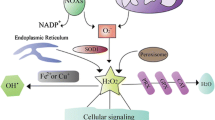
Abstract
Reactive species are frequently formed after viral infections. Antioxidant defences, including enzymatic and non-enzymatic components, protect against reactive species, but sometimes these defences are not completely adequate. An imbalance in the production of reactive species and the body’s inability to detoxify these reactive species is referred to as oxidative stress. The aim of this review is to analyse the role of oxidative stress in the pathogenesis of viral infections and highlight some major therapeutic approaches that have gained importance, with regards to controlling virus-induced oxidative injury. Attention will be focused on DNA viruses (papillomaviruses, hepadnaviruses), RNA viruses (flaviviruses, orthomyxoviruses, paramyxoviruses, togaviruses) and retroviruses (human immunodeficiency virus). In general, viruses cause an imbalance in the cellular redox environment, which depending on the virus and the cell can result in different responses, e.g. cell signaling, antioxidant defences, reactive species, and other processes. Therefore, the modulation of reactive species production and oxidative stress potentially represents a novel pharmacological approach for reducing the consequences of viral pathogenesis.
Similar content being viewed by others
References
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Akaike T (2001) Role of free radicals in viral pathogenesis and mutation. Rev Med Virol 11:87–101
Lander HM (1997) An essential role for free radicals and derived species in signal transduction. FASEB J 11(2):118–124
Gloire G, Legrand-Poels S, Piette J (2006) NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72(11):1493–1505
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, Oxford
Nakashima I, Liu W, Akhand AA et al (2003) 4-Hydroxynonenal triggers multistep signal transduction cascades for suppression of cellular functions. Mol Aspects Med 24:231–238
Forman HJ, Dickinson DA (2004) Introduction to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule. Free Radic Biol Med 37:594–596
Armogida M, Nisticò R, Mercuri NB (2012) Therapeutic potential of targeting hydrogen peroxide metabolism in the treatment of brain ischaemia. Br J Pharmacol 166:1211–1224
Ratnam DV, Ankola DD, Bhardwaj V et al (2006) Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Controll Release 113:189–207
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356
Zamocky M, Furtmuller PG, Obinger C (2008) Evolution of catalases from bacteria to humans. Antioxid Redox Signal 10:1527–1548
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8:1865–1879
Peterhans E (1979) Sendai virus stimulates chemiluminescence in mouse spleen cells. Biochem Biophys Res Commun 91:383–392
Peterhans E, Grob M, Burge T et al (1987) Virus-induced formation of reactive oxygen intermediates in phagocytic cells. Free Radic Res Commun 3(1–5):39–46
Muller F (1992) Reactive oxygen intermediates and human immunodeficiency virus (HIV) infection. Free Radic Biol Med 13(6):651–657
Reshi ML, Su Y-C, Hong J-R (2014) RNA viruses: ROS-mediated cell death. Int J Cell Biol 2014:467452-1--467452-16. doi:10.1155/2014/467452
Burdon RH (1995) Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18:775–794
Albrecht T, Boldogh I, Fons MP (1992) Receptor-initiated activation of cells and their oncogenes by herpes-family viruses. J Investig Dermatol 98(6 Suppl):29S–35S
Pace GW, Leaf CD (1995) The role of oxidative stress in HIV disease. Free Radic Biol Med 19:523–528
Stehbens WE (2004) Oxidative stress in viral hepatitis and AIDS. Exp Mol Pathol 77:121–132
Deramaudt TB, Dill C, Bonay M (2013) Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Médecine et maladies infectieuses 43:100–107
Gloenbock DT, Hampton RY, Qusesh RS et al (1991) Lipid Al-like molecule that antagonize the effects of endotoxin on human monocytes. J Biol Chem 266:19490–19498
Schwarz KB (1996) Oxidative stress during viral infection: a review. Free Radic Biol Med 21(5):641–649
Zhang Y, Wanga Z, Chen H et al (2014) Antioxidants: potential antiviral agents for Japanese encephalitis virus infection. Int J Infect Dis 24:30–36
Choi J, Ou JH (2006) Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol 290:G847–G851
Ha H-L, Shin H-J, Feitelson M et al (2010) Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 16(48):6035–6043
Peterhans E (1997) Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr 127:962S–965S
Gullberg RC, Jordan Steel J, Moon SL et al (2015) Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 475:219–229
Jacobson MD (1996) Reactive oxygen species and programmed cell death. Trends Biochem Sci 21:83–86
Peterhans E (1997) Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res 56:107–116
Akaike T, Suga M, Maeda H (1998) Free radicals in viral pathogenesis: molecular mechanisms involving superoxide and NO. Proc Soc Exp Biol Med 217:64–73
Beck MA (2000) Nutritionally induced oxidative stress: effect on viral disease. Am J Clin Nutr 71:1676S–1679S
Valyi-Nagy T, Dermody TS (2005) Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol Histopathol 20:957–967
Williams VM, Filippova M, Soto U et al (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6:45–57
Medvedev R, Ploen D, Hildt E (2016) HCV and oxidative stress: implications for HCV life cycle and HCV-associated pathogenesis. Oxid Med Cell Longev 2016:9012580
Huang H, Chen Y, Ye J (2007) Inhibition of hepatitis C virus replication by peroxidation of arachidonate and restoration by vitamin E. Proc Natl Acad Sci USA 104(47):18666–18670
Choi J, Lee KJ, Zheng Y et al (2004) Reactive oxygen species suppress hepatitis C virus RNA replication in human hepatoma cells. Hepatology 39(1):81–89
Ezzikouri S, Nishimura T, Kohara M et al (2015) Inhibitory effects of Pycnogenol® on hepatitis C virus replication. Antiviral Res 113:93–102
Dröge W (2003) Oxidative stress and aging. Adv Exp Med Biol 543:191–200
Perry G, Raina AK, Nunomura A et al (2000) How important is oxidative damage? Lessons from Alzheimer’s disease. Free Radic Biol Med 28:831–834
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44:479–496
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
Foppoli C, DeMarco F, Cini C et al (2015) Redox control of viral carcinogenesis: the human papillomavirus paradigm. Biochimica et Biophysica Acta 1850:1622–1632
Czaja MJ (2007) Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis 27:378–389
Storz P (2005) Reactive oxygen species in tumor progression. Front Biosci 10:1881–1896 (PubMed:15769673)
Hagen TM, Huang S, Curnutte J et al (1994) Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci USA 91:12808–12812
Demirdag K, Yilmaz S, Ozdarendeli A et al (2003) Levels of plasma malondialdehyde and erythrocyte antioxidant enzyme activities in patients with chronic hepatitis B. Hepatogastroenterology 50:766–770
Bolukbas C, Bolukbas FF, Horoz M et al (2005) Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis 5:95
Fujita N, Sugimoto R, Ma N et al (2008) Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat 15:498–507
Gwak GY, Lee DH, Moon TG et al (2008) The correlation of hepatitis B virus PreS mutation with cellular oxidative DNA damage in hepatocellular carcinoma. Hepatogastroenterology 55:2028–2032
Higgs MR, Chouteau P, Lerat H (2014) ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. J Gen Virol 95:991–1004
Ren JH, Chen X, Zhou L et al (2016) Protective role of Sirtuin3 (SIRT3) in oxidative stress mediated by hepatitis B virus X protein expression. PLoS One. doi:10.1371/journal.pone.0150961
Farinati F, Cardin R, Degan P et al (1999) Oxidative DNA damage in circulating leukocytes occurs as an early event in chronic HCV infection. Free Radic Biol Med 27:1284–1291
Sumida Y, Nakashima T, Yoh T et al (2000) Serum thioredoxin levels as an indicator of oxidative stress in patients with hepatitis C virus infection. J Hepatol 33:616–622
Mahmood S, Kawanaka M, Kamei A et al (2004) Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid Redox Signal 6:19–24
Sukowati Caecilia HC, El-Khobar Korri E, Ie Susan I et al (2016) Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J Gastroenterol 22(4):1497–1512
Gong G, Waris G, Tanveer R et al (2001) Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kB. Proc Natl Acad Sci USA 98:9599–9604
Pahl HL (1999) Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev 0:683–701
Berridge MJ, Bootman MD, Lipp P (1998) Calcium—a life and death signal. Nature (London) 395:645–648
Murphy A, Bredesen G, Cortopaasi C et al (1996) Proc Natl Acad Sci USA 93:9893–9898
Yen HH, Shih KL, Lin TT et al (2012) Decreased mitochondrial deoxyribonucleic acid and increased oxidative damage in chronic hepatitis C. World J Gastroenterol 18(36):5084–5089
Piccoli C, Quarato G, Ripoli M et al (2009) Review HCV infection induces mitochondrial bioenergetic unbalance: causes and effects. Biochim Biophys Acta 1787(5):539–546
Piccoli C, Scrima R, D’Aprile A et al (2006) Review mitochondrial dysfunction in hepatitis C virus infection. Biochim Biophys Acta 1757(9–10):1429–1437
Mercurio F, Manning A (1999) Oncogene 18:6163–6171
Qadri I, Iwahashi M, Capasso JM et al (2004) Induced oxidative stress and activated expression of manganese superoxide dismutase during hepatitis C virus replication: role of JNK, p38 MAPK and AP-1. Biochem J 378:919–928
Paracha UZ, Fatima K, Alqahtani M et al (2013) Oxidative stress and hepatitis C virus. Virol J 10:251
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26(Suppl 1):173–179
Fisher AB (2009) Redox signaling across cell membranes. Antioxid Redox Signal 11:1349–1356
Waris G, Turkson J, Hassanein T et al (2005) Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: role of STAT-3 in HCV replication. J Virol 79:1569–1580
Arzumanyan A, Reis HM, Feitelson MA (2013) Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer 13:123–135
Lozano-Sepulveda AS, Bryan-Marrugo OL, Cordova-Fletes C et al (2015) Oxidative stress modulation in hepatitis C virus infected cells. World J Hepatol 7(29):2880–2889
Gabbay E, Zigmond E, Pappo O et al (2007) Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol 13:5317–5323
Miura K, Taura K, Kodama Y et al (2008) Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology 48:1420–1429
Deng L, Shoji I, Ogawa W et al (2011) Hepatitis C virus infection promotes hepatic gluconeogenesis through an NS5A-mediated, FoxO1-dependent pathway. J Virol 85:8556–8568
Chambers TJ, Hahn CS, Galler R et al (1990) Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688
Burke DS, Lorsomrudee W, Leake CJ et al (1985) Fatal outcome in Japanese encephalitis. Am J Trop Med Hyg 34:1203–1210
Mathur A, Kulshreshtha R, Chaturvedi UC (1989) Evidence for latency of Japanese encephalitis virus in T lymphocytes. J Gen Virol 70:461–465
Raung SL, Kuo MD, Wang YM et al (2001) Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci Lett 315:9–12
Liao SL, Raung SL, Chen CJ (2002) Japanese encephalitis virus stimulates superoxide dismutase activity in rat glial cultures. Neurosci Lett 324:133–136
Srivastava R, Kalita J, Khan MY et al (2009) Free radical generation by neurons in rat model of Japanese encephalitis. Neurochem Res 34:2141–2146
Kumar S, Misra UK, Kalita J et al (2009) Imbalance in oxidant/ antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem Int 55:648–654
Yang TC, Lai CC, Shiu SL et al (2010) Japanese encephalitis virus down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38 MAPK activation in human promonocyte cells. Microbes Infect 12:643–651
Kaushik DK, Gupta M, Kumawat KL, Basu A (2012) NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS One 7:e32270
Gil L, Martínez G, Tápanes R et al (2004) Oxidative stress in adult dengue patients. Am J Trop Med Hyg 71:652–657
Klassen P, Biesalski HK, Mazariegos M et al (2004) Classic dengue fever affects levels of circulating antioxidants. Nutrition 20:542–547
Soundravally R, Sankar P, Bobby Z et al (2008) Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidation. Platelets 19(6):447–454
Soundravally R, Hoti SL, Patil SA et al (2014) Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int J Infect Dis 18:68–72
Yen YTCH, Lin YD, Shieh CC et al (2008) Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development. J Virol 82(24):12312–12324
Tian YJW, Gao N, Zhang J et al (2010) Inhibitory effects of glutathione on dengue virus production. Biochem Biophys Res Commun 397(3):420–424
Wang J, Chen Y, Gao N et al (2013) Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PloS One 8(1):e55407
Akaike T, Noguchi Y, Ijiri S et al (1996) Pathogenesis of influenza virus-induced pneumonia: Involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA 93:2448–2453
Choi AM, Knobil K, Otterbein SL et al (1996) Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am J Physiol 271(3 Pt 1):L383–L391
Suliman HB, Ryan LK, Bishop L et al (2001) Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am J Physiol Lung Cell Mol Physiol 280(1):L69–L78
Kobasa D, Jones SM, Shinya K et al (2007) Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445(7125):319–323
Walsh KB, Teijaro JR, Wilker PR et al (2011) Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci USA 108(29):12018–12023
Ng MP, Lee JC, Loke WM et al (2014) Does influenza A infection increase oxidative damage? Antioxid Redox Signal 21(7):1025–1031. doi:10.1089/ars.2014.5907 (Epub Jul 21)
Buffinton GD, Christen S, Peterhans E et al (1992) Oxidative stress in lungs of mice infected with influenza A virus. Free Radic Res Commun 16(2):99–110
Hennet T, Peterhans E, Stocker R (1992) Alterations in antioxidant defences in lung and liver of mice infected with influenza A virus. J Gen Virol 73(Pt 1):39–46
Cai J, Chen Y, Seth S et al (2003) Inhibition of influenza infection by glutathione. Free Radic Biol Med 34(7):928–936
Shi X, Shi Z, Huang H et al (2014) Ability of recombinant human catalase to suppress inflammation of the murine lung induced by influenza A. Inflammation 37(3):809–817. doi:10.1007/s10753-013-9800-2
Lin X, Wang R, Zou W et al (2016) The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses 8:13. doi:10.3390/v8010013
Mochizuki H, Todokoro M, Arakawa H (2009) RS virus-induced inflammation and the intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation 32(4):252–264. doi:10.1007/s10753-009-9128-0
Casola A, Burger N, Liu T et al (2001) Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem 276:19715–19722
Liu T, Shawn C, Brasier AR et al (2004) Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 279:2461–2469
Hosakote YM, Liu T, Castro SM et al (2009) Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am J Respir Cell Mol Biol 41:348–357
Castro SM, Guerrero-Plata A, Suarez-Real G et al (2006) Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am J Respir Crit Care Med 174:1361–1369
Hosakote YM, Jantzi PD, Esham DL et al (2011) Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 183:1550–1560
Huang SH, Cao XJ, Liu W et al (2010) Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res 48(2):109–116. doi:10.1111/j.1600-079X.2009.00733.x (Epub Jan 8)
Hosakote YM, Komaravelli N, Mautemps N et al (2012) Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol 303(11):L991–L1000. doi:10.1152/ajplung.00192.2012
Moreno-Solís G, de la Torre-Aguilar MJ, Torres-Borrego J et al (2015) Oxidative stress and inflamatory plasma biomarkers in respiratory syncytial virus bronchiolitis. Clin Respir J. doi:10.1111/crj.12425
Dhanwani R, Khan M, Alam SI et al (2011) Differential proteome analysis of Chikungunya virus infected new-born mice tissues reveal implication of stress, inflammatory and apoptotic pathways in disease pathogenesis. Proteomics 11:1936–1951
Dhanwani R, Khan M, Bhaskar AS et al (2012) Characterization of Chikungunya virus infection in human neuroblastoma SH-SY5Y cells: role of apoptosis in neuronal cell death. Virus Res 163(2012):563–572
Patil DR, Hundekar SL, Arankalle VA (2012) Expression profile of immune response genes during acute myopathy induced by chikungunya virus in a mouse model. Institut Pasteur Microbes Infect 14:457–469
Joubert PE, Werneke SW, de la Calle C et al (2012) Chikungunya virus-induced autophagy delays caspase-dependent cell death. J Exp Med 209(5):1029–1047
Nabel G, Baltimore D (1987) An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326(6114):711–713
Fuchs J, Ochsendorf F, Schofer H et al (1991) Oxidative imbalance in HIV infected patients. Med Hypotheses 36(1):60–64
Staal FJT, Roederer M, Herzenberg LA et al (1990) Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proc Natl Acad Sci USA 87(24):9943–9947
Meyer M, Pahl HL, Baeuerle PA (1994) Regulation of the transcription factors NF-κB and AP-1 by redox changes. Chem-Biol Interact 91(2–3):91–100
Sonnerborg A, Carlin G, Akerlund B et al (1988) Increased production of malondialdehyde in patients with HIV infection”. Scand J Infect Dis 20(3):287–290
Revillard J-P, Vincent CMA, Favier AE et al (1992) Lipid peroxidation in human immunodeficiency virus infection. J Acquired Immune Defic Syndromes 5(6):637–638
Favier A, Sappey C, Leclerc P et al (1994) Antioxidant status and lipid peroxidation in patients infected with HIV. Chem-Biol Interact 91(2–3):165–180
Malvy DJM, Richard M-J, Arnaud J et al (1994) Relationship of plasma malondialdehyde, vitamin E and antioxidant micronutrients to human immunodeficiency virus-1 seropositivity. Clin Chim Acta 224:89–94
Dröge W, Eck HP, Mihm S (1994) Oxidant-antioxidant status in human immunodeficiency virus infection. Oxygen Radic Biol Syst Methods Enzymol 233:594–601
Fuchs J, Emerit I, Levy A et al (1995) Clastogenic factors in plasma of HIV-1 infected patients. Free Radic Biol Med 19:843–848
De Rosa SC, Zaretsky MD, Dubs JG et al (2000) N-acetylcysteinereplenishes glutathione in HIV infection. Eur J Clin Investig 30:915–929
De La Fuente M, Miquel J, Catalan MP et al (2002) The amount of thiolic antioxidant ingestion related to improve several immune functions is higher in aged than in adult mice. Free Radic Res 36:119–126
Leff JA, Oppegard MA, Curiel TJ et al (1992) Progressive increases in serum catalase activity in advancing human immunodeficiency virus infection. Free Radic Biol Med 13(2):143–149
Greenspan HC (1994) Aruoma OI (1994) Oxidative stress and apoptosis in HIV infection: a role for plant-derived metabolites with synergistic antioxidant activity. Immunol Today 15(5):209–213
Mishra MK, Ghosh D, Duseja R et al (2009) Antioxidant potential of minocycline in Japanese encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int 54:464–470
Dutta K, Ghosh D, Basu A (2009) Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin–proteasome system. J Neuroimmune Pharmacol 4:328–337
Gansukh E, Kazibwe Z, Pandurangan M et al (2016) Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 23:958–967
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camini, F.C., da Silva Caetano, C.C., Almeida, L.T. et al. Implications of oxidative stress on viral pathogenesis. Arch Virol 162, 907–917 (2017). https://doi.org/10.1007/s00705-016-3187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-016-3187-y