure that struvite precipitation from wastewater is feasible [59]. For struvite precipitation, a wide variety of PO43− and Mg2+ ratios have been examined; with the effective ratio often found to be 1:1 or 1:1.2. Although phosphate removal is unaffected when Mg2+:NH4+:PO43− molar ratio is greater than 1.3:1.0:1.0 at pH 9.0 in a full-scale plant, most of the researchers have indicated that the ideal molar ratio of Mg2+:NH4+:PO43− for struvite precipitation is between 1:1:1 and 1.6:1:1 [63, 71].
The removal rate of phosphorus was found to be above 97% when the molar ratio of Mg2+ to PO43− was increased to 1.4:1 during the treatment of simulated wastewater (Fig. 6). When the molar ratio of Mg2+ to PO43− is less than 1.05:1, a combination of MAP and calcium phosphate is formed [19, 20, 71]. The presence of additional competing ions has a significant impact on struvite precipitation. Magnesium and calcium phosphate compounds and carbonates are the principal solid phases that can potentially precipitate alongside struvite [73]. The two primary magnesium phosphate compounds that can precipitate are Newberyite and Bobierrite. Their ability to generate a precipitate, however, is greatly dependent on operating conditions [61].

(Adapted from Kim et al. [63]).
Phosphorus and nitrogen removal according to PO4 3−:Mg.2+ molar ratio for anaerobic digester effluent at pH 9
Effect of temperature on struvite precipitation
Temperature affects struvite solubility in terms of the solubility constant and the rate of reaction and has an impact on crystal development [19, 20, 71]. Struvite precipitation is hindered at high temperatures because the solubility product and the supersaturation state of the solution in which crystals may form are linked. Another consideration is that the ammonia evaporation zone must be avoided. The relative speeds of diffusion surface integration are known to be affected by temperature, which impacts crystal formation [20]. The reaction temperature determines the solubility products and precipitation kinetics of the precipitates in the wastewater. The solubility and kinetics can affect recovery efficiency and potentially induce the simultaneous precipitation of struvite with calcium phosphates [74].
The influence of temperature on struvite precipitation was explored by conducting experiments with saltwater and synthetic refuse water at 20 °C and 30 °C, respectively. A higher P recovery was obtained at the lower temperatures of 20 °C as compared to 30 °C [64]. Increasing the temperature to 30 °C inhibited struvite nucleation at the Mg:P ratio of 1:1 during the 60-min reaction time in the investigation (Fig. 7). As the reaction temperature rises, the solubility product of struvite rises, reducing its supersaturation in solution [20, 64]. At other Mg:P ratios, increasing the temperature from 20 to 30 °C in line with decreasing supersaturation reduced Phosphate recovery percentage by 11% to 28%; however, no adverse effects on product quality were observed [64]. Figure 7 shows that a larger Mg:P molar ratio can compensate for the negative effect of increasing temperature on Phosphate recovery [20, 64, 75].

(Adapted from Shadda et al. [64]).
The effect of temperature on P recovery
Magnesium sources for struvite precipitation
The reagents added depend on the element of interest in terms of removal and recovery from the wastewater effluent. The chemical–physical characteristics and properties of the wastewater effluent dictate the number of reagents to be added [2]. Additional supplementation of alkaline compounds is required for adjusting the pH to the value able to minimise struvite solubility. The amount of alkaline medium to be added depends on the properties of the wastewater and the amount of magnesium and/or phosphorus added. Magnesium and phosphorous compounds tend to be acidic when added to an aqueous solution and, thus, their addition increases the consumption of alkaline compounds [65].
For the formation of struvite precipitates, pure reagents such as MgSO4, MgO, Mg(OH)2, and MgCl2 are commonly used [10]. Salts such as MgSO4 and MgCl2 are very soluble allowing for the recovery of a highly pure precipitate. However, the use of both reagents causes a significant increase in Cl− and SO42− concentrations, consequently negatively affecting the quality of the effluent [18, 76]. Alternatively, MgO does not produce a compromised effluent, however, it has low solubility in water. The solubilisation of MgO through acid dissolution pre-treatment complicates the struvite precipitation process. Phosphorus recovery as vivianite (Fe3(PO4)2⋅8H2O) is gaining traction given the higher economic value and recovery efficiency [3, 77].
Other methods for phosphorus recovery have been exploited in the past, such as electrolysis, and magnesium sacrificial anode as the source of Mg2+ has gained attention as a possible way for struvite precipitation. A high-purity magnesium alloy cast anode is very efficient for struvite precipitation formation of high purity from water solutions. In a novel approach to crystallisation and struvite precipitation of phosphate using pig wastewater, Huang et al. [78] used metal magnesium as an Mg2+ source. In this reaction, Mg0 is oxidised to Mg2+ with the production of H2 and OH−. The corrosion of the metal played two roles supplying Mg2+ and raising the solution pH. The metal dosage affects the efficiency of the process. The process was further improved by air bubbling and the addition of graphite pellets. The presence of graphite accelerates the rate of Mg0 corrosion, and the air bubbling reduces the passivation of the surface of the metal. Most of these practices are costly and thus researchers have sort after low-cost magnesium sources for the benefit of real-life application on a large scale. In particular, magnesite, bittern, seawater, and wood ash have been tested [64, 79, 80].
Benefits of struvite recovery
Through struvite precipitation of the liquid digestate, ammonium and phosphate may be effectively removed from waste streams and recovered as a solid compound, leaving only a negligible trace amount of impurities [2, 19]. Owing to the chemical characteristics of the precipitate produced, it presents a valuable multi-nutrient slow-release fertiliser for vegetable and plant growth. Distinct pH ranges result in different behaviours from struvite. If the pH is seven or above and the environment is neutral to basic, struvite dissolves very slowly [81]. Around plant roots, the soil becomes slightly more acidic (pH 7), thereby increasing the solubility of the struvite [73]. When the plants require the nutrients, the phosphorus, nitrogen, and magnesium in struvite slowly dissolve nearby plant roots and become available for uptake. As a result, when employed as a source of phosphorus for agriculture, struvite has slow-release characteristics in soil.
Struvite has been sprayed on fields or mixed into the ground to enhance crop production [82]. Currently, struvite precipitate is the most recovered compound in pilot and operational facilities in Europe with an estimated 15 000 tons per annum production rate from treating urban wastewater. Industrial wastewater and manure are being evaluated as potential struvite sources. Also, in China, Japan, and the USA significant struvite quantities are produced [83]. Even with the establishment of these applications, struvite recovery is still under continuous development.
Aquatic habitats benefit from wastewater phosphorus and nitrogen precipitation. Excess nitrogen and phosphorus can lead to eutrophication, eventually resulting in dead zones [4]. To thrive, algae require nutrients, majorly phosphorus and nitrogen. However, when either nutrient is in excess, particularly phosphorus, algae formation is higher than required, causing the surface of the water to be covered in algae [84]. The ensuing decomposition of the excess algae consumes oxygen, which may create an oxygen-depleted zone. Other plants and even fish may perish as a result. The quality of the water released back into the environment is improved by removing extra nutrients from wastewater, especially phosphorus and nitrogen [11, 64].
The impact of struvite on sludge disposal is crucial since phosphorus recovery can cut the volume of sludge produced by 49% [20]. If sludge disposal prices rise, phosphorus recovery could emerge to cut costs associated with sludge disposal. Another problem with sludge disposal is spreading more sludge per hectare of land by lowering the amounts of nitrogen and phosphorus in the sludge by controlled struvite precipitation. This is due to the implementation of precise nutrient application restrictions that limit the leaching of nitrates. [19].
Application of struvite on crops
A sustainable sludge management strategy that fosters a circular economy can benefit from struvite recovery [85], as depicted in Fig. 8. Struvite possesses qualities that are comparable to those of conventional ammonium phosphate fertiliser. By growing vegetable crops in pots, several struvite crystalline fertilisers have had their effectiveness assessed [84].
Recovered struvite has shown successful results in cultivating vegetable crops, particularly when used in the appropriate concentrations. Various experts have carried out investigations on the germination and growth of vegetable crops. The findings from studies on crop growth using recovered struvite and other commercial fertilisers are outlined in Table 3. The efficiency of struvite and commercial fertilisers for maize growth is comparable, according to Peeva et al. [82]. The application of carbamide + ammonium nitrate resulted in the maximum yield in the study of maize growth analysis (56.64 kg/ha), whereas the application of struvite alone resulted in a yield of 54.60 kg/ha. When struvite was the only source of phosphate/ammonium, V. radiata demonstrated the best plant development at lower struvite concentrations than other phosphate/ammonium source materials [85]. Thus from these findings, struvite can be produced as a renewable fertiliser from an abundant wastewater source for sustainable circular agricultural development [84].
Conclusion
Waste-activated sludge (WAS) from municipal wastewater treatment plants has significant environmental and operational concerns. Anaerobic digestion (AD) is commonly employed to stabilise the WAS, reduce solids, and recover energy. However, the decomposition of sludge during AD releases nutrients, which are then discharged in the anaerobic digestate, polluting recipient water bodies and increasing the nutrient burden. The nutrients, mainly phosphorus (P) and nitrogen (N) can be crystallised into struvite (magnesium ammonium phosphate, NH4MgPO4.6H2O) with numerous agricultural applications as fertiliser. Optimisation of the influencing variables is key for effective struvite recovery from wastewater streams. Struvite recovery can contribute to a sustainable sludge management approach, which creates a circular economy. In the circular economy, nutrients that are lost can be recycled back into the soil.
References
S. Kumari, S. Jose, M. Tyagi, S. Jagadevan, J. Clean. Prod. (2020). https://doi.org/10.1016/J.JCLEPRO.2020.120037
A. Siciliano, C. Limonti, G.M. Curcio, R. Molinari, Sustain 12, 18 (2020). https://doi.org/10.3390/su12187538
Y. Wang, K. Zheng, H. Guo, Y. Tong, T. Zhu, Y. Liu, Bioresour. Technol. (2022). https://doi.org/10.1016/j.biortech.2021.126045
D. Wang, X. Li, Y. Ding, T. Zeng, G. Zeng, Recent Pat. Food. Nutr. Agric. 1, 3 (2010). https://doi.org/10.2174/1876142910901030236
M. Qadir et al., Nat. Resour. Forum 44, 1 (2020). https://doi.org/10.1111/1477-8947.12187
M. Montwedi et al., J. Water Process Eng. (2021). https://doi.org/10.1016/j.jwpe.2021.101978
X. Meng, X. Liu, Q. Huang, H. Gao, K. Tay, J. Yan, Waste Manag. (2019). https://doi.org/10.1016/j.wasman.2019.04.045
F. Zeng, Q. Zhao, W. Jin, Y. Liu, K. Wang, D.J. Lee, Chem. Eng. J (2018). https://doi.org/10.1016/j.cej.2018.03.088
L. Li, H. Pang, J. He, J. Zhang, Chem. Eng. J (2019). https://doi.org/10.1016/j.cej.2019.05.146
X. Liu et al., Int. Biodeterior. Biodegrad. 148, 19 (2020). https://doi.org/10.1016/j.ibiod.2019.104878
T. Dalecha, E. Assefa, K. Krasteva, and G. Langergraber Fms2 (2012).
D. Lorick, B. Macura, M. Ahlström, A. Grimvall, R. Harder, Environ. Evid. 9, 1 (2020). https://doi.org/10.1186/s13750-020-00211-x
T.C. Lim, C. Welty, Front. Built Environ. (2018). https://doi.org/10.3389/fbuil.2018.00071
N. Krishnamoorthy, J. Clean. Prod (2021). https://doi.org/10.1016/j.jclepro.2022.131737
M. Marcińczyk, Y.S. Ok, P. Oleszczuk, Chemosphere (2022). https://doi.org/10.1016/j.chemosphere.2022.135310
B.J. Ni et al., CHAPTER 16: denitrification processes for wastewater treatment, 2017-Janua, 9. R. Soc. Chem. (2017). https://doi.org/10.1039/9781782623762-00368
C. Rodríguez, J. Cisternas, J. Serrano, E. Leiva, Water (Switzerland) (2021). https://doi.org/10.3390/w13233462
I. Kabdaşlı, O. Tünay, Environ. Technol. Rev. (2018). https://doi.org/10.1080/21622515.2018.1473504
I. Kabdaşlı, S. Kuşçuoğlu, O. Tünay, A. Siciliano, Sustainability (2022). https://doi.org/10.3390/su14031082
C. González-Morales, B. Fernández, F.J. Molina, D. Naranjo-Fernández, A. Matamoros-Veloza, M.A. Camargo-Valero, Sustainability (2021). https://doi.org/10.3390/su131910730
H. Shivaraju Int. J. Environ. Sci. 1, 5 (2011), [Online]. Available: http://www.indianjournals.com/ijor.aspx?target=ijor:ijes&volume=1&issue=5&article=020
R. Hreiz, M.A. Latifi, N. Roche, Chem. Eng. J (2015). https://doi.org/10.1016/J.CEJ.2015.06.125
Q. Wang et al., Sci. Total Environ (2017). https://doi.org/10.1016/j.scitotenv.2017.02.203
V. Naidoo PhD Thesis Submitt. to Univ. Natal (1999).
G. Zhen, X. Lu, H. Kato, Y. Zhao, Y.-Y.Y. Li, Renew. Sustain. Energy Rev. (2017). https://doi.org/10.1016/j.rser.2016.11.187
J. Abelleira, S.I.I. Pérez-Elvira, J. Sánchez-Oneto, J.R.R. Portela, E. Nebot, Resour Conserv. Recycl. (2012). https://doi.org/10.1016/J.RESCONREC.2011.03.008
J. Park et al., Bioresour Technol (2022). https://doi.org/10.1016/J.BIORTECH.2021.126594
G. Manterola, I. Uriarte, L. Sancho, Water Re. (2008). https://doi.org/10.1016/j.watres.2008.03.014
B. Otieno, M. Khune, J. Kabuba, P. Osifo, Phys. Sci. Rev (2023). https://doi.org/10.1515/psr-2022-0340
H. Yuan, B. Yu, P. Cheng, N. Zhu, C. Yin, L. Ying, Int. Biodeterior. Biodegrad (2016). https://doi.org/10.1016/j.ibiod.2016.04.001
X. Sun et al., Sci. Total Environ. (2022). https://doi.org/10.1016/J.SCITOTENV.2021.150773
M. Anjum, N.H. Al-Makishah, M.A. Barakat, Process Saf. Environ. Prot (2016). https://doi.org/10.1016/J.PSEP.2016.05.022
V. Naddeo, V. Belgiorno, M. Landi, T. Zarra, R.M.A. Napoli, Desalination (2009). https://doi.org/10.1016/j.desal.2009.02.061
G.B. Kim et al., Energy (2022). https://doi.org/10.1016/j.energy.2022.124345
L. Shao, T. Wang, T. Li, F. Lü, P. He, Bioresour. Technol. (2013). https://doi.org/10.1016/j.biortech.2013.04.081
J. Peccia, P. Westerhoff, Environ. Sci. Technol. (2015). https://doi.org/10.1021/acs.est.5b01931
B. Otieno, S. Apollo, J. Kabuba, B. Naidoo, G. Simate, A. Ochieng, J. Environ. Chem. Eng (2019). https://doi.org/10.1016/j.jece.2019.102945
B. Zhang, Z. Zhao, N. Chen, C. Feng, Z. Lei, Z. Zhang, Water Res. (2020). https://doi.org/10.1016/j.watres.2020.116427
M. Kuglarz, K. Grübel, J. Bohdziewicz, Arch. Environ. Prot. (2014). https://doi.org/10.2478/aep-2014-0030
Y. Xu, Q. Zhou, X. Wang, M. Yang, Y. Fang, Y. Lu, Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2020.129391
G. Lee, I. Lee, J.I. Han, J. Environ. Chem. Eng (2019). https://doi.org/10.1016/j.jece.2019.103329
H. Hou et al., Sci. Total Environ. (2020). https://doi.org/10.1016/j.scitotenv.2019.135274
M.A. Latif, C.M. Mehta, D.J. Batstone, Water Res. (2015). https://doi.org/10.1016/j.watres.2015.05.062
B. Otieno, A. Ochieng, J. Energy South. Africa (2018). https://doi.org/10.17159/2413-3051/2018/v29i1a3379
K.F. Adekunle, J.A. Okolie, Adv. Biosci. Biotechnol. (2015). https://doi.org/10.4236/abb.2015.63020
C. Chen et al., Renew. Energy (2016). https://doi.org/10.1016/j.renene.2016.03.095
J. Filer, H.H. Ding, S. Chang, Water (Switzerland) (2019). https://doi.org/10.3390/w11050921
T. Fenchel, G. M. King, and T. H. Blackburn in Bacterial Biogeochemistry (Third Edition), In: T. Fenchel, G. M. King, and T. H. Blackburn, (eds.), Third Edit.Boston: Academic Press, 2012. https://doi.org/10.1016/B978-0-12-415836-8.00001-3.
X. Cheng, B. Chen, Y. Cui, D. Sun, X. Wang, Sep. Purif. Technol. (2015). https://doi.org/10.1016/j.seppur.2015.01.002
G.U. Semblante, F.I. Hai, D.D. Dionysiou, K. Fukushi, W.E. Price, L.D. Nghiem, J. Environ. Manage (2017). https://doi.org/10.1016/j.jenvman.2016.10.022
B. Saerens, S. Geerts, M. Weemaes, J. Environ. Manage. (2021). https://doi.org/10.1016/j.jenvman.2020.111743
H. Xu et al., Sci. Total Environ. (2022). https://doi.org/10.1016/j.scitotenv.2022.154110
Y.H. Liu, J.H. Kwag, J.H. Kim, C.S. Ra, Desalination (2011). https://doi.org/10.1016/j.desal.2011.04.056
Z. Yin, Y. Fu, Q. Chen, E3S Web of Conf (2019). https://doi.org/10.1051/e3sconf/201911804031
K.P. Fattah, Int. J. Environ. Sci. Dev. (2012). https://doi.org/10.7763/ijesd.2012.v3.284
S. Harcum in Biologically Inspired Textiles, A. Abbott and M. Ellison, Eds., in Woodhead Publishing Series in Textiles. Woodhead Publishing, 2008. https://doi.org/10.1533/9781845695088.1.26.
M.I.H. Bhuiyan, D.S. Mavinic, F.A. Koch, Water Sci. Technol. (2008). https://doi.org/10.2166/wst.2008.002
T. Nakamuro, M. Sakakibara, H. Nada, K. Harano, E. Nakamura, J. Am. Chem. Soc. 143(4), 1763–1767 (2021). https://doi.org/10.1021/jacs.0c12100
Y. Wang, L.P. Qiu, M.F. Hu, IOP Conf. Ser. Mater. Sci. Eng. (2018). https://doi.org/10.1088/1757-899X/392/3/032032
A. Capdevielle, E. Sýkorová, B. Biscans, F. Béline, M.L. Daumer, J. Hazard. Mater. (2013). https://doi.org/10.1016/j.jhazmat.2012.11.054
N.Y. Acelas, E. Flórez, D. López, Desalin. Water Treat. (2015). https://doi.org/10.1080/19443994.2014.902337
J. Wang, J.G. Burken, X. Zhang, R. Surampalli, J. Environ. Eng. (2005). https://doi.org/10.1061/(asce)0733-9372(2005)131:10(1433)
D. Kim, K.J. Min, K. Lee, M.S. Yu, K.Y. Park, Environ. Eng. Res. (2017). https://doi.org/10.4491/eer.2016.037
S. Shaddel, T. Grini, S. Ucar, K. Azrague, J.P. Andreassen, S.W. Østerhus, Water Res. (2020). https://doi.org/10.1016/j.watres.2020.115572
A. Siciliano, S. De Rosa, Environ. Technol. (2014). https://doi.org/10.1080/09593330.2013.853088
X. Hao, C. Wang, M.C.M. Van Loosdrecht, Y. Hu, Environ. Sci. Technol. (2013). https://doi.org/10.1021/es401140s
Z. Bradford-Hartke, A. Razmjou, L. Gregory, Desalination (2021). https://doi.org/10.1016/j.desal.2021.114949
M.M. Thant Zin, D.J. Kim, Process Saf. Environ. Prot. (2019). https://doi.org/10.1016/j.psep.2019.04.018
H.-D. Ryu, Y.-D. Choo, M.-K. Kang, S.-I. Lee, Environ. Eng. Sci. (2014). https://doi.org/10.1089/ees.2013.0313
H. Xu et al., Process Saf. Environ. Prot. (2021). https://doi.org/10.1016/j.psep.2021.01.033
E. Qoku, M. Scheibel, T. Bier, A. Gerz, Constr. Build. Mater. (2021). https://doi.org/10.1016/j.conbuildmat.2020.121654
S. Dhakal, J. Environ. Eng. (2008). https://doi.org/10.2175/193864708790893594
S. Daneshgar, P.A. Vanrolleghem, C. Vaneeckhaute, A. Buttafava, A.G. Capodaglio, Sci. Total Environ. (2019). https://doi.org/10.1016/j.scitotenv.2019.03.055
M. Hanhoun, L. Montastruc, C. Azzaro-Pantel, B. Biscans, M. Frèche, L. Pibouleau, Chem. Eng. J. (2011). https://doi.org/10.1016/j.cej.2010.12.001
Y.H. Song et al., J. Hazard. Mater. 190, 1 (2011). https://doi.org/10.1016/j.jhazmat.2011.03.015
S. Kataki, H. West, M. Clarke, D.C. Baruah, Resour. Conserv. Recycl. (2016). https://doi.org/10.1016/j.resconrec.2015.12.009
X. Hao, W. Yu, T. Yuan, Y. Wu, M.C.M. van Loosdrecht, Water Res. (2022). https://doi.org/10.1016/j.watres.2022.118976
H. Huang, D. Xiao, J. Liu, L. Hou, L. Ding, Sci. Rep. (2015). https://doi.org/10.1038/srep10183
H.M. Huang, X.M. Xiao, L.P. Yang, B. Yan, Water Pract. Technol. (2010). https://doi.org/10.2166/wpt.2010.007
S.I. Lee, S.Y. Weon, C.W. Lee, B. Koopman, Chemosphere 51, 4 (2003). https://doi.org/10.1016/S0045-6535(02)00807-X
K.P. Fattah, Y. Zhang, D.S. Mavinic, F.A. Koch, Can. J. Civ. Eng. (2010). https://doi.org/10.1139/L10-055
G. Peeva, H. Yemendzhiev, R. Koleva, V. Nenov, J. Agric. Chem. Environ. (2021). https://doi.org/10.4236/jacen.2021.102014
D. Huygens, H. G. M. Saveyn, D. Tonini, P. Eder, and L. Delgado Sancho Technical proposals for selected new fertilising materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009)—Process and quality criteria, and assessment of environmental and market impacts for precipitated phosphate salts & derivate. 2019. doi: https://doi.org/10.2760/186684.
K.J. Min, D. Kim, J. Lee, K. Lee, K.Y. Park, Environ. Sci. Pollut. Res. (2019). https://doi.org/10.1007/s11356-019-05522-2
M. Prabhu, S. Mutnuri, Int. J. Recycl. Org. Waste Agric. (2014). https://doi.org/10.1007/s40093-014-0049-z
Funding
Open access funding provided by Vaal University of Technology. The research of this article was supported by the Water Research Commission (WRC, Project no. C2020/2021–00426) of South Africa and the German Academic Exchange Service (DAAD) within the framework of the climapAfrica programme with funds from the Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing or conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otieno, B., Funani, C.K., Khune, S.M. et al. Struvite recovery from anaerobically digested waste-activated sludge: A short review. Journal of Materials Research 38, 3815–3826 (2023). https://doi.org/10.1557/s43578-023-01108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01108-4