-
PDF
- Split View
-
Views
-
Cite
Cite
Esther M Ponnuraj, Jennifer Springer, Anthony R Hayward, Harry Wilson, Eric A. F Simoes, Antibody-Dependent Enhancement, a Possible Mechanism in Augmented Pulmonary Disease of Respiratory Syncytial Virus in the Bonnet Monkey Model, The Journal of Infectious Diseases, Volume 187, Issue 8, 15 April 2003, Pages 1257–1263, https://doi.org/10.1086/374604
Close - Share Icon Share
Abstract
Bonnet monkeys develop an enhanced disease after immunization with the formalin-inactivated (FI) respiratory syncytial virus (RSV) vaccine that is characterized by increased viral replication in perivascular sites of the lung. These sites contain many mononuclear cells, which are known to be permissive for RSV replication. To test the hypothesis that FI-RSV vaccine stimulates the production of enhancing antibodies that serve to increase the replication of RSV in macrophages, in vitro studies were done. Antibody-dependent enhancement was observed in animals immunized with FI-RSV but not in control animals with primary and tertiary infections or those immunized with FI-Vero cell culture. In the presence of serum samples from animals immunized with FI-RSV, an increased number of U937 cells was infected. The enhancement index correlated positively with the pathologic scores of the FI-RSV-vaccinated monkeys
Children immunized with the formalin-inactivated (FI) respiratory syncytial virus (RSV) vaccine during the 1960s developed a more severe disease after RSV infection that was characterized by increased pulmonary inflammation and, in some cases, death. However, nonimmune cohorts of these children who were exposed to natural RSV infection had a milder illness [1–,,4]. Efforts to develop an efficacious vaccine for preventing RSV disease requires an understanding of the mechanisms involved in the enhanced disease after immunization with the FI-RSV vaccine
In the Bonnet monkey, primary RSV infection created mild histological changes in the lung, with viral replication predominantly in epithelial cells [5]. However, after the administration of FI-RSV vaccine, inflammation accompanies the viral replication around blood vessels in the lung [6]. Furthermore, in FI-RSV-immunized animals, immunohistochemistry revealed a vast number of macrophages in the perivascular areas, where viral replication was demonstrated by in situ hybridization
Observations such as the enhanced disease after the administration of a FI vaccine and the demonstration of viral replication in macrophages in perivascular areas prompted the hypothesis that an antibody is responsible for the pathogenesis of enhanced RSV disease. Gimenez et al. [7] demonstrated that some of the monoclonal antibodies that they investigated could enhance the replication of RSV in U937 cells. IgG1 antibodies directed against epitopes of G or F glycoproteins that are on the surface of RSV may also be responsible for antibody-mediated endocytosis. If enhancing antibodies were produced actively after parenteral immunization with FI-RSV vaccine, it might increase the replication of RSV rather than neutralize the virus. However, evidence for such a mechanism in an experimental model is not available
To test the hypothesis that enhancing antibody produced by immunization with FI-RSV would augment viral uptake and increase replication in macrophages, we compared serum samples from animals preimmunized with FI-RSV vaccine with serum samples from control animals with a primary and tertiary RSV infection. In our in vitro study, we used a macrophage cell line (U937) to test for antibody-dependent enhancement (ADE). The present article reports, for the first time, levels of enhancing activity in the serum samples of monkeys with primary and tertiary natural RSV infections and those preimmunized with an FI-RSV vaccine
Materials and Methods
MonkeysWild-caught infant and juvenile Bonnet monkeys (Macaca radiata) weighing 500–2500 g were immunized as described in table 1 and infected with 106 pfu of RSV (long strain) after quarantine procedures described elsewhere [6]
Detection of ADEThe ADE assay was carried out with serum samples drawn from monkeys at various time points, as described in table 1, using the method of Gimenez et al. [7], with some minor modifications. In brief, a 1:10 dilution of serum was made in RPMI 1640 medium with 5% heat-inactivated fetal bovine serum (FBS) (RPMI-5), and then 100 μL of this serum dilution was mixed with 100 μL of A2 strain of RSV that contained ∼8.3×105 pfu. Virus control assays were prepared with 100 mL of RPMI-5 and 100 μL of RSV. The virus-antibody mixtures and virus controls were incubated for 30 min at 37°C before the addition of 2×105 U937 cells in RPMI-5. Virus adsorption was carried out at 37°C for 60 min, and the cells were then washed 3 times with RPMI-5, resuspended in fresh RPMI-5, and incubated for 24–48 h at 37°C. In the time-course experiment, the incubation period was extended to 96 h. The suspensions were then sonicated, and infectious virus titers were determined by an enzyme-linked immunostaining of plaques in a plaque assay. In brief, 200 μL of dilutions of virus suspensions were added to fresh monolayers of Hep-2 cells in 24-well plates in duplicate wells, along with 800 μL of maintenance medium that consisted of Dulbecco's modified minimal essential medium supplemented with 2% heat-inactivated FBS and antibiotics (100 U of penicillin, 100 μg of streptomycin, and 250 ng of amphotericin B/mL). The plates were spun at 1000 g for 45 min and incubated for 18 h at 37°C. The medium in the wells was aspirated and fixed with 80% methanol in PBS. The wells were then treated with blocking agent for 5 min (5% nonfat milk in PBS [PBSM]) and incubated with a 1:1000 dilution of anti-RSV pan-blend antibody (Chemicon) for 1 h at 37°C on a rocking platform. Plates were washed with PBSM and incubated with a 1:250 dilution of goat anti-mouse IgG (light and heavy chain) tagged to peroxidase (Kirkegard and Perry Laboratories) at 37°C for 1 h. The secondary antibody was washed with distilled water and a 0.04% solution of α-chloro-naphthol (Sigma), and 0.0009% H2O2 was added as a substrate for color development. Plates were then washed with distilled water, and the number of plaques was counted under an inverted microscope using a 10× objective lens
For calculating the enhancement index (EI), the infectious virus yields in the presence (x1) and absence (x0) of antibody were used in the equation EI=(x1-x0)/(x1+x0) [7]. On the basis of 2 Poisson-distributed observations, Giminez et al. [7] considered EI values ⩾1.96 to be significant
Estimation of the percentage of RSV-permissive cells by direct fluorescent antibody staining To determine whether increases in virus titers resulted from the infection of more U937 cells or from an increased permissiveness of a constant number of infected cells, we counted the number of infected cells by direct immunofluorescent staining. This experiment was carried out using a 1:10 dilution of serum derived from a monkey immunized with FI-RSV. At 24, 48, and 72 h, cytospin preparations were made. The slides were allowed to air dry, fixed in acetone at 4°C for 10 min, and stored wrapped in aluminum foil at −70°C until staining. The slides were incubated at 37°C for 30 min with a goat polyclonal anti-RSV antibody tagged to fluorescein (AB1128F; Chemicon). The slides were washed 3 times in PBS, counterstained with Evan's Blue, mounted, and viewed under a fluorescent microscope. From each cytospin preparation, 500 cells were counted, and the percentage of infected cells was calculated
Histological examination The lung tissues obtained on the seventh day of infection with RSV were examined for inflammation in peribronchiolar, perivascular, alveolar, and interstitial sites and were graded and scored as reported elsewhere [6]. To examine the relationship between EI and histological findings, a correlation analysis was performed using histological scores from the peribronchiolar, perivascular, alveolar, and interstitial sites, as well as total histological scores
Statistical analysisThe Kruskal-Wallis test was used to see whether there was a significant overall difference in EI among the different groups. Using combined group data, a correlation between EI and histological scores was performed, and Spearman's ρ was calculated using standard methods
Results
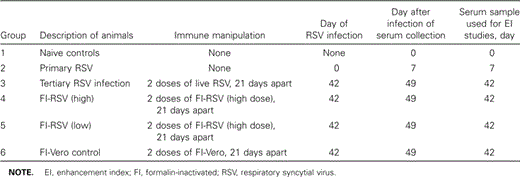
Significantly high EIs in U937 cells incubated with serum samples from monkeys preimmunized with FI-RSV and not from those that had mucosal exposure to live RSVBecause EI was a value derived from virus titers in U937 cells incubated with and without serum samples, values ranged from −44 to 203. With serum samples from naive control animals, the mean (±SE) EI observed was -20.51±10.75 (n=6). Serum samples collected from monkeys on the seventh day of primary RSV infection had a mean (±SE) EI of 1.14±0.1 (n=4). Serum from animals that had undergone 2 mucosal RSV exposures had a mean (±SE) EI of 1.49±0.39 (n=5), and the differences in these 2 groups of animals undergoing mucosal infection for the first or the third time were not statistically significant. Previous studies by Gimenez et al. [7] considered only EI>2 to be significant; none of the above 3 groups met this criterion; thus, they lacked enhancing antibodies. Serum from animals immunized with FI-RSV (high dose) had a mean (±SE) EI of 162±12.61 (n=6), and that from animals immunized with FI-RSV (low dose) had a mean (±SE) EI of 153±25.56 (n=3). The EI of animals immunized with either of the 2 doses of FI-RSV was significantly greater than that of controls (P<.0017, Dunn's procedure). Animals immunized with FI-Vero had a mean EI of 6.8 (n=5), which was significantly lower than that of animals immunized with FI-RSV (P<.005). Thus, animals immunized with the FI vaccine had very high EIs in a macrophage cell line, as shown in figure 1
Serum enhancement index values of monkeys. Group 1, naive monkeys; group 2, monkeys with primary infection; group 3, monkeys with tertiary infection; group 4, monkeys immunized with 2 (high) doses of formalin-inactivated (FI) respiratory syncytial virus (RSV) vaccine; group 5, monkeys immunized with 2 (low) doses of FI-RSV vaccine; and group 6, FI-Vero cell control vaccine prior to challenge with live RSV
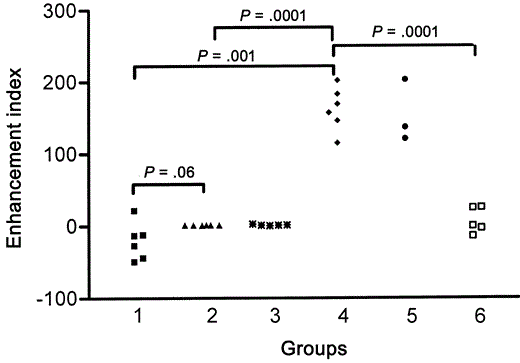
Positive correlation between the EI and pathologic scoresInflammation in the lungs of monkeys was graded in different anatomical sites in the 6 groups of monkeys [6]. Histological scores of monkeys belonging to all 6 groups and all available EIs were used in the correlation analysis (n=27). There were positive correlations between the enhancement index and peribronchiolar scores (r=0.60; P<.0009) (figure 2A), perivascular scores (r=0.62; P<.0005) (figure 2B), and interstitial score (r=0.52; P<.004) (figure 2C). The correlation between the EI and alveolar scores was the lowest (r=0.37; P<.05) (figure 2D), which resulted in a lower correlation in the total scores (r=0.54; P<.003) (figure 2E). When correlations were done, data points were found to cluster around 2 foci. One cluster was at the low end of the scale and was made up of all the serum samples from monkeys that were uninfected, those with primary or tertiary RSV infection, and those immunized with the control FI-Vero vaccine. The second cluster was seen on the high end of the scale and was made up of 9 monkeys that were immunized with FI-RSV (high and low dose). When these 2 clusters of animals were analyzed as 2 distinct groups (those with and those without FI-RSV immunization), there were significant differences in peribronchiolar (P<.0001), perivascular (P<.0001), interstitial (P<.0007), and total pathological scores (P<.0002) in the lung, according to analysis of variance. Both groups had distinct differences in EI (P<.0001)
Correlation analysis between various histological scores and enhancement indices of the 6 groups of monkeys. Because of overlap in data, some of the points represent multiple data points, which could not be shown in the graph
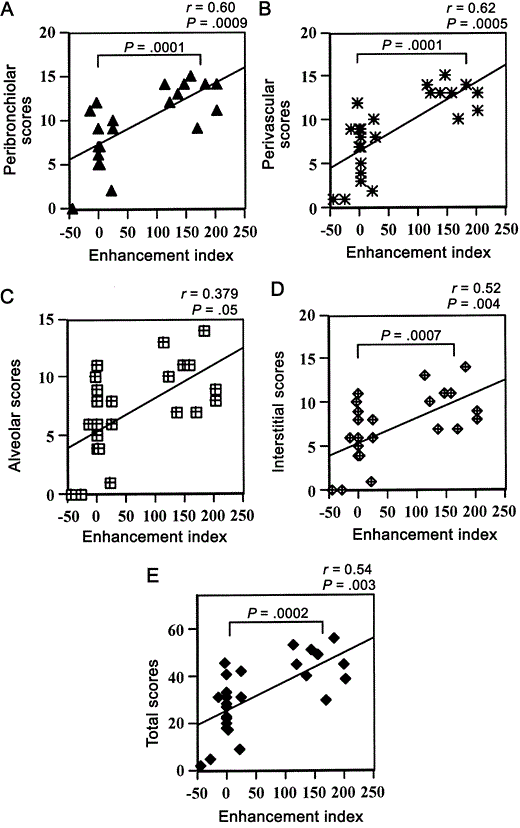
Proportion of macrophages infected in the presence of serum from animals preimmunized with FI-RSV When direct RSV fluorescent antibody staining was done and ⩾500 cells were examined to determine the proportion of cells that were infected, 29% of U937 cells were infected at 24 h. At the same time point in the presence of serum derived from a monkey preimmunized with FI-RSV, 52% of the U937 cells were infected. Figure 3 shows the RSV infection of U937 cells with and without enhancing antibody at 24, 48, and 72 h after fluorescent antibody staining for RSV
U937 cells infected with respiratory syncytial virus (RSV) in the absence (a, c and e) and presence (b, d and f) of enhancing antibody (serum from a monkey immunized with formalin-inactivated RSV at a dilution of 1:10) and stained with polyclonal RSV goat antibody tagged to fluorescein isothiocyanate after 24 (a and b) 48 (c and d) and 72 (e and f) h. Bar 10 μm
Discussion
Although enhanced disease in immunized subjects is a major concern in the development of a successful RSV vaccine, its immunologic basis is still not understood. Children immunized with the FI-RSV vaccine have an increased lymphoproliferative response [1], but whether this is a cause or a consequence of the enhanced pathology is not known. In the mouse model, CD4 cell depletion using monoclonal antibodies completely prevented the development of pulmonary lesions in FI-RSV-immunized mice, so CD4 cells were thought to contribute to the enhanced pathology [9]. Whether they might do so through the production of cytokines or the provision of help for antibody production is not known
Antibodies may play a major role in protection against viruses, or, when they mediate the transport of a virus into a permissive cell, they may enhance disease. All isotypes may not be protective in nature. When a virus can replicate in a cell bearing Fc receptors, antibodies that bind to Fc receptors may serve in the enhancement of disease. Halstead [10] suggested that ADE plays a major role in Dengue hemorrhagic fever. Antibodies that complex with the virus and then attach to an Fc receptor on the macrophage facilitate the uptake of virus particles by Fc receptor-mediated endocytosis [11]. Furthermore, high-affinity antibodies may be neutralizing, whereas low-affinity antibodies may be enhancing; therefore, the nature of antibodies is very important
The FI-RSV vaccine fiasco of the 1960s and the demonstration of increased viral replication in the lungs of monkeys preimmunized with FI-RSV vaccine set the stage for investigating a possible mechanism involving antibodies that may have enhancing activity. Peripheral blood mononuclear cells (PBMCs) bear RSV antigen during acute infection, and monocytes can be infected in vitro [12]. PBMCs of children with RSV disease have RSV transcripts, as is shown by polymerase chain reaction results [13]. Thus, this virus replicates not only in alveolar epithelial cells but also in mononuclear cells. When Bonnet monkeys are infected intratracheally with RSV, the virus replicates in alveolar epithelial cells in primary infection, whereas RSV replication is seen in inflammatory cells, consisting of macrophages around blood vessels, in monkeys that are preimmunized with FI-RSV vaccine. This argues for the induction of enhancing antibodies after FI-RSV vaccination
Animals preimmunized with FI-RSV vaccine had low levels of neutralizing antibody [6], and here it is shown that they have very high enhancement indices. Their perivascular and peribronchiolar scores correlated well with an increased EI, which suggests that the enhancing activity of certain antibodies probably played a role in the enhanced disease in this monkey model. On the contrary, animals with 2 prior live RSV infections had good neutralizing antibody that could mask the effect of the enhancing activity, and this could explain why these monkeys had very few pathologic abnormalities. Our results are not consistent with earlier results in the cotton rat model. To investigate whether ADE plays a role in the enhanced RSV disease, serum from cotton rats that were preimmunized with FI-RSV was transferred to naive cotton rats. The recipients did not develop enhanced pathology after RSV challenge [14], and this led to the conclusion that ADE does not play a role in RSV enhanced disease. The FI-RSV vaccine invariably induces neutralizing activity in cotton rats, and this could have protected these animals. It is also possible that the rats responded to different epitopes, that the range of cells in the lungs that are infected by RSV is different, or that the transfer of enhancing activity did not sustain physiological levels sufficient to cause enhanced disease at the time of RSV challenge
Other viruses that replicate within macrophages are also difficult to eliminate or eradicate by immunization. When human immunodeficiency virus (HIV) replicates in macrophages, either in vivo or in vitro, there is intracellular accumulation of the virus without the cells being killed. HIV-1-infected macrophages contribute to viral persistence by serving as a reservoir for virus, disseminating the virus, and promoting immunosuppression [15]. Macrophages not only play a role in antigen presentation but also produce tumor necrosis factor (TNF)-α, which is responsible for endothelial activation and local inflammation. When Lassa fever virus replicates in macrophages without damaging them, there is a down-regulation of lipopolysaccharide-stimulated TNF-α mRNA synthesis. A cumulative down-regulation of TNF-α and interleukin-8 expression could explain the absence of inflammation and effective immune response in severe cases of Lassa hemorrhagic fever [16]. On the contrary, viruses such as Marburg and Dengue replicate in macrophages, release TNF-α, and increase the permeability of endothelial cells [17–19]. RSV behaves like Marburg and Dengue viruses in producing TNF-α, as has been shown in mouse studies [20]. In Dengue hemorrhagic fever and Marburg virus disease, the infected macrophages are circulating monocytes; therefore, with the release of enormous amounts of TNF-α [21], there is a generalized vascular leak. In RSV-enhanced disease after FI-RSV preimmunization, it is conceivable that macrophages entering the lung get infected, which results in a vascular leak that is restricted to the lung. In RSV-enhanced disease, abundant amounts of TNF-α accompanied by the vascular leak is seen around the small vessels of the lung (E.M.P., A.R.H., H.W., and E.A.F.S., unpublished observations). We have not examined the systemic circulation of virus or the systemic production of TNF-α
Human alveolar macrophages get infected with RSV [21], and circulating human and bovine macrophages can be infected with RSV [12, 22]. Replication of RSV has been demonstrated in human and bovine mononuclear cells in vitro [23, 24], and subneutralizing concentrations of virus-specific antibodies can enhance the replication of human RSV in monocytic cell lines [25]
After the immunization of humans with FI-RSV vaccine, nonfunctional, nonneutralizing antibodies have been reported [26]. It is now evident that there are nonfunctional, nonneutralizing antibodies in the serum of Bonnet monkeys. Furthermore, there is a correlation between ADE and disease in the Bonnet monkey. In the RSV model of ADE, we show that there is an increase in the percentage of macrophages that are infected in the presence of enhancing antibodies
Enhanced disease after FI-RSV vaccine has been shown in African green [27] and cynomolgus [28] monkeys. However, in these animals, FI-RSV vaccine induced neutralizing antibodies that resulted in decreased viral replication in the lungs. In contrast, in our model, similar to responses that have been reported in human infants [26], there was a low neutralizing antibody response. Whether this was due to variations in the method of vaccine preparation in the different studies or differences in the host responses is not clear
Monoclonal antibodies against RSV surface glycoproteins have been shown to have an enhancing effect, not only by Giminez et al. [7] but also by others [29]. Antibodies against surface epitopes that are involved with viral attachment can bring about this Fc-mediated uptake of virus by the macrophages. It has been shown by Giminez et al. [7] that several monoclonal antibodies against RSV G protein and one against RSV F protein have enhancing activity. Thus, antibodies against the G and F proteins could have an enhancing effect, whereas antibodies against the nucleoproteins of RSV should not. Kirlov et al. [30] demonstrated that monoclonal antibodies against nucleoproteins do not participate in an Fc receptor-mediated enhancement, and, therefore, the development of newer vaccines for active immunization should use protective epitopes that will not result in the production of antibodies that would enhance infection
So far, live attenuated vaccines [31] and purified F protein vaccines [32] have both induced either no or low levels of neutralizing antibodies. High levels of neutralizing antibody protect the lower respiratory tract of cotton rats [33] and humans [34, 35]. It is for this reason that Palivizumab (Medimmune), an antibody directed against the F protein, which was selected for having the strongest binding affinity and neutralizing activity, has been a success in passive prophylaxis [34]. A 55% reduction in the hospitalization rate was observed after Palivizumab prophylaxis in children who were born prematurely or who had bronchopulmonary dysplasia [35]
Besides the enhancing activity, RSV antibodies may be capable of forming immune complexes and causing enhanced disease. Immune complex-mediated disease is complement mediated [36], and leukotrienes play a part in its pathogenesis [37–,39]. Thus, various mechanisms may be responsible for the enhanced disease of RSV, as has been recently reported [40]. We now show that ADE may also play a role in the immunopathogenesis of RSV enhanced disease. Therefore, when newer vaccines are being developed, testing for ADE in a suitable animal model may be essential
References
The study was approved by the Animal Welfare and Safety Committees of Christian Medical College Hospital, Vellore, and University of Colorado Health Sciences Center, Denver
Financial support: National Institute of Allergy and Infectious Diseases (grant AI-37271); Department of Biotechnology of the Government of India; United States-India Vaccine Action Program; Metropolitan State College of Denver (school to career program to J.S.)
Present affiliations: Gambro BCT Production, Lakewood, Colorado (J.S.); National Center for Research Resources, National Institutes of Health, Bethesda, Maryland (A.R.H.)