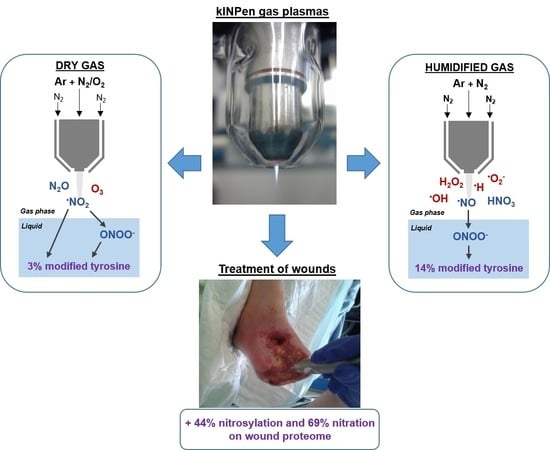
On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Model Solutions
2.1.2. Wound Exudates
2.2. Cold Plasma Treatments and Incubation with Control Oxidants
2.3. Cold Plasma-Induced Modifications of Tyrosine
2.3.1. Qualitative Screening via Direct Infusion High-Resolution Mass Spectrometry
2.3.2. HPLC-MS2 Quantitation of Tyrosine and 3-Nitrotyrosine
2.3.3. Data Analysis and Visualization
2.4. Characterization of Plasma-Induced Protein Modifications in Wound Exudates
3. Results and Discussion
3.1. Tyrosine Modification Induced by Plasma-Generated Reactive Oxygen and Nitrogen Species
3.2. Gas Composition: A Crucial Parameter to Regulate the NOx Generation
3.3. Tyrosine Modification Induced by Control Oxidants
3.4. Both Gas Phase- and Liquid Phase-Derived Species Contribute to Tyrosine Modification
3.5. Identification of Plasma-Derived Reactive Nitrogen Species
3.6. Plasma Induces Oxidative and Nitrosative Protein Modifications in Wounds
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef] [PubMed]
- Törnvall, U. Pinpointing oxidative modifications in proteins—Recent advances in analytical methods. Anal. Methods 2010, 2. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yan, L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013, 1, 15–26. [Google Scholar] [PubMed]
- Halliwell, B. Free Radicals and Other Reactive Species in Disease. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015, 240, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29 Pt 2, 345–350. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci. Technol. 2012, 21, 043001. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Semmler, M.L.; Bekeschus, S.; Schafer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Pasqual-Melo, G.; Sagwal, S.K.; Freund, E.; Gandhirajan, R.K.; Frey, B.; von Woedtke, T.; Gaipl, U.; Bekeschus, S. Combination of Gas Plasma and Radiotherapy Has Immunostimulatory Potential and Additive Toxicity in Murine Melanoma Cells in Vitro. Int. J. Mol. Sci. 2020, 21, 1379. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemiere, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [Green Version]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs. Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Bekeschus, S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants 2018, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preissner, S.; Kastner, I.; Schutte, E.; Hartwig, S.; Schmidt-Westhausen, A.M.; Paris, S.; Preissner, R.; Hertel, M. Adjuvant antifungal therapy using tissue tolerable plasma on oral mucosa and removable dentures in oral candidiasis patients: A randomised double-blinded split-mouth pilot study. Mycoses 2016, 59, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, H.; Santos Sousa, J.; Weltmann, K.D.; Wende, K.; Reuter, S. Quantification of the ozone and singlet delta oxygen produced in gas and liquid phases by a non-thermal atmospheric plasma with relevance for medical treatment. Sci. Rep. 2018, 8, 12195. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Heusler, T.; Lackmann, J.-W.; von Woedtke, T.; Weltmann, K.-D.; Wende, K. Cold physical plasma-induced oxidation of cysteine yields reactive sulfur species (RSS). Clin. Plasma Med. 2019, 14, 100083. [Google Scholar] [CrossRef]
- Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S. Chemistry and biochemistry of cold physical plasma derived reactive species in liquids. Biol. Chem. 2018, 400, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; Williams, P.; Dalluge, J.; Gaens, W.V.; Aboubakr, H.; Bischof, J.; von Woedtke, T.; Goyal, S.M.; Weltmann, K.D.; Bogaerts, A.; et al. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 2015, 10, 029518. [Google Scholar] [CrossRef] [Green Version]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Bruno, G.; Jablonowski, H.; Kogelheide, F.; Offerhaus, B.; Held, J.; Schulz-von der Gathen, V.; Stapelmann, K.; von Woedtke, T.; Wende, K. Nitrosylation vs. oxidation—How to modulate cold physical plasmas for biological applications. PLoS ONE 2019, 14, e0216606. [Google Scholar] [CrossRef] [Green Version]
- Lackmann, J.W.; Baldus, S.; Steinborn, E.; Edengeiser, E.; Kogelheide, F.; Langklotz, S.; Schneider, S.; Leichert, L.I.O.; Benedikt, J.; Awakowicz, P.; et al. A dielectric barrier discharge terminally inactivates RNase A by oxidizing sulfur-containing amino acids and breaking structural disulfide bonds. J. Phys. D Appl. Phys. 2015, 48, 494003. [Google Scholar] [CrossRef]
- Striesow, J.; Lackmann, J.W.; Ni, Z.; Wenske, S.; Weltmann, K.D.; Fedorova, M.; von Woedtke, T.; Wende, K. Oxidative modification of skin lipids by cold atmospheric plasma (CAP): A standardizable approach using RP-LC/MS(2) and DI-ESI/MS(2). Chem. Phys. Lipids 2020, 226, 104786. [Google Scholar] [CrossRef] [PubMed]
- Lackmann, J.W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhou, R.; Zhuang, J.; Zong, Z.; Zhang, X.; Liu, D.; Bazaka, K.; Ostrikov, K. Interaction of Atmospheric-Pressure Air Microplasmas with Amino Acids as Fundamental Processes in Aqueous Solution. PLoS ONE 2016, 11, e0155584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, E.; Kitamura, T.; Kuwabara, J.; Ikawa, S.; Yoshizawa, S.; Shiraki, K.; Kawasaki, H.; Arakawa, R.; Kitano, K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J. Phys. D Appl. Phys. 2014, 47, 285403. [Google Scholar] [CrossRef]
- Wende, K.; Bruno, G.; Lalk, M.; Weltmann, K.-D.; von Woedtke, T.; Bekeschus, S.; Lackmann, J.-W. On a heavy path—Determining cold plasma-derived short-lived species chemistry using isotopic labelling. RSC Adv. 2020, 10, 11598–11607. [Google Scholar] [CrossRef]
- Benedikt, J.; Mokhtar Hefny, M.; Shaw, A.; Buckley, B.R.; Iza, F.; Schakermann, S.; Bandow, J.E. The fate of plasma-generated oxygen atoms in aqueous solutions: Non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O(aq). Phys. Chem. Chem. Phys. 2018, 20, 12037–12042. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rodder, K.; Jablonowski, H.; Schmidt, A.; Woedtke, T.V.; Weltmann, K.D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.; Tresp, H.; Hammer, M.U.; Iseni, S.; Kupsch, S.; Schmidt-Bleker, A.; Wende, K.; Dunnbier, M.; Masur, K.; Weltmannan, K.D.; et al. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Reuter, S.; Winter, J.; Iseni, S.; Schmidt-Bleker, A.; Dunnbier, M.; Masur, K.; Wende, K.; Weltmann, K.D. The Influence of Feed Gas Humidity Versus Ambient Humidity on Atmospheric Pressure Plasma Jet-Effluent Chemistry and Skin Cell Viability. IEEE Trans. Plasma Sci. 2015, 43, 3185–3192. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Bansemer, R.; Reuter, S.; Weltmann, K.-D. How to produce an NOx- instead of Ox-based chemistry with a cold atmospheric plasma jet. Plasma Process Polym. 2016, 13, 1120–1127. [Google Scholar] [CrossRef]
- Schmidt-Bleker, A.; Winter, J.; Bosel, A.; Reuter, S.; Weltmann, K.D. On the plasma chemistry of a cold atmospheric argon plasma jet with shielding gas device. Plasma Sources Sci. Technol. 2016, 25, 015005. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Naïtali, M.; Herry, J.-M.; Hnatiuc, E.; Kamgang, G.; Brisset, J.-L. Kinetics and Bacterial Inactivation Induced by Peroxynitrite in Electric Discharges in Air. Plasma Chem. Plasma Process. 2012, 32, 675–692. [Google Scholar] [CrossRef]
- Yamashiro, R.; Misawa, T.; Sakudo, A. Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci. Rep. 2018, 8, 17947. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hugel, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Emmert, S.; Brehmer, F.; Haenßle, H.; Helmke, A.; Mertens, N.; Ahmed, R.; Simon, D.; Wandke, D.; Maus-Friedrichs, W.; Daeschlein, G.; et al. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clin. Plasma Med. 2013, 1, 24–29. [Google Scholar] [CrossRef]
- van Gils, C.A.J.; Hofmann, S.; Boekema, B.K.H.L.; Brandenburg, R.; Bruggeman, P.J. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46, 175203. [Google Scholar] [CrossRef]
- Bekeschus, S.; Freund, E.; Spadola, C.; Privat-Maldonado, A.; Hackbarth, C.; Bogaerts, A.; Schmidt, A.; Wende, K.; Weltmann, K.D.; von Woedtke, T.; et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers 2019, 11, 1237. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; von Woedtke, T.; Weltmann, K.-D.; Metelmann, H.-R. Plasma, Cancer, Immunity. Clin. Plasma Med. 2018, 9, 13–14. [Google Scholar] [CrossRef]
- Bekeschus, S.; Favia, P.; Robert, E.; von Woedtke, T. White paper on plasma for medicine and hygiene: Future in plasma health sciences. Plasma Process Polym. 2019, 16, 1800033. [Google Scholar] [CrossRef] [Green Version]
- Shekhter, A.B.; Pekshev, A.V.; Vagapov, A.B.; Butenko, A.V.; Fayzullin, A.L.; Rudenko, T.G.; Sharapov, N.A.; Serejnikova, N.B.; Vasilets, V.N. Dose-dependent effect of plasma-chemical NO-containing gas flow on wound healing. An experimental study. Clin. Plasma Med. 2020, 19–20. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Broker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Ikawa, S.; Tani, A.; Nakashima, Y.; Kitano, K. Physicochemical properties of bactericidal plasma-treated water. J. Phys. D Appl. Phys. 2016, 49, 425401. [Google Scholar] [CrossRef]
- Girard, F.; Badets, V.; Blanc, S.; Gazeli, K.; Marlin, L.; Authier, L.; Svarnas, P.; Sojic, N.; Clement, F.; Arbault, S. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv. 2016, 6, 78457–78467. [Google Scholar] [CrossRef]
- Breen, C.; Pal, R.; Elsegood, M.R.J.; Teat, S.J.; Iza, F.; Wende, K.; Buckley, B.R.; Butler, S.J. Time-resolved luminescence detection of peroxynitrite using a reactivity-based lanthanide probe. Chem. Sci. 2020, 11, 3164–3170. [Google Scholar] [CrossRef] [Green Version]
- Calcerrada, P.; Peluffo, G.; Radi, R. Nitric oxide-derived oxidants with a focus on peroxynitrite: Molecular targets, cellular responses and therapeutic implications. Curr. Pharm. Des. 2011, 17, 3905–3932. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Ischiropoulos, H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003, 305, 776–783. [Google Scholar] [CrossRef]
- Betts, M.J.; Russell, R.B. Amino-Acid Properties and Consequences of Substitutions. In Bioinformatics for Geneticists; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 311–342. [Google Scholar]
- Bartesaghi, S.; Peluffo, G.; Zhang, H.; Joseph, J.; Kalyanaraman, B.; Radi, R. Tyrosine nitration, dimerization, and hydroxylation by peroxynitrite in membranes as studied by the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester. Methods Enzymol. 2008, 441, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Radi, R. Protein and lipid nitration: Role in redox signaling and injury. Biochim. Biophys. Acta 2008, 1780, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, T.R.; Garcia-Rama, C.; Tarin, C.; Saura, M.; Calvo, E.; Lopez, J.A.; Lopez-Otin, C.; Folgueras, A.R.; Lamas, S.; Zaragoza, C. Nitric oxide elicits functional MMP-13 protein-tyrosine nitration during wound repair. FASEB J. 2008, 22, 3207–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakovlev, V.A.; Bayden, A.S.; Graves, P.R.; Kellogg, G.E.; Mikkelsen, R.B. Nitration of the tumor suppressor protein p53 at tyrosine 327 promotes p53 oligomerization and activation. Biochemistry 2010, 49, 5331–5339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballal, S.; Bartesaghi, S.; Radi, R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim. Biophys. Acta 2014, 1840, 768–780. [Google Scholar] [CrossRef] [Green Version]
- Abaffy, P.; Tomankova, S.; Naraine, R.; Kubista, M.; Sindelka, R. The role of nitric oxide during embryonic wound healing. BMC Genom. 2019, 20, 815. [Google Scholar] [CrossRef]
- Kitano, T.; Yamada, H.; Kida, M.; Okada, Y.; Saika, S.; Yoshida, M. Impaired Healing of a Cutaneous Wound in an Inducible Nitric Oxide Synthase-Knockout Mouse. Dermatol. Res. Pract. 2017, 2017, 2184040. [Google Scholar] [CrossRef] [Green Version]
- Sekar, Y.; Moon, T.C.; Slupsky, C.M.; Befus, A.D. Protein tyrosine nitration of aldolase in mast cells: A plausible pathway in nitric oxide-mediated regulation of mast cell function. J. Immunol. 2010, 185, 578–587. [Google Scholar] [CrossRef]
- Masters, K.S.; Leibovich, S.J.; Belem, P.; West, J.L.; Poole-Warren, L.A. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen. 2002, 10, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, S.; Doll, C.; Voss, J.O.; Hertel, M.; Preissner, S.; Raguse, J.D. Treatment of Wound Healing Disorders of Radial Forearm Free Flap Donor Sites Using Cold Atmospheric Plasma: A Proof of Concept. J. Oral Maxillofac. Surg. 2017, 75, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef] [Green Version]
- Aruoma, O.I.; Whiteman, M.; England, T.G.; Halliwell, B. Antioxidant action of ergothioneine: Assessment of its ability to scavenge peroxynitrite. Biochem. Biophys. Res. Commun. 1997, 231, 389–391. [Google Scholar] [CrossRef]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with *NO, *NO2, and O2-*. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [Green Version]
- Franzoni, F.; Colognato, R.; Galetta, F.; Laurenza, I.; Barsotti, M.; Di Stefano, R.; Bocchetti, R.; Regoli, F.; Carpi, A.; Balbarini, A.; et al. An in vitro study on the free radical scavenging capacity of ergothioneine: Comparison with reduced glutathione, uric acid and trolox. Biomed. Pharmacother. 2006, 60, 453–457. [Google Scholar] [CrossRef]
- Winter, J.; Wende, K.; Masur, K.; Iseni, S.; Dunnbier, M.; Hammer, M.U.; Tresp, H.; Weltmann, K.D.; Reuter, S. Feed gas humidity: A vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J. Phys. D Appl. Phys. 2013, 46. [Google Scholar] [CrossRef]
- Jablonowski, H.; Schmidt-Bleker, A.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Non-touching plasma-liquid interaction—Where is aqueous nitric oxide generated? Phys. Chem. Chem. Phys. 2018, 20, 25387–25398. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Squadrito, G.L.; Pryor, W.A. Oxidative chemistry of nitric oxide: The roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic. Biol. Med. 1998, 25, 392–403. [Google Scholar] [CrossRef]
- Wenske, S.; Lackmann, J.-W.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bent, D.V.; Hayon, E. Excited state chemistry of aromatic amino acids and related peptides. I. Tyrosine. J. Am. Chem. Soc. 1975, 97, 2599–2606. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Parsons-Mair, H.N.; Gebicki, S.; Gebicki, J.M.; Davies, M.J. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem. J. 2004, 381, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Koppenol, W.H.; Moreno, J.J.; Pryor, W.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992, 5, 834–842. [Google Scholar] [CrossRef]
- Ramezanian, M.S.; Padmaja, S.; Koppenol, W.H. Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem. Res. Toxicol. 1996, 9, 232–240. [Google Scholar] [CrossRef]
- Jablonowski, H.; Bussiahn, R.; Hammer, M.U.; Weltmann, K.D.; von Woedtke, T.; Reuter, S. Impact of plasma jet vacuum ultraviolet radiation on reactive oxygen species generation in bio-relevant liquids. Phys. Plasmas 2015, 22, 122008. [Google Scholar] [CrossRef] [Green Version]
- Snyder, H.L.; Smtih, B.T.; Parr, T.P.; Martin, R.M. Dissociative Excitation of Water by Metastable Argon. Chem. Phys. 1982, 65, 397–406. [Google Scholar] [CrossRef]
- Zvereva, G.N. Using vacuum ultraviolet radiation to obtain highly reactive radicals. J. Opt. Technol. 2012, 79, 477–483. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef] [PubMed]
- Plowman, J.E.; Deb-Choudhury, S.; Grosvenor, A.J.; Dyer, J.M. Protein oxidation: Identification and utilisation of molecular markers to differentiate singlet oxygen and hydroxyl radical-mediated oxidative pathways. Photochem. Photobiol. Sci. 2013, 12, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Li, Y.K.; He, S.G.; Bierbaum, V.M. Reactivity of amino acid anions with nitrogen and oxygen atoms. Phys. Chem. Chem. Phys. 2018, 20, 4990–4996. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.L.; Carroll, K.S. The chemistry of thiol oxidation and detection. In Oxidative Stress and Redox Regulation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–42. [Google Scholar]
- Schmidt-Bleker, A.; Winter, J.; Iseni, S.; Dunnbier, M.; Weltmann, K.D.; Reuter, S. Reactive species output of a plasma jet with a shielding gas device-combination of FTIR absorption spectroscopy and gas phase modelling. J. Phys. D Appl. Phys. 2014, 47, 145201. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants for Inorganic and Organic Species of Potential Importance in Environmental Chemistry; Max-Planck Institute of Chemistry, Air Chemistry Department: Mainz, Germany, 1999. [Google Scholar]
- Koppenol, W.H.; Bounds, P.L.; Nauser, T.; Kissner, R.; Ruegger, H. Peroxynitrous acid: Controversy and consensus surrounding an enigmatic oxidant. Dalton Trans. 2012, 41, 13779–13787. [Google Scholar] [CrossRef]
- Shilov, V.P.; Fedoseev, A.M. Role of peroxynitrite in oxidation of f-element ions in HNO3 solutions. Radiochemistry 2013, 55, 366–368. [Google Scholar] [CrossRef]
- Lobachev, V.L.; Rudakov, E.S. The chemistry of peroxynitrite. Reaction mechanisms and kinetics. Usp. Khim. 2006, 75, 422–444. [Google Scholar] [CrossRef]
- Robinson, K.M.; Beckman, J.S. Synthesis of peroxynitrite from nitrite and hydrogen peroxide. Methods Enzymol. 2005, 396, 207–214. [Google Scholar] [CrossRef]
- Gorbanev, Y.; O’Connell, D.; Chechik, V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chemistry (Easton) 2016, 22, 3496–3505. [Google Scholar] [CrossRef] [Green Version]
- Stoffels, C.; Oumari, M.; Perrou, A.; Termath, A.; Schlundt, W.; Schmalz, H.G.; Schafer, M.; Wewer, V.; Metzger, S.; Schomig, E.; et al. Ergothioneine stands out from hercynine in the reaction with singlet oxygen: Resistance to glutathione and TRIS in the generation of specific products indicates high reactivity. Free Radic. Biol. Med. 2017, 113, 385–394. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid. J. Proteom. 2017, 152, 22–32. [Google Scholar] [CrossRef] [PubMed]













| Functional Group(s) on Tyrosine | Formula | [M+H]+ | Compound Code | Group Letter * |
|---|---|---|---|---|
| None | C9H11NO3 | 182.081725 | 1 | - |
| 1 × OH | C9H11NO4 | 198.0766 | 2 | - |
| 2 × OH | C9H11NO5 | 214.071525 | 3 | - |
| 3 × OH | C9H11NO6 | 230.066425 | 4 | - |
| 4 × OH | C9H11NO7 | 246.061425 | 5 | - |
| 1 × NO | C9H10N2O4 | 211.071925 | 6 | e |
| 2 × NO | C9H9N3O5 | 240.062025 | 7 | a, d |
| 3 × NO | C9H8N4O6 | 269.052225 | 8 | b, d |
| 4 × NO | C9H7N5O7 | 298.042325 | 9 | b, d |
| 1 × NO2 + 1 × OH, 1 × NO | C9H10N2O5 | 227.066825 | 10 | c, e |
| 2 × NO2 + 2 × OH, 2 × NO | C9H9N3O7 | 272.051825 | 11 | b, e |
| 3 × NO2 | C9H8N4O9 | 317.036925 | 12 | b, e |
| 4 × NO2 | C9H7N5O11 | 362.022025 | 13 | b, e |
| 1 × Y | C18H20N2O6 | 361.139925 | 14 | - |
| 1 × Y, 1 × OH | C18H20N2O7 | 377.134925 | 15 | - |
| 1 × Y, 2 × OH | C18H20N2O8 | 393.129825 | 16 | - |
| 1 × Y, 3 × OH | C18H20N2O9 | 409.124725 | 17 | - |
| 1 × Y, 1 × NO | C18H19N3O7 | 390.130125 | 18 | c, d |
| 1 × Y, 1 × NO2 + 1 × Y, 1 × OH, 1 × NO | C18H19N3O8 | 406.125025 | 19 | e |
| 1 × OH, 2 × NO | C9H9N3O6 | 256.056925 | 20 | a, f |
| 1 × OH, 3 × NO | C9H8N4O7 | 285.047125 | 21 | b, f |
| 1 × OH, 1 × NO2 + 2 × OH, 1 × NO | C9H10N2O6 | 243.061725 | 22 | c, f |
| 2 × OH, 1 × NO2 + 3 × OH, 1 × NO | C9H10N2O7 | 259.056625 | 23 | f |
| 3 × OH, 1 × NO2 | C9H10N2O8 | 275.051525 | 24 | f |
| 1 × OH, 2 × NO2 | C9H9N3O8 | 288.046825 | 25 | f |
| 2 × OH, 2 × NO2 | C9H9N3O9 | 304.041725 | 26 | a, f |
| 1 × OH, 3 × NO2 | C9H8N4O10 | 333.031825 | 27 | f |
| Added Functional Groups on Tyrosine | Plasma Components in the Gas Phase | Proposed Reactions in Liquid | |
|---|---|---|---|
| Ar-only | 1 × OH | Ar2*, VUV | H2O → ˙H + ˙OH |
| Ar + N2 | 1–2 × OH | Ar2*, VUV | H2O + hv → ˙H + ˙OH |
| Ar + N2 + O2 | 1–2 × OH; 1, 4 × NO; 1–2 × NO2 |
˙NO2(g), ˙O(g) | ˙O + H2O → ˙OH + ˙OH ˙NO2 + ˙OH → ONOOH |
| Ar + O2 | 1–4 × OH; 1, 4 × NO; 1–2 × NO2; mi×ed |
˙NO2(g), ˙O(g), O3(g) N2O5(g) |
˙O + H2O → ˙OH + ˙OH ˙NO2 + ˙OH → ONOOH |
| Ar-only hum | 1 × OH; 1, 4 × NO; 1–2 × NO2; mi×ed |
˙NO(g), HNO3(g), H2O2 (aq), ˙O2−(aq), ˙OH (aq) | ˙NO + ˙O2− → ONOOH HNO2 + H2O2 → ONOOH |
| Ar + N2 hum | 1 × OH; 1, 4 × NO; 1–2 × NO2; mi×ed |
˙NO(g), HNO3(g), H2O2 (aq), ˙O2−(aq), ˙OH (aq) | ˙NO + ˙O2− → ONOOH HNO2 + H2O2 → ONOOH |
| Ar + N2 + O2 hum | 1 × OH; 1 × NO; 1 × NO2 |
˙NO2(g), N2O5(g), H2O2 (aq), ˙O2−(aq), ˙OH (aq) | ˙NO2 + ˙OH → ONOOH HNO2 + H2O2 → ONOOH |
| Ar + O2 hum | 1 × OH; 1 × NO; 1 × NO2 |
˙NO2(g), N2O5(g), H2O2 (aq), ˙O2−(aq), ˙OH (aq) | ˙NO2 + ˙OH → ONOOH HNO2 + H2O2 → ONOOH |
| Uniprot Identifier | Abbreviated Protein Name | Protein Name | Modified Tyrosine Residue (Bold Denotes Significant Site) |
|---|---|---|---|
| P02768 | ALBU | Albumin | Y54, Y172, Y174, Y287, Y377, Y425, Y356, Y365, Y521 |
| P01009 | A1AT | Alpha-1-antitrypsin | Y184 |
| P01023 | A2MG | Alpha-2-macroglobulin | Y544, Y818, Y1152, Y1264 |
| O43299 | AP5Z1 | AP-5 complex subunit zeta-1 | Y344 |
| P02647 | APOA1 | Apolipoprotein A-I | Y124, Y190 |
| P00915 | CAH1 | Carbonic anhydrase 1 | Y21 |
| P00450 | CERU | Ceruloplasmin | Y55, Y539 |
| P01024 | CO3 | Complement C3 | Y139, Y1447, Y1620 |
| P0C0L4 | CO4A | Complement C4-A | Y1612 |
| P02751 | FINC | Fibronectin | Y841, Y2362 |
| P00738 | HPT | Haptoglobin | Y224, Y242, Y280, Y386, Y389 |
| P69905 | HBA | Hemoglobin subunit alpha | Y25, Y43 |
| P68871 | HBB | Hemoglobin subunit beta | Y36, Y131 |
| P02042 | HBD | Hemoglobin subunit delta | Y36, Y131 |
| P01876 | IGHA1 | Immunoglobulin heavy constant alpha 1 | Y276 |
| P01857 | IGHG1 | Immunoglobulin heavy constant gamma 1 | Y161, Y319 |
| Q14624 | ITIH4 | Inter-alpha-trypsin inhibitor heavy chain H4 | Y157 |
| P13645 | K1C10 | Keratin, type I cytoskeletal 10 | Y172, Y325 |
| P02533 | K1C14 | Keratin, type I cytoskeletal 14 | Y46 |
| P35527 | K1C9 | Keratin, type I cytoskeletal 9 | Y330, Y345 |
| P04264 | K2C1 | Keratin, type II cytoskeletal 1 | Y266, Y295, Y358, Y373 |
| Q7Z794 | K2C1B | Keratin, type II cytoskeletal 1b | Y361 |
| P35908 | K22E | Keratin, type II cytoskeletal 2 | Y563, Y589 |
| P02788 | TRFL | Lactotransferrin | Y211, Y545 |
| P32119 | PRDX2 | Peroxiredoxin-2 | Y126 |
| P00747 | PLMN | Plasminogen | Y66 |
| P02787 | TRFE | Serotransferrin | Y114, Y333, Y533 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, G.; Wenske, S.; Lackmann, J.-W.; Lalk, M.; von Woedtke, T.; Wende, K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules 2020, 10, 1687. https://doi.org/10.3390/biom10121687
Bruno G, Wenske S, Lackmann J-W, Lalk M, von Woedtke T, Wende K. On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas. Biomolecules. 2020; 10(12):1687. https://doi.org/10.3390/biom10121687
Chicago/Turabian StyleBruno, Giuliana, Sebastian Wenske, Jan-Wilm Lackmann, Michael Lalk, Thomas von Woedtke, and Kristian Wende. 2020. "On the Liquid Chemistry of the Reactive Nitrogen Species Peroxynitrite and Nitrogen Dioxide Generated by Physical Plasmas" Biomolecules 10, no. 12: 1687. https://doi.org/10.3390/biom10121687