Key Points
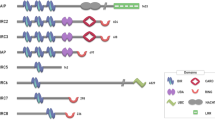
BH3-only (B-cell lymphoma 2 (BCL-2)-homology domain 3 only) proteins are a pro-apoptotic subgroup of the BCL-2 family. They share with each other and the rest of the BCL-2 family only the short (9–16 amino acid) BH3 region.
The BH3 domain of BH3-only proteins is required for the ability of these proteins to bind BCL-2-like pro-survival proteins and to trigger apoptosis.
BH3-only proteins are essential for the initiation of programmed cell death and stress-induced apoptosis in species as distantly related as nematodes and mice.
Mammals have at least eight BH3-only proteins, and these are activated by different apoptotic stimuli. There is also evidence for cell-type-restricted functions of mammalian BH3-only proteins.
BH3-only proteins trigger apoptosis by a mechanism that requires BAX- (BCL-2-associated X protein)/BAK (BCL-2-antagonist/killer)-like members of the multi-BH-domain pro-apoptotic subgroup of the BCL-2 family.
The BH3-only protein BIM (BCL-2-interacting mediator of cell death) is essential for lymphocyte homeostasis, for negative selection of autoreactive T and B cells and for shut-down of immune responses.
The BH3-only protein PUMA (p53-upregulated modulator of apoptosis), and to a lesser extent NOXA, is required for DNA-damage-induced apoptosis, which is mediated by the tumour-suppressor protein p53.
The BH3-only protein BID (BH3-interacting-domain death agonist) is activated by caspase-mediated proteolysis and has a cell-type-restricted role in death-receptor-induced apoptosis.
Defects in BH3-only proteins can cause autoimmune disease or cancer, particularly in combination with mutations that dysregulate cell-cycle control.
Loss of BIM prevents the immunodeficiency and the other degenerative disorders that are caused by BCL-2 deficiency, and it partially restores B- and T-cell numbers and enhances immune responses in mice that lack the α-chain of the interleukin-7 receptor.
BH3-only proteins, in particular PUMA and BIM, are required for the apoptosis that is induced in lymphocytes and fibroblasts by γ-rays or by certain chemotherapeutic drugs, indicating that these proteins have a role in anticancer therapy of human malignancies.
Abstract
Programmed cell death — also known as apoptosis — has a crucial role in the immune system of mammals and other animals. It removes useless cells and potentially dangerous cells, including lymphocytes, and is involved in killing pathogen-infected or damaged cells. Defects in this process have been found to cause or contribute to diseases of the immune system, including immunodeficiency, autoimmunity, lymphoma and leukaemia. This review describes BH3-only proteins, a pro-apoptotic subgroup of the BCL-2 family, and their role in the development and function of the immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marsden, V. & Strasser, A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21, 71–105 (2003).
Strasser, A., O'Connor, L. & Dixit, V. M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Strasser, A., Harris, A. W., Huang, D. C. S., Krammer, P. H. & Cory, S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 14, 6136–6147 (1995).
Nagata, S. Fas ligand-induced apoptosis. Annu. Rev. Genet. 33, 29–55 (1999).
Strasser, A., Harris, A. W. & Cory, S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell 67, 889–899 (1991).
Strasser, A. et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl Acad. Sci. USA 88, 8661–8665 (1991).
Strasser, A., Harris, A. W., Corcoran, L. M. & Cory, S. Bcl-2 expression promotes B but not T lymphoid development in scid mice. Nature 368, 457–460 (1994).
Strasser, A., Harris, A. W., Von Boehmer, H. & Cory, S. Positive and negative selection of T cells in T cell receptor transgenic mice expressing a bcl-2 transgene. Proc. Natl Acad. Sci. USA 91, 1376–1380 (1994).
Maraskovsky, E. et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89, 1011–1019 (1997).
Newton, K., Harris, A. W., Bath, M. L., Smith, K. G. C. & Strasser, A. A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17, 706–718 (1998).
Newton, K., Harris, A. W. & Strasser, A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. EMBO J. 19, 931–941 (2000).
Kraus, M., Alimzhanov, M. B., Rajewsky, N. & Rajewsky, K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell 117, 787–800 (2004).
Sprent, J. & Tough, D. F. T cell death and memory. Science 293, 245–248 (2001).
Strasser, A. & Pellegrini, M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 25, 610–615 (2004).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Metcalf, D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 339, 27–30 (1989).
Vaux, D. L. & Strasser, A. The molecular biology of apoptosis. Proc. Natl Acad. Sci. USA 93, 2239–2244 (1996).
Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9, 459–470 (2002).
Enari, M. et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391, 43–50 (1998).
Liu, X., Zou, H., Slaughter, C. & Wang, X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89, 175–184 (1997).
Zhang, J., Cado, D., Chen, A., Kabra, N. H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Varfolomeev, E. E. et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267–276 (1998).
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001).
Marsden, V. et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419, 634–637 (2002).
Hara, H. et al. The apoptotic protease-activating factor 1-mediated pathway of apoptosis is dispensable for negative selection of thymocytes. J. Immunol. 168, 2288–2295 (2002).
Marsden, V. S. et al. Bcl-2-regulated apoptosis and cytochrome c release can occur independently of both caspase-2 and caspase-9. J. Cell Biol. 165, 775–780 (2004).
Ricci, J. E. et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117, 773–786 (2004).
Yin, X.-M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999). This study shows that BID is required for death-receptor-induced apoptosis of hepatocytes but not lymphocytes.
Yin, X.-M., Oltvai, Z. N. & Korsmeyer, S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369, 321–323 (1994).
Chittenden, T. et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 14, 5589–5596 (1995).
Huang, D. C. S., Adams, J. M. & Cory, S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 17, 1029–1039 (1998).
Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993).
Nakayama, K. et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science 261, 1584–1588 (1993).
Motoyama, N. et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science 267, 1506–1510 (1995).
Ross, A. J. et al. Testicular degeneration in Bclw-deficient mice. Nature Genet. 18, 251–256 (1998).
Print, C. G. et al. Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc. Natl Acad. Sci. USA 95, 12424–12431 (1998).
Xiang, Z. et al. Essential role of the prosurvival Bcl-2 homologue A1 in mast cell survival after allergic activation. J. Exp. Med. 194, 1561–1569 (2001).
Opferman, J. T. et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 (2003).
Suzuki, M., Youle, R. J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654 (2000).
Lindsten, T. et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 (2000).
Rathmell, J. C., Lindsten, T., Zong, W.-X., Cinalli, R. M. & Thompson, C. B. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nature Immunol. 3, 932–939 (2002). References 42 and 43 show that BAX and BAK have an overlapping essential role in programmed cell death and stress-induced apoptosis of lymphocytes and certain other cell types.
Ekert, P. G. et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J. Cell Biol. 165, 835–842 (2004).
Conradt, B. & Horvitz, H. R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93, 519–529 (1998). This study shows that the BH3-only protein EGL-1 is required for developmentally programmed cell death in C. elegans.
Bouillet, P. et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 (1999). This study shows that BIM is required for cytokine-withdrawal-induced and calcium-flux-induced apoptosis of lymphoid cells. It also shows that loss of BIM results in increased numbers of lymphoid and myeloid cells and leads to autoimmune disease.
Huang, D. C. S. & Strasser, A. BH3-only proteins — essential initiators of apoptotic cell death. Cell 103, 839–842 (2000). This is a review about BH3-only proteins.
Yang, E. et al. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell 80, 285–291 (1995). This study describes the discovery of BAD.
Boyd, J. M. et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11, 1921–1928 (1995).
Han, J., Sabbatini, P. & White, E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell. Biol. 16, 5857–5864 (1996).
Hegde, R., Srinivasula, S. M., Ahmad, M., Fernandes-Alnemri, T. & Alnemri, E. S. Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J. Biol. Chem. 273, 7783–7786 (1998).
Wang, K., Yin, X.-M., Chao, D. T., Milliman, C. L. & Korsmeyer, S. J. BID: a novel BH3 domain-only death agonist. Genes Dev. 10, 2859–2869 (1996). This study describes the discovery of BID.
Inohara, N., Ding, L., Chen, S. & Nu–ez, G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-XL . EMBO J. 16, 1686–1694 (1997).
Imaizumi, K. et al. Molecular cloning of a novel polypeptide, DP5, induced during programmed neuronal death. J. Biol. Chem. 272, 18842–18848 (1997).
O'Connor, L. et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17, 384–395 (1998).
Hsu, S. Y., Lin, P. & Hsueh, A. J. W. BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members. Mol. Endocrinol. 12, 1432–1440 (1998). References 55 and 56 describe the discovery of BIM.
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000). This study describes the discovery of NOXA.
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Yu, J., Zhang, L., Hwang, P. M., Kinzler, K. W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001). References 58 and 59 describe the discovery of PUMA.
Puthalakath, H. et al. Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Petros, A. M. et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9, 2528–2534 (2000).
Liu, X., Dai, S., Zhu, Y., Marrack, P. & Kappler, J. W. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity 19, 341–352 (2003). This study presents a structural analysis of a complex between a fragment of BIM with BCL-X L.
Cheng, E. H. et al. BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8, 705–711 (2001).
Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R. & Thompson, C. B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15, 1481–1486 (2001). References 63 and 64 show that BAX and/or BAK are required for the apoptosis that is induced by BH3-only proteins.
Marani, M., Tenev, T., Hancock, D., Downward, J. & Lemoine, N. R. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol. Cell. Biol. 22, 3577–3589 (2002).
Letai, A. et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2, 183–192 (2002).
Oltvai, Z. N., Milliman, C. L. & Korsmeyer, S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619 (1993).
Wolter, K. G. et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281–1292 (1997).
Cory, S. & Adams, J. M. The Bcl2 family: regulators of the cellular life-or-death switch. Nature Rev. Cancer 2, 647–656 (2002).
Puthalakath, H., Huang, D. C. S., O'Reilly, L. A., King, S. M. & Strasser, A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 (1999). This study shows that the pro-apoptotic activity of BIM EL and BIM L can be regulated by their sequestration to the microtubular dynein-motor complex.
Zhu, Y. et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl Acad. Sci. USA 101, 7681–7686 (2004).
O'Reilly, L. A. et al. The pro-apoptotic BH3-only protein Bim is expressed in hemopoietic, epithelial, neuronal and germ cells. Am. J. Path. 157, 449–461 (2000).
Villunger, A., Scott, C., Bouillet, P. & Strasser, A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood 101, 2393–2400 (2003).
Bouillet, P. et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature 415, 922–926 (2002). This study and reference 77 show that BIM is required for the apoptosis of autoreactive thymocytes (that is, for negative selection).
Villunger, A. et al. Negative selection of semimature CD4+8−HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc. Natl Acad. Sci. USA 101, 7052–7057 (2004).
Davey, G. M. et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J. Exp. Med. 196, 947–955 (2002).
Enders, A. et al. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J. Exp. Med. 198, 1119–1126 (2003).
Hildeman, D. A. et al. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity 16, 759–767 (2002).
Pellegrini, M., Belz, G., Bouillet, P. & Strasser, A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl Acad. Sci. USA 100, 14175–14180 (2003). References 78 and 79 show that BIM is required for termination of T-cell responses.
Krammer, P. H. CD95's deadly mission in the immune system. Nature 407, 789–795 (2000).
Russell, J. H., Rush, B., Weaver, C. & Wang, R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc. Natl Acad. Sci. USA 90, 4409–4413 (1993).
Alderson, M. R. et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J. Exp. Med. 181, 71–77 (1995).
Puthalakath, H. & Strasser, A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9, 505–512 (2002).
Dijkers, P. F., Medemadagger, R. H., Lammers, J. J., Koenderman, L. & Coffer, P. J. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10, 1201–1204 (2000). This study shows that BIM can be regulated transcriptionally by the forkhead transcription factor FOXO3A.
Ley, R., Balmanno, K., Hadfield, K., Weston, C. & Cook, S. J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278, 18811–18816 (2003).
Akiyama, T. et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 22, 6653–6664 (2003).
Luciano, F. et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene 22, 6785–6793 (2003). References 85–87 show that BIM can be regulated by ERK-mediated phosphorylation, which controls BIM ubiquitylation and proteasomal degradation.
Lei, K. & Davis, R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA 100, 2432–2437 (2003).
Putcha, G. V. et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38, 899–914 (2003).
Putcha, G. V. et al. Induction of Bim, a proapoptotic BH3-only Bcl-2 family member, is critical for neuronal apoptosis. Neuron 29, 615–628 (2001).
Whitfield, J., Neame, S. J., Paquet, L., Bernard, O. & Ham, J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 29, 629–643 (2001).
Han, J. et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc. Natl Acad. Sci. USA 98, 11318–11323 (2001).
Coultas, L., Huang, D. C. S., Adams, J. M. & Strasser, A. Pro-apoptotic BH3-only Bcl-2 family members in vertebrate model organisms suitable for genetic experimentation. Cell Death Differ. 9, 1163–1166 (2002).
Strasser, A., Harris, A. W., Jacks, T. & Cory, S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell 79, 329–339 (1994).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science 302, 1036–1038 (2003).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 (2003).
Shibue, T. et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 17, 2233–2238 (2003). References 95–97 show that PUMA, and to a lesser extent NOXA, are essential for DNA-damage-induced apoptosis mediated by p53.
Zha, J., Harada, H., Yang, E., Jockel, J. & Korsmeyer, S. J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not Bcl-xL . Cell 87, 619–628 (1996).
del Peso, L., González-Garcia, M., Page, C., Herrera, R. & Nuñez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997). References 98–100 show that stimulation of cells with growth factors causes inactivation of BAD through AKT-mediated phosphorylation.
Ranger, A. M. et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc. Natl Acad. Sci. USA 100, 9324–9329 (2003).
Chou, J. J., Li, H., Salvesen, G. S., Yuan, J. & Wagner, G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 (1999).
McDonnell, J. M., Fushman, D., Milliman, C. L., Korsmeyer, S. J. & Cowburn, D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96, 625–634 (1999).
Luo, X., Budlhardjo, I., Zou, H., Slaughter, C. & Wang, X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 (1998).
Li, H., Zhu, H., Xu, C.-J. & Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 (1998). References 104 and 105 show that BID can be activated by caspase-8-mediated proteolysis.
Sutton, V. R. et al. Initiation of apoptosis by granzyme B requires direct cleavage of Bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 192, 1403–1414 (2000).
Heibein, J. A. et al. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J. Exp. Med. 192, 1391–1402 (2000). References 106 and 107 show that the pro-apoptotic activity of BID can be activated by granzyme-B-mediated proteolysis.
Jiang, A. & Clark, E. A. Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J. Immunol. 166, 6025–6033 (2001).
Coultas, L. et al. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol. Cell. Biol. 24, 1570–1581 (2004).
Sanz, C. et al. Specific and rapid induction of the proapoptotic protein Hrk after growth factor withdrawal in hematopoietic progenitor cells. Blood 95, 2742–2747 (2000).
Imaizumi, K. et al. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J. Neurosci. 24, 3721–3725 (2004).
Vaux, D. L., Cory, S. & Adams, J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988).
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348, 331–333 (1990).
Zinkel, S. S. et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 17, 229–239 (2003).
Egle, A., Harris, A. W., Bouillet, P. & Cory, S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc. Natl Acad. Sci. USA 101, 6164–6169 (2004). References 114 and 115 show that loss of BID or BIM can promote lymphomagenesis.
Kuribara, R. et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Mol. Cell. Biol. 24, 6172–6183 (2004). This study shows that BIM is required for killing of a CML-derived cell line with the designer anticancer drug imatinib (Gleevec).
Wakeland, E. K., Liu, K., Graham, R. R. & Behrens, T. W. Delineating the genetic basis of systemic lupus erythematosus. Immunity 15, 397–408 (2001).
Bouillet, P., Cory, S., Zhang, L.-C., Strasser, A. & Adams, J. M. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev. Cell 1, 645–653 (2001). This study shows that loss of BIM prevents the immunodeficiency and other degenerative disorders that are caused by loss of BCL-2.
Pellegrini, M. et al. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 200, 1189–1195 (2004).
Oliver, P. M. et al. Loss of Bim allows precursor B cell survival but not precursor B cell differentiation in the absence of interleukin 7. J. Exp. Med. 200, 1179–1187 (2004).
Akashi, K., Kondo, M., von Freeden-Jeffry, U., Murray, R. & Weissman, I. L. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89, 1033–1041 (1997).
Smith, K. G. C., Strasser, A. & Vaux, D. L. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO J. 15, 5167–5176 (1996).
Hsu, S. Y., Kaipia, A., McGee, E., Lomeli, M. & Hsueh, A. J. W. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc. Natl Acad. Sci. USA 94, 12401–12406 (1997).
Inohara, N. et al. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-xL . J. Biol. Chem. 273, 8705–8710 (1998).
Guo, B., Godzik, A. & Reed, J. C. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J. Biol. Chem. 276, 2780–2785 (2001).
Coultas, L. et al. Bfk: a novel weakly proapoptotic member of the Bcl-2 protein family with a BH3 and a BH2 region. Cell Death Differ. 10, 185–192 (2003).
Acknowledgements
I thank J. Adams, S. Cory, D. Vaux, J. Miller, D. Metcalf, A. Harris, P. Bouillet, D. Huang, H. Puthalakath, L. O'Reilly, S. Bath, A. Villunger, C. Scott, L. O'Connor, K. Newton, V. Marsden, L. Coultas, E. Michalak, P. Kelly and E. Naik for all of their work and for many fruitful discussions. I apologize to the many scientists whose excellent work was not cited directly in the text but only referred to indirectly through reviews. Work in my laboratory is supported by fellowships and grants from the National Health and Medical Research Council (Australia), the Leukemia & Lymphoma Society (United States), the National Institutes of Health (United States), the Juvenile Diabetes Research Foundation International (United States), The Cancer Council Victoria (Australia) and the Cancer Research Institute (United States).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- NEGATIVE SELECTION IN THE THYMUS
-
The deletion of self-reactive thymocytes. Thymocytes expressing T-cell receptors that strongly bind self-peptide bound to self-MHC molecules undergo apoptosis in response to the signalling generated by high-affinity binding.
- TUMOUR-NECROSIS-FACTOR-RECEPTOR FAMILY
-
(TNFR family). A family of cell-surface receptors that binds members of the TNF-ligand family. The receptors all contain cysteine-rich extracellular regions that are involved in ligand binding.
- DEATH DOMAIN
-
A protein–protein interaction domain found in many proteins that are involved in signalling and apoptosis.
- LYMPHADENOPATHY
-
Enlargement of the lymph nodes.
- BCL-2 HOMOLOGY DOMAIN
-
(BH domain). These domains are regions of amino-acid sequence and structural similarity in members of the B-cell lymphoma 2 (BCL-2) family. These regions are involved in interactions between pro- and anti-apoptotic members of this family.
- POSITIVE SELECTION
-
The process in the thymus that selects thymocytes expressing T-cell receptors (TCRs) that can interact weakly with self-MHC molecules. This weak interaction generates differentiation and survival signals in these lymphocytes, the TCRs of which later recognize foreign peptides bound to self-MHC. Positive selection establishes the MHC-restricted T-cell repertoire.
- IMMUNOLOGICAL MEMORY
-
A consequence of the ability of the adaptive arm of the immune system to respond more rapidly and efficiently after a primary immune response to a subsequent challenge with the same pathogen or experimentally administered antigen. Immunological memory is maintained by long-term survival of antigen-specific B and T cells.
- ASPARTIC-ACID-SPECIFIC CYSTEINE PROTEASES
-
A family of enzymes that has a cysteine residue in the active site and cleaves substrates after an aspartic acid residue. Members of this family are also known as caspases. Many of them are involved in apoptosis, but some (for example, caspase-1) are also required for processing of certain cytokines (such as interleukin-1β and interleukin-18).
- DEATH EFFECTOR DOMAIN
-
(DED). A domain that is found in certain initiator caspases (for example, mammalian caspase-8) and their adaptor protein FADD. This domain mediates protein–protein interactions.
- CASPASE-RECRUITMENT DOMAIN
-
(CARD). A domain that is found in certain initiator caspases (for example, mammalian caspase-9) and their adaptor proteins (for example, APAF1). This domain mediates protein–protein interactions.
- APOPTOSOME
-
An apoptotic-protein complex formed by the interaction of APAF1 (apoptotic-protease-activating factor 1), cytochrome c and dATP with pro-caspase-9. Complex formation leads to the cleavage and activation of caspase-9, which activates caspase-3 and other effector caspases, leading to cell death.
- p53
-
A tumour suppressor that is found to be mutated in ∼50% of all human cancers. The p53 protein is a transcription factor that is activated by damage to DNA, anoxia, expression of certain oncogenes, and several other stress stimuli. Target genes activated by p53 regulate cell-cycle arrest, apoptosis, cell senescence and DNA repair.
- λ-PHAGE EXPRESSION LIBRARY
-
A library that is used to find binding partners for known proteins. Cellular proteins are expressed from a λ-phage vector in infected Escherichia coli, and probing is carried out using tagged (for example, with 32P) 'bait' protein, usually produced in E. coli.
- YEAST TWO-HYBRID LIBRARY
-
A library that is used to determine the existence of direct interactions between proteins. It involves the use of plasmids that encode two hybrid proteins: one protein is fused to the DNA-binding domain of GAL4, and the other is fused to the activation domain of GAL4. The two proteins are expressed together in yeast, and if they interact, the resulting complex then drives the expression of a reporter gene, commonly β-galactosidase.
- CALCIUM IONOPHORE
-
A drug that promotes calcium flux into cells.
- ETOPOSIDE
-
A chemotherapeutic drug that is widely used. It is a semi-synthetic podophyllotoxin derived from the root of Podophyllum peltatum (may apple). It induces single-stranded DNA breaks, as well as DNA damage, through inhibition of DNA topoisomerase II.
- GLUCOCORTICOIDS
-
A group of compounds that belongs to the corticosteroid family. These compounds can be naturally produced (hormones) or synthetic. They affect metabolism and have anti-inflammatory and immunosuppressive effects. Some synthetic glucocorticoids (for example, dexamethasone) are used as chemotherapeutic drugs.
- UBIQUITYLATION
-
The attachment of the small protein ubiquitin to lysine residues that are present in other proteins. This tags these proteins for rapid cellular degradation.
- 14-3-3 SCAFFOLD PROTEINS
-
A family of conserved proteins that is present in all eukaryotic organisms. These proteins are involved in diverse cellular processes, such as apoptosis and stress, as well as in intracellular signalling and cell-cycle regulation. They function as adaptors in protein interactions and can regulate protein localization and enzymatic activity. Approximately 100 binding partners have been reported for the 14-3-3 proteins.
- Eμ-MYC TRANSGENE
-
A transgene construct that subjugates expression of the proto-oncogene MYC (which promotes cell cycling) under the control of the immunoglobulin heavy-chain gene enhancer Eμ. This drives expression of the MYC oncogene in B cells and causes development of pre-cursor (pre)-B-cell- and B-cell lymphoma in mice.
- HAPLOINSUFFICIENT
-
A condition caused when loss of a single allele of a gene causes a noticeable phenotypic abnormality. This indicates that the levels of the corresponding protein are limiting under normal circumstances.
- BCR–ABL KINASE
-
An oncogenic kinase that is formed by the fusion of the Abelson leukaemia-virus protein (ABL) and the breakpoint-cluster region (BCR). This occurs as a consequence of the t(9; 22) chromosomal translocation (known as the Philadelphia chromosome), which is found in most cases of chronic myeloid leukaemia (CML). The BCR–ABL kinase is the target of the anticancer drug imatinib (Gleevec), which has proven highly successful in the treatment of CML.
Rights and permissions
About this article
Cite this article
Strasser, A. The role of BH3-only proteins in the immune system. Nat Rev Immunol 5, 189–200 (2005). https://doi.org/10.1038/nri1568
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1568
This article is cited by
Emerging biomarkers and potential therapeutics of the BCL-2 protein family: the apoptotic and anti-apoptotic context
Egyptian Journal of Medical Human Genetics (2024)
Identification of the Bcl-2 and Bax homologs from Rhipicephalus haemaphysaloides and their function in the degeneration of tick salivary glands
Parasites & Vectors (2021)
Absence of Bim sensitizes mice to experimental Trypanosoma cruzi infection
Cell Death & Disease (2021)
Venetoclax imparts distinct cell death sensitivity and adaptivity patterns in T cells
Cell Death & Disease (2021)
Loss of BIM in T cells results in BCL-2 family BH3-member compensation but incomplete cell death sensitivity normalization
Apoptosis (2020)