Dosing & Uses
Dosage Forms & Strengths
injectable solution: Schedule II
- 10mg/mL
tablet: Schedule II
- 5mg
- 10mg
dispersible tablet: Schedule II
- 40mg
oral solution: Schedule II
- 5mg/5mL
- 10mg/5mL
oral concentrate solution: Schedule II
- 10mg/mL
Pain Management
Indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate
Opioid-naive patients: 2.5 mg PO q8-12hr; titrate slowly with dose increases no more frequent than every 3-5 days
Conversion from parenteral methadone to oral methadone
- 1:2 Parenteral-to-PO ratio: 5 mg parenteral = 10 mg PO
Conversion from other oral opioids to oral or intravenous methadone
- Total daily baseline oral estimated daily oral and intravanous methadone requirement as percent of morphine equivalent dose
- < 100 mg morphine equivalent dose: Administer 20% to 30% oral methadone or 10-15% IV methdone as percent of morphine equivalent dose
- 100-300 mg morphine equivalent dose: Administer 10% to 20% oral methadone or 5-10% IV methadone as percent of morphine equivalent dose
- 300-600 mg morphine equivalent dose: administer 8% to 12% oral methadone or 4-6% IV methadone as percent of morphine equivalent dose
- 600-1,000 mg morphine equivalent dose: administer 5% to 10% oral methadone or 3-5% IV methadone as percent of morphine equivalent dose
- > 1,000 mg morphine equivalent dose: Administer < 5 % oral methadone or <3% IV methadone as percent of morphine equivalent dose
- For patients on a single opioid, sum the current total daily dose of the opioid, convert it to a morphine equivalent dose according to specific conversion factor for that specific opioid, then multiply the Morphine equivalent dose by the corresponding percentage in the above conversion description to calculate the approximate oral and IV methadone daily dose; divide the total daily methadone dose derived from the conversion description above to reflect the intended dosing schedule (i.e., for administration every 8 hours, divide total daily methadone dose by 3)
- For patients on a regimen of more than one opioid, calculate the approximate oral methadone dose for each opioid and sum the totals to obtain the approximate total methadone daily dose. Divide the total daily methadone dose derived from the conversion description above to reflect the intended dosing schedule (i.e., for administration every 8 hours, divide total daily methadone dose by 3)
Parenteral morphine to intravenous methadone conversion for chronic administration
- Total daily baseline parenteral estimated daily parenteral methadone requirement as percent of total daily morphine dose
- 10-30 mg total daily baseline parenteral morphine dose: Administer 40-66% parenteral methadone as percent of morphine dose
- 30-50 mg total daily baseline parenteral morphine dose: Administer 27-66% parenteral methadone as percent of morphine dose
- 50-100 mg total daily baseline parenteral morphine dose: Administer 22-50% parenteral methadone as percent of morphine dose
- 100-200 mg total daily baseline parenteral morphine dose: Administer 15-34% parenteral methadone as percent of morphine dose
- 200-500 mg total daily baseline parenteral morphine dose: Administer 10% to 20% parenteral methadone as percent of morphine dose
- The total daily methadone dose derived from the conversion calculations above may be divided to reflect the intended dosing schedule (i.e., for administration every 8 hours, divide total daily methadone dose by 3)
- Methadone dosing should not be based solely on these methadone conversion calculations; dose titration methods should always be individualized to account for the patient's prior opioid exposure, general medical condition, concomitant medication, and anticipated breakthrough medication use; the endpoint of titration is achievement of adequate pain relief, balanced against tolerability of opioid side effects; if a patient develops intolerable opioid related side effects, the methadone dose, or dosing interval, may need to be decreased; for patients on a regimen of fixed-ratio opioid/non-opioid analgesic products, use only the opioid component of these products in the conversion; always round the dose down, if necessary, to the appropriate methadone tablet or IV formulation strength(s) available
Opioid-tolerant patients
- Discontinue all other around-the-clock opioids
- Substantial interpatient variability, see prescribing information for guidance
Opioid-tolerant definition
- Patients who are opioid tolerant are those receiving, for 1 week or longer, at least 60 mg/day PO morphine, 25 mcg/hr transdermal fentanyl, 30 mg/day PO oxycodone, 8 mg/day PO hydromorphone, 25 mg/day PO oxymorphone, or an equianalgesic dose of another opioid
- Use of higher starting doses in patients who are not opioid tolerant may cause fatal respiratory depression
Detoxification
20-30 mg PO once daily or minimum dosage necessary to suppress withdrawal; may be titrated to 40 mg/day in divided doses and continued for 2-3 days, then decreased 20% daily as tolerated
Dosing Modifications
Renal impairment (CrCl <10mL/min): 50-75% of normal dose
Hepatic impairment: Not recommended in severe liver disease:
Dosing Considerations
Do not abruptly discontinue methadone in a physically dependent patient
Limitations of use
- Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, and because of the greater risks of overdose and death with extended-release opioid formulations, reserve for patients whom alternative treatment options (eg, nonopioid analgesics or immediate-release opioids) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain
- Not indicated for acute pain or as a PRN analgesic
Access to naloxone for opioid overdose
- Assess need for naloxone upon initiating and renewing treatment
Consider prescribing naloxone
- Based on patient’s risk factors for overdose (eg, concomitant use of CNS depressants, a history of opioid use disorder, prior opioid overdose); presence of risk factors should not prevent proper pain management
- Household members (including children) or other close contacts at risk for accidental ingestion or overdose
Consult patients and caregivers on the following:
- Availability of naloxone for emergency treatment of opioid overdose
- Ways differ on how to obtain naloxone as permitted by individual state dispensing and prescribing requirements or guidelines (eg, by prescription, directly from a pharmacist, as part of a community-based program)
Dosage Forms & Strengths
injected solution: Schedule II
- 10mg/mL
tablet: Schedule II
- 5mg
- 10mg
- 40mg
dispersible tablet: Schedule II
- 40mg
oral solution: Schedule II
- 5mg/5mL
- 10mg/5mL
Pain (Off-label)
0.7 mg/kg/day PO/SC/IV/IM divided q6hr PRN; not to exceed 10 mg/dose
Opiate Withdrawal (Off-label)
Neonates: 0.05-0.2 mg/kg PO q12-24hr; reduce dose by 10-20% per week over 4-6 weeks; adjust tapering on signs and symptoms of withdrawal
Pain
2.5 mg PO/IM q8-12hr; titrate slowly with dose increases no more frequent than every 3-5 days
Detoxification
20-30 mg PO once daily or minimum dosage necessary to suppress withdrawal; may be titrated to 40 mg/day in divided doses and continued for 2-3 days, then decreased 20% daily as tolerated
Interactions
Interaction Checker
No Results

Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor

Contraindicated (11)
- alvimopan
alvimopan, methadone. receptor binding competition. Contraindicated. Alvimopan is contraindicated in opioid tolerant patients (ie, those who have taken therapeutic doses of opioids for >7 consecutive days immediately prior to taking alvimopan). Patients recently exposed to opioids are expected to be more sensitive to the effects of alvimopan and therefore may experience abdominal pain, nausea and vomiting, and diarrhea. No significant interaction is expected with concurrent use of opioid analgesics and alvimopan in patients who received opioid analgesics for 7 or fewer consecutive days prior to alvimopan.
- eliglustat
methadone increases levels of eliglustat by affecting hepatic enzyme CYP2D6 metabolism. Contraindicated. If coadministered with strong or moderate CYP2D6 inhibitors, reduce eliglustat dose from 84 mg BID to 84 mg once daily in extensive and intermediate metabolizers; eliglustat is contraindiated if strong or moderate CYP2D6 inhibitors are given concomitantly with strong or moderate CYP3A inhibitors.
- itraconazole
itraconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated. QT interval prolongation and serious arrhythmia (torsades de pointes) have occurred in patients using methadone concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- ketoconazole
ketoconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated.
ketoconazole and methadone both increase QTc interval. Contraindicated. - lefamulin
lefamulin will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated. Lefamulin is contraindicated with CYP3A substrates know to prolong the QT interval.
- levoketoconazole
levoketoconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated.
levoketoconazole and methadone both increase QTc interval. Contraindicated. - olanzapine/samidorphan
olanzapine/samidorphan will decrease the level or effect of methadone by pharmacodynamic antagonism. Contraindicated. Samidorphan elicits opioid antagonistic effects and increases risk of precipitating acute opioid withdrawal in patients dependent on opioids. Prescribing information recommends at least a 7-day opioid-free interval for short-acting opioids and at least a 14-day opioid-free interval for long-acting opioids before starting olanzapine/samidorphan.
- rasagiline
rasagiline increases toxicity of methadone by unknown mechanism. Contraindicated. Risk of hypotension, hyperpyrexia, somnolence, or death.
- ribociclib
ribociclib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Contraindicated.
- safinamide
methadone, safinamide. Either increases toxicity of the other by serotonin levels. Contraindicated. Concomitant use could result in life-threatening serotonin syndrome.
- selegiline
selegiline increases toxicity of methadone by unknown mechanism. Contraindicated. At least 14 days should elapse between discontinuation of selegiline and initiation of analgesic.
Serious - Use Alternative (137)
- acrivastine
acrivastine and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- adagrasib
adagrasib, methadone. Either increases effects of the other by QTc interval. Avoid or Use Alternate Drug. Each drug prolongs the QTc interval, which may increased the risk of Torsade de pointes, other serious arryhthmias, and sudden death. If coadministration unavoidable, more frequent monitoring is recommended for such patients.
- alfuzosin
alfuzosin and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- amiodarone
amiodarone and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- amisulpride
amisulpride and methadone both increase QTc interval. Avoid or Use Alternate Drug. ECG monitoring is recommended if coadministered.
amisulpride and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - anagrelide
anagrelide and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- apalutamide
apalutamide will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Coadministration of apalutamide, a strong CYP3A4 inducer, with drugs that are CYP3A4 substrates can result in lower exposure to these medications. Avoid or substitute another drug for these medications when possible. Evaluate for loss of therapeutic effect if medication must be coadministered. Adjust dose according to prescribing information if needed.
- apomorphine
apomorphine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- aripiprazole
aripiprazole and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- arsenic trioxide
arsenic trioxide and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- artemether
artemether and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- asenapine
asenapine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
asenapine and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - asenapine transdermal
asenapine transdermal and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- atomoxetine
atomoxetine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- avapritinib
avapritinib and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- benzhydrocodone/acetaminophen
benzhydrocodone/acetaminophen, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
benzhydrocodone/acetaminophen and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - bremelanotide
bremelanotide will decrease the level or effect of methadone by Other (see comment). Avoid or Use Alternate Drug. Bremelanotide may slow gastric emptying and potentially reduces the rate and extent of absorption of concomitantly administered oral medications. Avoid use when taking any oral drug that is dependent on threshold concentrations for efficacy. Interactions listed are representative examples and do not include all possible clinical examples.
- brexpiprazole
brexpiprazole and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- brimonidine
brimonidine and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- brivaracetam
brivaracetam and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- buprenorphine
buprenorphine, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Mixed opiate agonist/antagonists usually produce additive sedation with narcotics; however, in narcotic addicted pts., the antagonist activity may provoke withdrawal Sx.
buprenorphine and methadone both increase QTc interval. Avoid or Use Alternate Drug. - buprenorphine buccal
buprenorphine buccal, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Mixed opiate agonist/antagonists usually produce additive sedation with narcotics; however, in narcotic addicted pts., the antagonist activity may provoke withdrawal Sx.
buprenorphine buccal and methadone both increase QTc interval. Avoid or Use Alternate Drug. - buprenorphine subdermal implant
buprenorphine subdermal implant and methadone both increase QTc interval. Avoid or Use Alternate Drug.
buprenorphine subdermal implant and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - buprenorphine transdermal
buprenorphine transdermal and methadone both increase QTc interval. Avoid or Use Alternate Drug.
buprenorphine transdermal and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - buprenorphine, long-acting injection
buprenorphine, long-acting injection and methadone both increase QTc interval. Avoid or Use Alternate Drug.
buprenorphine, long-acting injection and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - butorphanol
butorphanol, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Mixed opiate agonist/antagonists usually produce additive sedation with narcotics; however, in narcotic addicted pts., the antagonist activity may provoke withdrawal Sx.
- calcium/magnesium/potassium/sodium oxybates
methadone, calcium/magnesium/potassium/sodium oxybates. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
- carbamazepine
carbamazepine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- cariprazine
cariprazine and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- ceritinib
ceritinib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
ceritinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. - chloroquine
chloroquine increases toxicity of methadone by QTc interval. Avoid or Use Alternate Drug.
- cimetidine
cimetidine will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
cimetidine increases effects of methadone by decreasing metabolism. Avoid or Use Alternate Drug. - clarithromycin
clarithromycin will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- clonidine
clonidine, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration enhances CNS depressant effects.
- clozapine
clozapine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- crizotinib
crizotinib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- degarelix
degarelix and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- diazepam intranasal
diazepam intranasal, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
- disopyramide
disopyramide and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- donepezil
donepezil and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- efavirenz
efavirenz and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- eliglustat
eliglustat and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- eluxadoline
methadone, eluxadoline. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Avoid coadministration with other drugs that cause constipation. Increases risk for constipation related serious adverse reactions. .
- encorafenib
encorafenib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- entrectinib
methadone and entrectinib both increase QTc interval. Avoid or Use Alternate Drug.
- enzalutamide
enzalutamide will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- eribulin
eribulin and methadone both increase QTc interval. Avoid or Use Alternate Drug. Potential for enhanced QTc-prolonging effects; if concurrent use is necessary then ECG monitoring is recommended.
- erythromycin base
erythromycin base will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- erythromycin ethylsuccinate
erythromycin ethylsuccinate will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- erythromycin lactobionate
erythromycin lactobionate will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- erythromycin stearate
erythromycin stearate will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- escitalopram
escitalopram increases toxicity of methadone by QTc interval. Avoid or Use Alternate Drug.
- ethotoin
ethotoin decreases levels of methadone by increasing metabolism. Contraindicated.
- fentanyl
fentanyl, methadone. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration with other CNS depressants, such as skeletal muscle relaxants, may cause respiratory depression, hypotension, profound sedation, coma, and/or death. Consider dose reduction of either or both agents to avoid serious adverse effects. Monitor for hypotension, respiratory depression, and profound sedation.
fentanyl and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - fentanyl intranasal
fentanyl intranasal, methadone. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration with other CNS depressants, such as skeletal muscle relaxants, may cause respiratory depression, hypotension, profound sedation, coma, and/or death. Consider dose reduction of either or both agents to avoid serious adverse effects. Monitor for hypotension, respiratory depression, and profound sedation.
fentanyl intranasal and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - fentanyl iontophoretic transdermal system
fentanyl iontophoretic transdermal system and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- fentanyl transdermal
fentanyl transdermal, methadone. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration with other CNS depressants, such as skeletal muscle relaxants, may cause respiratory depression, hypotension, profound sedation, coma, and/or death. Consider dose reduction of either or both agents to avoid serious adverse effects. Monitor for hypotension, respiratory depression, and profound sedation.
fentanyl transdermal and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - fentanyl transmucosal
fentanyl transmucosal, methadone. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration with other CNS depressants, such as skeletal muscle relaxants, may cause respiratory depression, hypotension, profound sedation, coma, and/or death. Consider dose reduction of either or both agents to avoid serious adverse effects. Monitor for hypotension, respiratory depression, and profound sedation.
fentanyl transmucosal and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate - fexinidazole
fexinidazole and methadone both increase QTc interval. Avoid or Use Alternate Drug. Avoid coadministration of fexinidazole with drugs known to block potassium channels and/or prolong QT interval.
fexinidazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Fexinidazole inhibits CYP3A4. Coadministration may increase risk for adverse effects of CYP3A4 substrates. - fingolimod
fingolimod and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- fosphenytoin
fosphenytoin decreases levels of methadone by increasing metabolism. Contraindicated.
- gadobenate
gadobenate and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- gemifloxacin
gemifloxacin and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- gilteritinib
gilteritinib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- givinostat
methadone and givinostat both increase QTc interval. Avoid or Use Alternate Drug. If unable to avoid coadministration, obtain ECGs when initiating, during concomitant use, and as clinically indicated. Withhold if QTc interval >500 ms or a change from baseline >60 ms.
- glasdegib
methadone and glasdegib both increase QTc interval. Avoid or Use Alternate Drug. If coadministration unavoidable, monitor for increased risk of QTc interval prolongation.
- granisetron
granisetron and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- hydrocodone
hydrocodone, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
- hydroxychloroquine sulfate
hydroxychloroquine sulfate and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- ibutilide
ibutilide and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- idelalisib
idelalisib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Idelalisib is a strong CYP3A inhibitor; avoid coadministration with sensitive CYP3A substrates
- indapamide
indapamide and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- inotuzumab
inotuzumab and methadone both increase QTc interval. Avoid or Use Alternate Drug. If unable to avoid concomitant use, obtain ECGs and electrolytes before and after initiation of any drug known to prolong QTc, and periodically monitor as clinically indicated during treatment.
- isocarboxazid
isocarboxazid increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. Risk of hypotension, hyperpyrexia, somnolence, or death; separate by 14 d.
- isoflurane
isoflurane and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- ivosidenib
ivosidenib and methadone both increase QTc interval. Avoid or Use Alternate Drug. Avoid coadministration of QTc prolonging drugs with ivosidenib or replace with alternate therapies. If coadministration of a QTc prolonging drug is unavoidable, monitor for increased risk of QTc interval prolongation.
ivosidenib will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Avoid coadministration of sensitive CYP3A4 substrates with ivosidenib or replace with alternative therapies. If coadministration is unavoidable, monitor patients for loss of therapeutic effect of these drugs. - levetiracetam
levetiracetam and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- linezolid
linezolid increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. Risk of hypotension, hyperpyrexia, somnolence, or death; separate by 14 d.
- lithium
lithium and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- lonafarnib
methadone will increase the level or effect of lonafarnib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. If coadministration of lonafarnib (a sensitive CYP3A substrate) with weak CYP3A inhibitors is unavoidable, reduce to, or continue lonafarnib at starting dose. Closely monitor for arrhythmias and events (eg, syncope, heart palpitations) since lonafarnib effect on QT interval is unknown.
- lopinavir
lopinavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- macimorelin
macimorelin and methadone both increase QTc interval. Avoid or Use Alternate Drug. Macimorelin causes an increase of ~11 msec in the corrected QT interval. Avoid coadministration with drugs that prolong QT interval, which could increase risk for developing torsade de pointes-type ventricular tachycardia. Allow sufficient washout time of drugs that are known to prolong the QT interval before administering macimorelin.
- methohexital
methohexital and methadone both increase sedation. Avoid or Use Alternate Drug. Limit use to patients for whom alternative treatment options are inadequate
- methylene blue
methylene blue and methadone both increase serotonin levels. Avoid or Use Alternate Drug. If drug combination must be administered, monitor for evidence of serotonergic or opioid-related toxicities
- metoclopramide intranasal
methadone, metoclopramide intranasal. Either increases effects of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Avoid use of metoclopramide intranasal or interacting drug, depending on importance of drug to patient.
- mifepristone
mifepristone will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- mirtazapine
mirtazapine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- mobocertinib
mobocertinib and methadone both increase QTc interval. Avoid or Use Alternate Drug. If coadministration unavoidable, reduce mobocertinib dose and monitor QTc interval more frequently.
- nalbuphine
nalbuphine, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Mixed opiate agonist/antagonists usually produce additive sedation with narcotics; however, in narcotic addicted pts., the antagonist activity may provoke withdrawal Sx.
- nefazodone
nefazodone will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- olanzapine
olanzapine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- olopatadine intranasal
methadone and olopatadine intranasal both increase sedation. Avoid or Use Alternate Drug. Coadministration increases risk of CNS depression, which can lead to additive impairment of psychomotor performance and cause daytime impairment.
- ondansetron
methadone and ondansetron both increase QTc interval. Avoid or Use Alternate Drug. Avoid with congenital long QT syndrome; ECG monitoring recommended with concomitant medications that prolong QT interval, electrolyte abnormalities, CHF, or bradyarrhythmias.
- oxaliplatin
oxaliplatin and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- ozanimod
ozanimod and methadone both increase sympathetic (adrenergic) effects, including increased blood pressure and heart rate. Avoid or Use Alternate Drug. Because the active metabolite of ozanimod inhibits MAO-B in vitro, there is a potential for serious adverse reactions, including hypertensive crisis. Therefore, coadministration of ozanimod with drugs that can increase norepinephrine or serotonin is not recommended. Monitor for hypertension with concomitant use.
- panobinostat
methadone and panobinostat both increase QTc interval. Avoid or Use Alternate Drug. Panobinostat is known to significantly prolong QT interval. Panobinostat prescribing information states use with drugs known to prolong QTc is not recommended.
- pentamidine
methadone and pentamidine both increase QTc interval. Avoid or Use Alternate Drug.
- pentazocine
pentazocine, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Mixed opiate agonist/antagonists usually produce additive sedation with narcotics; however, in narcotic addicted pts., the antagonist activity may provoke withdrawal Sx.
- phenelzine
phenelzine increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. Risk of hypotension, hyperpyrexia, somnolence, or death; separate by 14 d.
- phenytoin
phenytoin decreases levels of methadone by increasing metabolism. Contraindicated.
- pimozide
methadone and pimozide both increase QTc interval. Avoid or Use Alternate Drug.
- pitolisant
methadone and pitolisant both increase QTc interval. Avoid or Use Alternate Drug.
- ponesimod
ponesimod, methadone. Either increases effects of the other by QTc interval. Avoid or Use Alternate Drug. Consult cardiologist if considering treatment. Generally, should not be initiated in patients who are concurrently taking QT prolonging drugs with known arrhythmogenic properties, such as HR-lowering calcium channel blockers (eg, verapamil, diltiazem).
- primaquine
primaquine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- procainamide
methadone and procainamide both increase QTc interval. Avoid or Use Alternate Drug.
- procarbazine
procarbazine increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. MAOIs may potentiate CNS depression and hypotension. Do not use within 14 days of MAOI use. .
- quinidine
quinidine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- ribociclib
ribociclib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- rifabutin
rifabutin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- rifampin
rifampin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- romidepsin
methadone and romidepsin both increase QTc interval. Avoid or Use Alternate Drug.
- ropeginterferon alfa 2b
ropeginterferon alfa 2b and methadone both increase Other (see comment). Avoid or Use Alternate Drug. Narcotics, hypnotics or sedatives can produce additive neuropsychiatric side effects. Avoid use and monitor patients receiving the combination for effects of excessive CNS toxicity.
- saquinavir
saquinavir, methadone. Either increases toxicity of the other by QTc interval. Avoid or Use Alternate Drug. Increased risk of PR prolongation and cardiac arrhythmias.
saquinavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. - selegiline transdermal
selegiline transdermal increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. Risk of hypotension, hyperpyrexia, somnolence, or death.
- selinexor
selinexor, methadone. unspecified interaction mechanism. Avoid or Use Alternate Drug. Patients treated with selinexor may experience neurological toxicities. Avoid taking selinexor with other medications that may cause dizziness or confusion.
- sertraline
sertraline and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- sevoflurane
sevoflurane and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- siponimod
siponimod and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- sodium oxybate
methadone, sodium oxybate. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
- solifenacin
solifenacin and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- sotalol
methadone and sotalol both increase QTc interval. Avoid or Use Alternate Drug.
- St John's Wort
St John's Wort will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug.
- sufentanil SL
sufentanil SL, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration may result in hypotension, profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation.
- sunitinib
sunitinib and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- tacrolimus
tacrolimus and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- tetrabenazine
tetrabenazine and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- tramadol
tramadol, methadone. Other (see comment). Avoid or Use Alternate Drug. Comment: Tramadol may reinitiate opiate dependence in pts. previously addicted to other opiates; it may also provoke withdrawal Sx. in pts. who are currently opiate dependent.
- tranylcypromine
tranylcypromine increases toxicity of methadone by unknown mechanism. Avoid or Use Alternate Drug. Risk of hypotension, hyperpyrexia, somnolence, or death; separate by 14 d.
- tucatinib
tucatinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Avoid concomitant use of tucatinib with CYP3A substrates, where minimal concentration changes may lead to serious or life-threatening toxicities. If unavoidable, reduce CYP3A substrate dose according to product labeling.
- umeclidinium bromide/vilanterol inhaled
methadone increases toxicity of umeclidinium bromide/vilanterol inhaled by QTc interval. Avoid or Use Alternate Drug. Exercise extreme caution when vilanterol coadministered with drugs that prolong QTc interval; adrenergic agonist effects on the cardiovascular system may be potentiated.
- valerian
valerian and methadone both increase sedation. Avoid or Use Alternate Drug.
- vandetanib
methadone, vandetanib. Either increases toxicity of the other by QTc interval. Avoid or Use Alternate Drug. Avoid coadministration with drugs known to prolong QT interval; if a drug known to prolong QT interval must be used, more frequent ECG monitoring is recommended.
- vemurafenib
vemurafenib and methadone both increase QTc interval. Avoid or Use Alternate Drug. Concomitant use of vemurafenib with drugs that prolong QT interval is not recommended.
- vilanterol/fluticasone furoate inhaled
methadone increases toxicity of vilanterol/fluticasone furoate inhaled by QTc interval. Avoid or Use Alternate Drug. Exercise extreme caution when vilanterol coadministered with drugs that prolong QTc interval; adrenergic agonist effects on the cardiovascular system may be potentiated.
- vorinostat
vorinostat and methadone both increase QTc interval. Avoid or Use Alternate Drug.
- voxelotor
voxelotor will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Avoid or Use Alternate Drug. Voxelotor increases systemic exposure of sensitive CYP3A4 substrates. Avoid coadministration with sensitive CYP3A4 substrates with a narrow therapeutic index. Consider dose reduction of the sensitive CYP3A4 substrate(s) if unable to avoid.
- zuranolone
methadone, zuranolone. Either increases effects of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Coadministration of zuranolone with other CNS depressants may increase impairment of psychomotor performance or CNS depressant effects. If unavoidable, consider dose reduction. .
Monitor Closely (345)
- abacavir
abacavir will decrease the level or effect of methadone by unknown mechanism. Use Caution/Monitor. Monitor for opioid withdrawal symptoms.
- albuterol
methadone increases and albuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
albuterol and methadone both increase QTc interval. Use Caution/Monitor. - alfentanil
alfentanil and methadone both increase sedation. Use Caution/Monitor.
- alfuzosin
methadone and alfuzosin both increase QTc interval. Use Caution/Monitor.
- alprazolam
alprazolam and methadone both increase sedation. Use Caution/Monitor.
- amitriptyline
amitriptyline and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and amitriptyline both increase sedation. Use Caution/Monitor. - amobarbital
amobarbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
amobarbital and methadone both increase sedation. Use Caution/Monitor. - amoxapine
amoxapine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and amoxapine both increase sedation. Use Caution/Monitor. - apomorphine
methadone and apomorphine both increase sedation. Use Caution/Monitor.
- aprepitant
aprepitant will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- arformoterol
methadone increases and arformoterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
arformoterol and methadone both increase QTc interval. Use Caution/Monitor. - aripiprazole
methadone and aripiprazole both increase sedation. Use Caution/Monitor.
methadone, aripiprazole. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - armodafinil
armodafinil will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone increases and armodafinil decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. - artemether/lumefantrine
artemether/lumefantrine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
artemether/lumefantrine and methadone both increase QTc interval. Modify Therapy/Monitor Closely. - asenapine
methadone, asenapine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction).
- asenapine transdermal
asenapine transdermal and methadone both increase sedation. Use Caution/Monitor.
- atazanavir
atazanavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- atogepant
methadone will increase the level or effect of atogepant by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- avapritinib
methadone will increase the level or effect of avapritinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- axitinib
methadone increases levels of axitinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- azelastine
azelastine and methadone both increase sedation. Use Caution/Monitor.
- baclofen
baclofen and methadone both increase sedation. Use Caution/Monitor.
- bedaquiline
methadone and bedaquiline both increase QTc interval. Modify Therapy/Monitor Closely. ECG should be monitored closely
- belladonna and opium
methadone and belladonna and opium both increase sedation. Use Caution/Monitor.
- benperidol
methadone and benperidol both increase sedation. Use Caution/Monitor.
- benzphetamine
methadone increases and benzphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- bosentan
bosentan will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- bosutinib
bosutinib and methadone both increase QTc interval. Use Caution/Monitor.
- brexanolone
brexanolone, methadone. Either increases toxicity of the other by sedation. Use Caution/Monitor.
- brexpiprazole
methadone will increase the level or effect of brexpiprazole by affecting hepatic enzyme CYP2D6 metabolism. Modify Therapy/Monitor Closely. Administer a quarter of brexpiprazole dose if coadministered with a moderate CYP2D6 inhibitor PLUS a strong/moderate CYP3A4 inhibitor.
- brompheniramine
brompheniramine and methadone both increase sedation. Use Caution/Monitor.
- budesonide
budesonide will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- buprenorphine
buprenorphine and methadone both increase sedation. Use Caution/Monitor.
- buprenorphine buccal
buprenorphine buccal and methadone both increase sedation. Use Caution/Monitor.
- buprenorphine, long-acting injection
methadone increases toxicity of buprenorphine, long-acting injection by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of buprenorphine and benzodiazepines or other CNS depressants increases risk of adverse reactions including overdose, respiratory depression, and death. Cessation of benzodiazepines or other CNS depressants is preferred in most cases. In some cases, monitoring at a higher level of care for tapering CNS depressants may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
- butabarbital
butabarbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
butabarbital and methadone both increase sedation. Use Caution/Monitor. - butalbital
butalbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
butalbital and methadone both increase sedation. Use Caution/Monitor. - butorphanol
butorphanol and methadone both increase sedation. Use Caution/Monitor.
- caffeine
methadone increases and caffeine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- cannabidiol
cannabidiol will increase the level or effect of methadone by decreasing metabolism. Modify Therapy/Monitor Closely. Cannabidiol may potentially inhibit CYP2C8 activity. Consider reducing the dose when concomitantly using CYP2C8 substrates.
- capecitabine
capecitabine and methadone both increase QTc interval. Use Caution/Monitor.
- carbinoxamine
carbinoxamine and methadone both increase sedation. Use Caution/Monitor.
- cariprazine
methadone, cariprazine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction).
- carisoprodol
carisoprodol and methadone both increase sedation. Use Caution/Monitor.
- cenobamate
cenobamate will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Increase dose of CYP3A4 substrate, as needed, when coadministered with cenobamate.
- chloral hydrate
chloral hydrate and methadone both increase sedation. Use Caution/Monitor.
- chlordiazepoxide
chlordiazepoxide and methadone both increase sedation. Use Caution/Monitor.
- chlorpheniramine
chlorpheniramine and methadone both increase sedation. Use Caution/Monitor.
- chlorpromazine
chlorpromazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and chlorpromazine both increase sedation. Use Caution/Monitor. - chlorzoxazone
chlorzoxazone and methadone both increase sedation. Use Caution/Monitor.
- cinnarizine
cinnarizine and methadone both increase sedation. Use Caution/Monitor.
- ciprofloxacin
ciprofloxacin and methadone both increase QTc interval. Use Caution/Monitor. Ciprofloxacin elicits minimal effects on QT interval. Caution if used in combination with other drugs known to affect QT interval or in patients with other risk factors.
- citalopram
methadone and citalopram both increase QTc interval. Use Caution/Monitor. Combination may increase risk of serotonin syndrome or neuroleptic malignant syndrome like reactions. ECG monitoring is recommended, along with drugs that may prolong the QT interval.
- clarithromycin
clarithromycin and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- clemastine
clemastine and methadone both increase sedation. Use Caution/Monitor.
- clobazam
methadone, clobazam. Other (see comment). Use Caution/Monitor. Comment: Concomitant administration can increase the potential for CNS effects (e.g., increased sedation or respiratory depression).
- clomipramine
clomipramine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and clomipramine both increase sedation. Use Caution/Monitor. - clonazepam
clonazepam and methadone both increase sedation. Use Caution/Monitor.
- clorazepate
clorazepate and methadone both increase sedation. Use Caution/Monitor.
- clozapine
methadone and clozapine both increase sedation. Use Caution/Monitor.
methadone, clozapine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - cobicistat
cobicistat will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Methadone dose may require an adjustment
- codeine
codeine and methadone both increase sedation. Use Caution/Monitor.
- conivaptan
conivaptan will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- cortisone
cortisone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- crizotinib
crizotinib increases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Dose reduction may be needed for coadministered drugs that are predominantly metabolized by CYP3A. ECG monitoring is recommended, along with drugs that may prolong the QT interval.
- crofelemer
crofelemer increases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Crofelemer has the potential to inhibit CYP3A4 at concentrations expected in the gut; unlikely to inhibit systemically because minimally absorbed.
- cyclizine
cyclizine and methadone both increase sedation. Use Caution/Monitor.
- cyclobenzaprine
cyclobenzaprine and methadone both increase sedation. Use Caution/Monitor.
- cyclosporine
cyclosporine will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- cyproheptadine
cyproheptadine and methadone both increase sedation. Use Caution/Monitor.
- dabrafenib
dabrafenib will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely.
- danazol
danazol will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- dantrolene
dantrolene and methadone both increase sedation. Use Caution/Monitor.
- daridorexant
methadone and daridorexant both increase sedation. Modify Therapy/Monitor Closely. Coadministration increases risk of CNS depression, which can lead to additive impairment of psychomotor performance and cause daytime impairment.
- darifenacin
darifenacin will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- darunavir
darunavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Carefully titrate dose when initiating buprenorphine, buprenorphine/naloxone, or methadone with patients taking darunavir/cobicstat. When initiating cobicistat in patients taking buprenorphine, buprenorphine/naloxone, or methadone, adjust dose for buprenorphine, buprenorphine/naloxone, or methadone and monitor clinical signs and symptoms.
- dasatinib
dasatinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
dasatinib and methadone both increase QTc interval. Use Caution/Monitor. - deferasirox
deferasirox will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- desflurane
desflurane and methadone both increase sedation. Use Caution/Monitor. Opioids may decrease MAC requirements, less inhalation anesthetic may be required.
- desipramine
desipramine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and desipramine both increase sedation. Use Caution/Monitor. - deutetrabenazine
methadone and deutetrabenazine both increase sedation. Use Caution/Monitor.
deutetrabenazine and methadone both increase QTc interval. Use Caution/Monitor. At the maximum recommended dose, deutetrabenazine does not prolong QT interval to a clinically relevant extent. Certain circumstances may increase risk of torsade de pointes and/or sudden death in association with drugs that prolong the QTc interval (eg, bradycardia, hypokalemia or hypomagnesemia, coadministration with other drugs that prolong QTc interval, presence of congenital QT prolongation). - dexamethasone
dexamethasone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- dexchlorpheniramine
dexchlorpheniramine and methadone both increase sedation. Use Caution/Monitor.
- dexfenfluramine
methadone increases and dexfenfluramine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dexmedetomidine
dexmedetomidine and methadone both increase sedation. Use Caution/Monitor.
- dexmethylphenidate
methadone increases and dexmethylphenidate decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dextroamphetamine
methadone increases and dextroamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dextromoramide
dextromoramide and methadone both increase sedation. Use Caution/Monitor.
- DHEA, herbal
DHEA, herbal will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- diamorphine
diamorphine and methadone both increase sedation. Use Caution/Monitor.
- diazepam
diazepam and methadone both increase sedation. Use Caution/Monitor.
- dichlorphenamide
dichlorphenamide and methadone both decrease serum potassium. Use Caution/Monitor.
- didanosine
methadone decreases levels of didanosine by inhibition of GI absorption. Applies only to oral form of both agents. Use Caution/Monitor.
- diethylpropion
methadone increases and diethylpropion decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- difelikefalin
difelikefalin and methadone both increase sedation. Use Caution/Monitor.
- difenoxin hcl
difenoxin hcl and methadone both increase sedation. Use Caution/Monitor.
- diltiazem
diltiazem will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- dimenhydrinate
dimenhydrinate and methadone both increase sedation. Use Caution/Monitor.
- diphenhydramine
diphenhydramine and methadone both increase sedation. Use Caution/Monitor.
- diphenoxylate hcl
diphenoxylate hcl and methadone both increase sedation. Use Caution/Monitor.
- dipipanone
dipipanone and methadone both increase sedation. Use Caution/Monitor.
- dobutamine
methadone increases and dobutamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dofetilide
dofetilide and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- dolasetron
dolasetron and methadone both increase QTc interval. Use Caution/Monitor.
- dopamine
methadone increases and dopamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dopexamine
methadone increases and dopexamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dosulepin
methadone and dosulepin both increase sedation. Use Caution/Monitor.
- doxepin
doxepin and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and doxepin both increase sedation. Use Caution/Monitor. - doxylamine
doxylamine and methadone both increase sedation. Use Caution/Monitor.
- dronedarone
dronedarone will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
dronedarone and methadone both increase QTc interval. Modify Therapy/Monitor Closely. - droperidol
droperidol and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and droperidol both increase sedation. Use Caution/Monitor. - duvelisib
duvelisib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Coadministration with duvelisib increases AUC of a sensitive CYP3A4 substrate which may increase the risk of toxicities of these drugs. Consider reducing the dose of the sensitive CYP3A4 substrate and monitor for signs of toxicities of the coadministered sensitive CYP3A substrate.
- efavirenz
efavirenz will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- elagolix
elagolix decreases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Elagolix is a weak-to-moderate CYP3A4 inducer. Monitor CYP3A substrates if coadministered. Consider increasing CYP3A substrate dose if needed.
- eltrombopag
eltrombopag increases levels of methadone by decreasing metabolism. Use Caution/Monitor. UGT inhibition; significance of interaction unclear.
- elvitegravir
elvitegravir decreases levels of methadone by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. Dosage of methadone may need to be increased.
- elvitegravir/cobicistat/emtricitabine/tenofovir DF
elvitegravir/cobicistat/emtricitabine/tenofovir DF increases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Cobicistat is a CYP3A4 inhibitor; contraindicated with CYP3A4 substrates for which elevated plasma concentrations are associated with serious and/or life-threatening events.
- encorafenib
encorafenib, methadone. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Encorafenib both inhibits and induces CYP3A4 at clinically relevant plasma concentrations. Coadministration of encorafenib with sensitive CYP3A4 substrates may result in increased toxicity or decreased efficacy of these agents.
- ephedrine
methadone increases and ephedrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- epinephrine
epinephrine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone increases and epinephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. - epinephrine racemic
epinephrine racemic and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone increases and epinephrine racemic decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. - erythromycin base
erythromycin base and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- erythromycin ethylsuccinate
erythromycin ethylsuccinate and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- erythromycin lactobionate
erythromycin lactobionate and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- erythromycin stearate
erythromycin stearate and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- esketamine intranasal
esketamine intranasal, methadone. Either increases toxicity of the other by sedation. Modify Therapy/Monitor Closely.
- eslicarbazepine acetate
eslicarbazepine acetate will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- estazolam
estazolam and methadone both increase sedation. Use Caution/Monitor.
- ethanol
methadone and ethanol both increase sedation. Use Caution/Monitor.
- etomidate
etomidate and methadone both increase sedation. Use Caution/Monitor.
- etrasimod
etrasimod, methadone. Either increases effects of the other by QTc interval. Modify Therapy/Monitor Closely. Transient decrease in heart rate and AV conduction delays may occur when initiating etrasimod. Owing to potential of additive effect on heart rate, etrasimod may increase risk of QT prolongation and Torsades de Pointes when coadministered with Class Ia or Class III antiarrhythmic drugs, or other drugs that prolong the QT interval. .
- etravirine
etravirine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- ezogabine
ezogabine, methadone. Either increases toxicity of the other by QTc interval. Use Caution/Monitor. Slight and transient QT-prolongation observed with ezogabine, particularly when dose titrated to 1200 mg/day. QT interval should be monitored when ezogabine is prescribed with agents known to increase QT interval.
- fedratinib
fedratinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Adjust dose of drugs that are CYP3A4 substrates as necessary.
- fenfluramine
methadone increases and fenfluramine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- finerenone
methadone will increase the level or effect of finerenone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Monitor serum potassium during initiation and dosage adjustment of either finererone or weak CYP3A4 inhibitors. Adjust finererone dosage as needed.
- flecainide
flecainide and methadone both increase QTc interval. Use Caution/Monitor.
- flibanserin
methadone and flibanserin both increase sedation. Modify Therapy/Monitor Closely. Risk for sedation increased if flibanserin is coadministration with other CNS depressants.
methadone will increase the level or effect of flibanserin by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Increased flibanserin adverse effects may occur if coadministered with multiple weak CYP3A4 inhibitors. - floxuridine
floxuridine and methadone both increase QTc interval. Use Caution/Monitor.
- fluconazole
fluconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
fluconazole and methadone both increase QTc interval. Modify Therapy/Monitor Closely. - fludrocortisone
fludrocortisone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fluoxetine
fluoxetine and methadone both increase QTc interval. Use Caution/Monitor.
- fluphenazine
fluphenazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and fluphenazine both increase sedation. Use Caution/Monitor.
methadone, fluphenazine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - flurazepam
flurazepam and methadone both increase sedation. Use Caution/Monitor.
- fluvoxamine
fluvoxamine and methadone both increase QTc interval. Use Caution/Monitor.
- formoterol
formoterol and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone increases and formoterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. - fosamprenavir
fosamprenavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- fosaprepitant
fosaprepitant will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- foscarnet
foscarnet and methadone both increase QTc interval. Use Caution/Monitor.
- fosphenytoin
fosphenytoin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- gabapentin
gabapentin, methadone. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
- gabapentin enacarbil
gabapentin enacarbil, methadone. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
- ganaxolone
methadone and ganaxolone both increase sedation. Use Caution/Monitor.
- gemtuzumab
methadone and gemtuzumab both increase QTc interval. Use Caution/Monitor.
- gepirone
gepirone and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- goserelin
goserelin increases toxicity of methadone by QTc interval. Use Caution/Monitor. Increases risk of torsades de pointes.
- grapefruit
grapefruit will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- griseofulvin
griseofulvin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- haloperidol
haloperidol and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and haloperidol both increase sedation. Use Caution/Monitor.
methadone, haloperidol. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - histrelin
histrelin increases toxicity of methadone by QTc interval. Use Caution/Monitor. Increases risk of torsades de pointes.
- hydrocortisone
hydrocortisone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- hydromorphone
hydromorphone and methadone both increase sedation. Use Caution/Monitor.
- hydroxyzine
hydroxyzine and methadone both increase sedation. Use Caution/Monitor.
hydroxyzine increases toxicity of methadone by QTc interval. Use Caution/Monitor. Increases risk of torsades de pointes. - iloperidone
iloperidone and methadone both increase QTc interval. Use Caution/Monitor.
methadone and iloperidone both increase sedation. Use Caution/Monitor.
iloperidone increases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Iloperidone is a time-dependent CYP3A inhibitor and may lead to increased plasma levels of drugs predominantly eliminated by CYP3A4.
methadone, iloperidone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - imipramine
imipramine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and imipramine both increase sedation. Use Caution/Monitor. - indacaterol, inhaled
indacaterol, inhaled, methadone. QTc interval. Use Caution/Monitor. Drugs that are known to prolong the QTc interval may have an increased the risk of ventricular arrhythmias.
- indinavir
indinavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- isavuconazonium sulfate
methadone will increase the level or effect of isavuconazonium sulfate by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- isoniazid
isoniazid will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- isoproterenol
methadone increases and isoproterenol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- istradefylline
istradefylline will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Istradefylline 40 mg/day increased peak levels and AUC of CYP3A4 substrates in clinical trials. This effect was not observed with istradefylline 20 mg/day. Consider dose reduction of sensitive CYP3A4 substrates.
- ketamine
ketamine and methadone both increase sedation. Use Caution/Monitor.
- ketotifen, ophthalmic
methadone and ketotifen, ophthalmic both increase sedation. Use Caution/Monitor.
- lapatinib
lapatinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
lapatinib and methadone both increase QTc interval. Use Caution/Monitor. - larotrectinib
larotrectinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- lasmiditan
lasmiditan, methadone. Either increases effects of the other by sedation. Use Caution/Monitor. Coadministration of lasmiditan and other CNS depressant drugs, including alcohol have not been evaluated in clinical studies. Lasmiditan may cause sedation, as well as other cognitive and/or neuropsychiatric adverse reactions.
- lemborexant
methadone will increase the level or effect of lemborexant by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Lower nightly dose of lemborexant recommended if coadministered with weak CYP3A4 inhibitors. See drug monograph for specific dosage modification.
lemborexant, methadone. Either increases effects of the other by sedation. Modify Therapy/Monitor Closely. Dosage adjustment may be necessary if lemborexant is coadministered with other CNS depressants because of potentially additive effects. - lenacapavir
lenacapavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. When initiating methadone while on lenacapavir, use lowest feasible initial or maintenance dose of methadone and carefully titrate dose to desired effect. When initiating lenacapavir while taking methadone, consider adjusting dose for methadone. Monitor clinical signs and symptoms.
- lenvatinib
methadone and lenvatinib both increase QTc interval. Use Caution/Monitor. Lenvatinib prescribing information recommends monitoring ECG closely when coadministered with QT prolonging drugs.
- letermovir
letermovir increases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- leuprolide
leuprolide increases toxicity of methadone by QTc interval. Use Caution/Monitor. Increases risk of torsades de pointes.
- levalbuterol
methadone increases and levalbuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- levofloxacin
levofloxacin and methadone both increase QTc interval. Use Caution/Monitor.
- levorphanol
levorphanol and methadone both increase sedation. Use Caution/Monitor.
- lisdexamfetamine
methadone increases and lisdexamfetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- lofepramine
lofepramine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and lofepramine both increase sedation. Use Caution/Monitor. - lofexidine
methadone and lofexidine both increase sedation. Use Caution/Monitor.
methadone and lofexidine both increase QTc interval. Use Caution/Monitor. ECG monitoring is recommended. - lomitapide
methadone increases levels of lomitapide by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Lomitapide dose should not exceed 30 mg/day.
- loprazolam
loprazolam and methadone both increase sedation. Use Caution/Monitor.
- lorazepam
lorazepam and methadone both increase sedation. Use Caution/Monitor.
- lorlatinib
lorlatinib will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- lormetazepam
lormetazepam and methadone both increase sedation. Use Caution/Monitor.
- loxapine
methadone and loxapine both increase sedation. Use Caution/Monitor.
methadone, loxapine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - loxapine inhaled
methadone and loxapine inhaled both increase sedation. Use Caution/Monitor.
methadone, loxapine inhaled. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - lumefantrine
lumefantrine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
lumefantrine and methadone both increase QTc interval. Modify Therapy/Monitor Closely. - lurasidone
lurasidone, methadone. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: Potential for increased CNS depressant effects when used concurrently; monitor for increased adverse effects and toxicity.
methadone, lurasidone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - maprotiline
maprotiline and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and maprotiline both increase sedation. Use Caution/Monitor. - maraviroc
maraviroc increases levels of methadone by QTc interval. Use Caution/Monitor. Potential for increased toxicity. Increased risk of QT prolongation and cardiac arrhythmias.
- marijuana
marijuana will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone and marijuana both increase sedation. Use Caution/Monitor. - melatonin
methadone and melatonin both increase sedation. Use Caution/Monitor.
- meperidine
meperidine and methadone both increase sedation. Use Caution/Monitor.
- meprobamate
methadone and meprobamate both increase sedation. Use Caution/Monitor.
- metaproterenol
methadone increases and metaproterenol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- metaxalone
metaxalone and methadone both increase sedation. Use Caution/Monitor.
- methamphetamine
methadone increases and methamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- methocarbamol
methocarbamol and methadone both increase sedation. Use Caution/Monitor.
- methylenedioxymethamphetamine
methadone increases and methylenedioxymethamphetamine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- methylprednisolone
methylprednisolone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- metronidazole
metronidazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- miconazole vaginal
miconazole vaginal will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- midazolam
midazolam and methadone both increase sedation. Use Caution/Monitor.
- midazolam intranasal
methadone will increase the level or effect of midazolam intranasal by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Coadministration of mild CYP3A4 inhibitors with midazolam intranasal may cause higher midazolam systemic exposure, which may prolong sedation.
midazolam intranasal, methadone. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of benzodiazepines and opioids increases risk of respiratory depression. Use only in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. - midodrine
methadone increases and midodrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- mifepristone
mifepristone will increase the level or effect of methadone by Other (see comment). Modify Therapy/Monitor Closely. Inhibits CYP2C8/2C9; use smallest recommended doses for substrates and monitor; combination may increase QT interval. Use alternatives if available
- mirtazapine
methadone and mirtazapine both increase sedation. Use Caution/Monitor.
- mitotane
mitotane decreases levels of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Mitotane is a strong inducer of cytochrome P-4503A4; monitor when coadministered with CYP3A4 substrates for possible dosage adjustments.
- modafinil
methadone increases and modafinil decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- molindone
methadone, molindone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction).
- morphine
methadone and morphine both increase sedation. Use Caution/Monitor.
- motherwort
methadone and motherwort both increase sedation. Use Caution/Monitor.
- moxifloxacin
methadone and moxifloxacin both increase QTc interval. Modify Therapy/Monitor Closely.
- moxonidine
methadone and moxonidine both increase sedation. Use Caution/Monitor.
- nabilone
methadone and nabilone both increase sedation. Use Caution/Monitor.
- nafcillin
nafcillin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- nalbuphine
methadone and nalbuphine both increase sedation. Use Caution/Monitor.
- nelfinavir
nelfinavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- nevirapine
nevirapine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- nifedipine
nifedipine will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- nilotinib
nilotinib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone and nilotinib both increase QTc interval. Modify Therapy/Monitor Closely. - nirmatrelvir
nirmatrelvir will decrease the level or effect of methadone by affecting hepatic enzyme CYP2B6 metabolism. Use Caution/Monitor. Methadone is metabolized by CYP2B6 and CYP3A4. Ritonavir may decrease AUC and peak plasma concentration by CYP2B6 induction. This may be partially offset by ritonavir inhibiting CYP3A4.
- nirmatrelvir/ritonavir
nirmatrelvir/ritonavir will decrease the level or effect of methadone by affecting hepatic enzyme CYP2B6 metabolism. Use Caution/Monitor. Methadone is metabolized by CYP2B6 and CYP3A4. Ritonavir may decrease AUC and peak plasma concentration by CYP2B6 induction. This may be partially offset by ritonavir inhibiting CYP3A4.
- norepinephrine
methadone increases and norepinephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- nortriptyline
nortriptyline and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and nortriptyline both increase sedation. Use Caution/Monitor. - octreotide
methadone and octreotide both increase QTc interval. Modify Therapy/Monitor Closely.
- octreotide (Antidote)
methadone and octreotide (Antidote) both increase QTc interval. Modify Therapy/Monitor Closely.
- ofloxacin
methadone and ofloxacin both increase QTc interval. Use Caution/Monitor.
- olanzapine
methadone and olanzapine both increase sedation. Use Caution/Monitor.
methadone, olanzapine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - oliceridine
methadone will increase the level or effect of oliceridine by affecting hepatic enzyme CYP2D6 metabolism. Modify Therapy/Monitor Closely. If concomitant use is necessary, may require less frequent oliceridine dosing. Closely monitor for respiratory depression and sedation and titrate subsequent doses accordingly. If inhibitor is discontinued, consider increase oliceridine dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal.
oliceridine, methadone. Either increases toxicity of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Profound sedation, respiratory depression, coma, and death may result if coadministered. Reserve concomitant prescribing of these drugs in patients for whom other treatment options are inadequate. Limit dosages and durations to the minimum required. Monitor closely for signs of respiratory depression and sedation. - olodaterol inhaled
methadone and olodaterol inhaled both increase QTc interval. Use Caution/Monitor. Drugs that prolong the QTc interval and may potentiate the effects of beta2 agonists on the cardiovascular system; increased risk of ventricular arrhythmias
- omaveloxolone
omaveloxolone will decrease the level or effect of methadone by Other (see comment). Use Caution/Monitor. Omaveloxolone may reduce systemic exposure of sensitive CYP2C8 substrates. Check prescribing information of substrate if dosage modification is needed.
- opium tincture
methadone and opium tincture both increase sedation. Use Caution/Monitor.
- orphenadrine
orphenadrine and methadone both increase sedation. Use Caution/Monitor.
- osilodrostat
osilodrostat and methadone both increase QTc interval. Use Caution/Monitor.
- osimertinib
osimertinib and methadone both increase QTc interval. Use Caution/Monitor. Conduct periodic monitoring with ECGs and electrolytes in patients taking drugs known to prolong the QTc interval.
- oxaliplatin
oxaliplatin will increase the level or effect of methadone by Other (see comment). Use Caution/Monitor. Monitor for ECG changes if therapy is initiated in patients with drugs known to prolong QT interval.
- oxazepam
oxazepam and methadone both increase sedation. Use Caution/Monitor.
- oxcarbazepine
oxcarbazepine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- oxycodone
methadone and oxycodone both increase sedation. Use Caution/Monitor.
- oxymorphone
methadone and oxymorphone both increase sedation. Use Caution/Monitor.
- ozanimod
ozanimod and methadone both increase QTc interval. Modify Therapy/Monitor Closely. The potential additive effects on heart rate, treatment with ozanimod should generally not be initiated in patients who are concurrently treated with QT prolonging drugs with known arrhythmogenic properties.
- paliperidone
methadone and paliperidone both increase QTc interval. Use Caution/Monitor.
methadone and paliperidone both increase sedation. Use Caution/Monitor.
methadone, paliperidone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - papaveretum
methadone and papaveretum both increase sedation. Use Caution/Monitor.
- papaverine
methadone and papaverine both increase sedation. Use Caution/Monitor.
- paroxetine
methadone and paroxetine both increase QTc interval. Use Caution/Monitor.
- pasireotide
methadone and pasireotide both increase QTc interval. Modify Therapy/Monitor Closely.
- peginterferon alfa 2b
peginterferon alfa 2b will increase the level or effect of methadone by unknown mechanism. Use Caution/Monitor. Methadone levels may be elevated, increasing the pharmacologic effects and adverse reactions. Monitor patients for signs and symptoms of increased narcotic effects and adjust methadone dose as needed.
- pegvisomant
methadone decreases effects of pegvisomant by unknown mechanism. Use Caution/Monitor.
- pentazocine
methadone and pentazocine both increase sedation. Use Caution/Monitor.
- pentobarbital
pentobarbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
pentobarbital and methadone both increase sedation. Use Caution/Monitor. - perampanel
perampanel and methadone both decrease sedation. Use Caution/Monitor.
- perphenazine
perphenazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and perphenazine both increase sedation. Use Caution/Monitor.
methadone, perphenazine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - phendimetrazine
methadone increases and phendimetrazine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- phenobarbital
phenobarbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
phenobarbital and methadone both increase sedation. Use Caution/Monitor. - phentermine
methadone increases and phentermine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- phenylephrine
methadone increases and phenylephrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- phenylephrine PO
methadone increases and phenylephrine PO decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor. .
- phenytoin
phenytoin will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- pholcodine
methadone and pholcodine both increase sedation. Use Caution/Monitor.
- pimavanserin
methadone, pimavanserin. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction).
- pimozide
methadone and pimozide both increase sedation. Use Caution/Monitor.
methadone, pimozide. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - pirbuterol
methadone increases and pirbuterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- posaconazole
posaconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone and posaconazole both increase QTc interval. Use Caution/Monitor. - prednisone
prednisone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- pregabalin
pregabalin, methadone. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Coadministration of CNS depressants can result in serious, life-threatening, and fatal respiratory depression. Use lowest dose possible and monitor for respiratory depression and sedation.
- primidone
primidone will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
primidone and methadone both increase sedation. Use Caution/Monitor. - prochlorperazine
prochlorperazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and prochlorperazine both increase sedation. Use Caution/Monitor. - promazine
promazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
- promethazine
promethazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
promethazine and methadone both increase sedation. Use Caution/Monitor. - propofol
propofol and methadone both increase sedation. Use Caution/Monitor.
- propylhexedrine
methadone increases and propylhexedrine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- protriptyline
protriptyline and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and protriptyline both increase sedation. Use Caution/Monitor. - quazepam
quazepam and methadone both increase sedation. Use Caution/Monitor.
- quetiapine
methadone and quetiapine both increase sedation. Use Caution/Monitor.
quetiapine, methadone. Either increases toxicity of the other by QTc interval. Use Caution/Monitor. Avoid use with drugs that prolong QT and in patients with risk factors for prolonged QT interval. Postmarketing cases show QT prolongation with overdose in patients with concomitant illness or with drugs known to cause electrolyte imbalance or prolong QT.
methadone, quetiapine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - quinine
methadone and quinine both increase QTc interval. Use Caution/Monitor.
- quinupristin/dalfopristin
quinupristin/dalfopristin will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- quizartinib
quizartinib, methadone. Either increases effects of the other by QTc interval. Modify Therapy/Monitor Closely. Monitor patients more frequently with ECG if coadministered with QT prolonging drugs.
- ramelteon
methadone and ramelteon both increase sedation. Use Caution/Monitor.
- ranolazine
methadone and ranolazine both increase QTc interval. Use Caution/Monitor.
- remimazolam
remimazolam, methadone. Either increases toxicity of the other by sedation. Modify Therapy/Monitor Closely. Coadministration may result in profound sedation, respiratory depression, coma, and/or death. Continuously monitor vital signs during sedation and recovery period if coadministered. Carefully titrate remimazolam dose if administered with opioid analgesics and/or sedative/hypnotics.
- rifampin
rifampin decreases levels of methadone by increasing metabolism. Use Caution/Monitor.
- rifapentine
rifapentine will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- rilpivirine
rilpivirine, methadone. Other (see comment). Use Caution/Monitor. Comment: No dose adjustments are required when initiating co-administration of methadone with rilpivirine. However, clinical monitoring is recommended as methadone maintenance therapy may need to be adjusted in some patients.
rilpivirine increases toxicity of methadone by QTc interval. Use Caution/Monitor. Rilpivirine should be used with caution when co-administered with a drug with a known risk of Torsade de Pointes. - risperidone
methadone and risperidone both increase QTc interval. Use Caution/Monitor.
methadone and risperidone both increase sedation. Use Caution/Monitor.
methadone, risperidone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - ritonavir
ritonavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
ritonavir decreases effects of methadone by Other (see comment). Use Caution/Monitor. Comment: Ritonavir may induce the metabolism of methadone and/or induce the p glycoprotein pathway that pumps drugs out of the CNS. - rucaparib
rucaparib will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Adjust dosage of CYP3A4 substrates, if clinically indicated.
- rufinamide
rufinamide will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- salmeterol
methadone increases and salmeterol decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- schisandra
schisandra will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- scullcap
methadone and scullcap both increase sedation. Use Caution/Monitor.
- secobarbital
secobarbital will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
secobarbital and methadone both increase sedation. Use Caution/Monitor. - selpercatinib
selpercatinib increases toxicity of methadone by QTc interval. Use Caution/Monitor.
- sevoflurane
sevoflurane and methadone both increase sedation. Use Caution/Monitor.
- shepherd's purse
methadone and shepherd's purse both increase sedation. Use Caution/Monitor.
- sodium sulfate/?magnesium sulfate/potassium chloride
sodium sulfate/?magnesium sulfate/potassium chloride increases toxicity of methadone by QTc interval. Use Caution/Monitor. Consider predose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias. .
- sodium sulfate/potassium sulfate/magnesium sulfate
sodium sulfate/potassium sulfate/magnesium sulfate increases toxicity of methadone by QTc interval. Use Caution/Monitor. Consider predose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias. .
- sorafenib
sorafenib and methadone both increase QTc interval. Use Caution/Monitor.
- stiripentol
stiripentol, methadone. affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Stiripentol is a CYP3A4 inhibitor and inducer. Monitor CYP3A4 substrates coadministered with stiripentol for increased or decreased effects. CYP3A4 substrates may require dosage adjustment.
stiripentol will increase the level or effect of methadone by Other (see comment). Modify Therapy/Monitor Closely. Stiripentol is a CYP2C8 inhibitor. Consider dosage reduction for CYP2C8 substrates if adverse effects are experienced when coadministered.
stiripentol, methadone. Either increases effects of the other by sedation. Use Caution/Monitor. Concomitant use stiripentol with other CNS depressants, including alcohol, may increase the risk of sedation and somnolence. - sufentanil
methadone and sufentanil both increase sedation. Use Caution/Monitor.
- sulfamethoxazole
sulfamethoxazole and methadone both increase QTc interval. Use Caution/Monitor.
- suvorexant
suvorexant and methadone both increase sedation. Modify Therapy/Monitor Closely. Dosage adjustments of suvorexant and concomitant CNS depressants may be necessary
- tamsulosin
methadone increases levels of tamsulosin by affecting hepatic enzyme CYP2D6 metabolism. Use Caution/Monitor.
- tapentadol
methadone and tapentadol both increase sedation. Use Caution/Monitor.
- tazemetostat
tazemetostat will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone will increase the level or effect of tazemetostat by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. - tecovirimat
tecovirimat will increase the level or effect of methadone by affecting hepatic enzyme CYP2C19 metabolism. Use Caution/Monitor. Tecovirimat is a weak inhibitor of CYP2C8 and CYP2C19. Monitor for adverse effects if coadministered with sensitive substrates of these enzymes.
tecovirimat will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Tecovirimat is a weak CYP3A4 inducer. Monitor sensitive CYP3A4 substrates for effectiveness if coadministered. - telavancin
methadone and telavancin both increase QTc interval. Use Caution/Monitor.
- temazepam
temazepam and methadone both increase sedation. Use Caution/Monitor.
- terbutaline
methadone increases and terbutaline decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- teriflunomide
teriflunomide increases levels of methadone by Other (see comment). Use Caution/Monitor. Comment: Teriflunomide inhibits CYP2C8; caution when coadministered with CYP2C8 substrates.
- thioridazine
thioridazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and thioridazine both increase sedation. Use Caution/Monitor. - thiothixene
methadone and thiothixene both increase sedation. Use Caution/Monitor.
methadone, thiothixene. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - tinidazole
methadone will increase the level or effect of tinidazole by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- tipranavir
tipranavir decreases levels of methadone by increasing metabolism. Use Caution/Monitor.
tipranavir will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. - topiramate
topiramate will decrease the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
methadone and topiramate both increase sedation. Modify Therapy/Monitor Closely. - tramadol
methadone and tramadol both increase sedation. Use Caution/Monitor.
- trazodone
trazodone and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and trazodone both increase sedation. Use Caution/Monitor. - triazolam
triazolam and methadone both increase sedation. Use Caution/Monitor.
- triclabendazole
triclabendazole and methadone both increase QTc interval. Use Caution/Monitor.
- triclofos
triclofos and methadone both increase sedation. Use Caution/Monitor.
- trifluoperazine
trifluoperazine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and trifluoperazine both increase sedation. Use Caution/Monitor.
methadone, trifluoperazine. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - trimethoprim
methadone and trimethoprim both increase QTc interval. Use Caution/Monitor.
- trimipramine
trimipramine and methadone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and trimipramine both increase sedation. Use Caution/Monitor. - triprolidine
triprolidine and methadone both increase sedation. Use Caution/Monitor.
- triptorelin
triptorelin increases toxicity of methadone by QTc interval. Use Caution/Monitor. Increases risk of torsades de pointes.
- tropisetron
methadone and tropisetron both increase QTc interval. Use Caution/Monitor.
- valbenazine
valbenazine and methadone both increase QTc interval. Use Caution/Monitor.
- venlafaxine
methadone and venlafaxine both increase QTc interval. Use Caution/Monitor.
- verapamil
verapamil will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- voclosporin
voclosporin, methadone. Either increases effects of the other by QTc interval. Use Caution/Monitor.
- voriconazole
voriconazole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Modify Therapy/Monitor Closely. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed.
methadone and voriconazole both increase QTc interval. Use Caution/Monitor. - xylometazoline
methadone increases and xylometazoline decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- yohimbine
methadone increases and yohimbine decreases sedation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- zafirlukast
zafirlukast will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor.
- ziconotide
methadone and ziconotide both increase sedation. Use Caution/Monitor.
- ziprasidone
methadone and ziprasidone both increase QTc interval. Modify Therapy/Monitor Closely.
methadone and ziprasidone both increase sedation. Use Caution/Monitor.
methadone, ziprasidone. unspecified interaction mechanism. Use Caution/Monitor. Serotonin modulators may enhance dopamine blockade, possibly increasing the risk for neuroleptic malignant syndrome. Antipsychotics may enhance serotonergic effect of serotonin modulators, which may result in serotonin syndrome. Monitor for evidence of serotonin toxicity (eg, mental status changes, autonomic instability, and neuromuscular hyperactivity) or neuroleptic malignant syndrome (eg, hyperthermia, muscle rigidity, autonomic dysfunction). - zotepine
methadone and zotepine both increase sedation. Use Caution/Monitor.
Minor (17)
- acetazolamide
acetazolamide will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- anastrozole
anastrozole will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- azithromycin
azithromycin and methadone both increase QTc interval. Minor/Significance Unknown.
- brimonidine
brimonidine increases effects of methadone by pharmacodynamic synergism. Minor/Significance Unknown. Increased CNS depression.
- cyclophosphamide
cyclophosphamide will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- dextroamphetamine
dextroamphetamine increases effects of methadone by unspecified interaction mechanism. Minor/Significance Unknown.
- drospirenone
drospirenone will increase the level or effect of methadone by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- eucalyptus
methadone and eucalyptus both increase sedation. Minor/Significance Unknown.
- lidocaine
lidocaine increases toxicity of methadone by pharmacodynamic synergism. Minor/Significance Unknown. Risk of increased CNS depression.
- octreotide
octreotide decreases levels of methadone by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown.
- octreotide (Antidote)
octreotide (Antidote) decreases levels of methadone by inhibition of GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown.
- pazopanib
methadone and pazopanib both increase QTc interval. Minor/Significance Unknown.
- ruxolitinib
methadone will increase the level or effect of ruxolitinib by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- ruxolitinib topical
methadone will increase the level or effect of ruxolitinib topical by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
- sage
methadone and sage both increase sedation. Minor/Significance Unknown.
- ziconotide
ziconotide, methadone. Mechanism: unspecified interaction mechanism. Minor/Significance Unknown. Additive decreased GI motility. Additive analgesia. Ziconotide does NOT potentiate opioid induced respiratory depression.
- zidovudine
methadone increases levels of zidovudine by decreasing renal clearance. Minor/Significance Unknown.
Adverse Effects
Frequency Not Defined
Agitation
Angina pectoris
Anticholinergic effects (dry mouth, palpitation, tachycardia)
Bradycardia
Cardiac arrest
Coma
Constipation
Dizziness
Dysphoria
Euphoria
Faintness
Mental clouding or depression
Myocardial infarction
Nausea
Pruritus, urticaria
Nervousness
QT-interval prolongation
Respiratory arrest
Respiratory/circulatory depression
Restlessness
Sedation
Seizures
Severe cardiac arrhythmias
Shock
ST-segment elevation
Sweating, flushing, warmness of face/neck/upper thorax
Syncope
Urinary retention, oliguria
Ventricular tachycardia
Visual disturbances
Vomiting
Weakness
Postmarketing Reports
Confusion
Disorientation
Congenital oculomotor disorders
Insomnia
Hallucinations
Hypoglycemia
Warnings
Black Box Warnings
Opioid analgesic risk evaluation and mitigation strategy (REMS)
- To ensure that benefits of opioid analgesics outweigh risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a REMS for these products; under requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers
Healthcare providers are strongly encouraged to:
- Complete a REMS-compliant education program
- Counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products
- Emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist
- Consider other tools to improve patient, household, and community safety
Detoxification and maintenance of dependence
- For detoxification and maintenance of opioid dependence, methadone should be administered in accordance with the treatment standards cited in 42 CFR Section 8, including limitations on unsupervised administration
Addiction, abuse, and misuse
- Risk of opioid addiction, abuse, and misuse, which can lead to overdose and death
- Assess each patient’s risk prior to prescribing and monitor all patients regularly for the development of these behaviors or conditions
Life-threatening respiratory depression
- Serious, life-threatening, or fatal respiratory depression may occur
- Monitor for respiratory depression, especially during initiation or following a dose increase
- Instruct patients to swallow tablet/capsule whole; crushing, chewing, snorting, injecting or dissolving can cause rapid release and absorption of a potentially fatal dose
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death
Concomitant use with benzodiazepines or other CNS depressants
- Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death
- Reserve concomitant prescribing with benzodiazepines or other CNS depressants for patients for whom alternatives to benzodiazepines or other CNS depressants are inadequate; limit dosages and durations to minimum required for patients being treated for pain; follow patients for signs and symptoms of respiratory depression and sedation; if patient is visibly sedated, evaluate cause of sedation, and consider delaying or omitting the daily methadone dose
Accidental exposure
- Accidental of even 1 dose, especially by children, can result in a fatal overdose
Neonatal opioid withdrawal syndrome
- Prolonged use during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts
- Syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight
- Onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn
- If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available
Life-threatening QT prolongation
- QT interval prolongation and serious arrhythmia (torsades de pointes) have occurred during treatment with methadone; closely monitor patients with risk factors for development of prolonged QT interval, a history of cardiac conduction abnormalities, and those taking medications affecting cardiac conduction
Cytochrome CYP450 interactions
- Concomitant use with CYP3A4, 2B6, 2C19, 2C9 or 2D6 inhibitors or discontinuation of concomitantly used CYP3A4 2B6, 2C19, or 2C9 inducers can result in fatal overdose
Treatment for opioid addiction
- Methadone products, when used for the treatment of opioid addiction in detoxification or maintenance programs, shall be dispensed only by certified opioid treatment programs as stipulated in 42 CFR 8.12
Contraindications
Hypersensitivity to methadone or formulation components; acute abdominal condition, toxin-mediated diarrhea, pseudomembranous colitis, respiratory depression; concurrent use of selegiline, hypercarbia, known or suspected gastrointestinal obstruction, including paralytic ileus, asthma (acute), significant respiratory impairment
Acute pain or postoperative pain; pain that is mild or not expected to persist
Cautions
Schedule II opioid analgesics expose users to the risks of addiction, abuse, and misuse; there is a greater risk for overdose and death with extended-release opioids due to the larger amount of active opioid present (see Black Box Warnings)
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia; opioid use increases risk of CSA in a dose-dependent fashion; in patients who present with CSA, consider decreasing opioid dosage using best practices for opioid taper
Addiction, abuse, and misuse risks are increased in patients with a personal or family history of substance abuse or mental illness (eg, major depression); the potential for these risks should not, however, prevent the prescribing of proper pain management in any given patient; intensive monitoring is necessary (see Black Box Warnings)
Serious, life-threatening, or fatal respiratory depression reported (see Black Box Warnings)
May cause constipation, which could cause problems in patients with unstable angina and patients post-myocardial infarction; consider preventive measures (sool softener, increased fiber in diet) to reduce potential for constipation
Accidental exposure reported, including fatalities (see Black Box Warnings)
Do not abruptly discontinue buprenorphine in a patient physically dependent on opioids; when discontinuing therapy, in a physically dependent patient, gradually taper the dosage; rapid tapering in a patient physically dependent on opioids may lead to a withdrawal syndrome and return of pain
Neonatal opioid withdrawal syndrome reported with long-term use during pregnancy (see Black Box Warnings)
Interactions with CNS depressants (eg, alcohol, sedatives, anxiolytics, hypnotics, neuroleptics, other opioids) can cause additive effects and increase risk for respiratory depression, profound sedation, and hypotension; deaths reported due to methadone abuse in conjunction with benzodiazepines
Monitor for hypotension during dose initiation and titration; use with caution in patients with hypovolemia, cardiovascular disease (including acute MI), or drugs which may significantly increase hypotensive effects
In patients with circulatory shock, therapy may cause vasodilation that can further reduce cardiac output and blood pressure; avoid therapy in patients with circulatory shock
Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients
Serotonin syndrome may occur with concomitant administration of serotonergic agents; monitor patients for symptoms of serotonin syndrome such as mental status changes, autonomic instability, neuromuscular changes, and/or GI symptoms
Risk of QT interval prolongation at high doses
Use caution in cardiac arrhythmias, drug abuse or dependence, emotional lability, gallbladder disease, head injury, prostatic hyperplasia,/urethral stricture, hepatic impairment, thyroid dysfunction, increased intracranial pressure, prostatic hypertrophy, adrenal insufficiency, history of depression or suicidal tendencies, renal function impairment, seizures with epilepsy, urethral stricture, patients who are morbidly obese, urinary tract surgery
May cause CNS depression; use caution in performing tasks, which require mental alertness
Not recommended to treat abdominal conditions; may obscure diagnosis or clinical course of patients with acute abdominal conditions
Avoid the use of mixed agonist/antagonist (i.e., pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving a full opioid agonist; mixed agonists/antagonist and partial agonist analgesics may reduce analgesic effect and/or may precipitate withdrawal symptoms; when discontinuing therapy, gradually taper dosage; do not abruptly discontinue therapy
May obscure diagnosis or clinical course of patients with acute abdominal conditions; avoid use in patients with obstruction
Use with caution in patients with biliary tract dysfunction, including acute pancreatitis; may cause constriction of sphincter of Oddi
Use with caution in patients with delirium tremens
Hypoglycemia
- Cases of methadone-associated hypoglycemia reported, some resulting in hospitalization; in many cases, patients had predisposing risk factors (eg, diabetes)
- The relationship between this drug and hypoglycemia is not fully understood but may be dose dependent
- If hypoglycemia suspected, monitor blood glucose levels, and manage the patient as clinically appropriate
Opioid-induce hyperalgesia and allodynia
- Opioid-induced hyperalgesia (OIH) occurs when opioid analgesic paradoxically causes increase in pain, or increase in sensitivity to pain; this condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect
- Symptoms include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia); these symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior
- Cases of OIH reported, both with short-term and longer-term use of opioid analgesics; though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated; medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia; if a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing dose of current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety)
FDA-approved safety considerations for immediate-release (IR) and extended-release/long-acting (ER/LA) opioid analgesics addressing opioid crisis
- The risk of overdose increases as the dosage increases for all opioid pain medicines
- IR opioids should not be used for an extended period of time unless a patient’s pain remains severe enough to require them and alternative treatment options continue to be inadequate
- Numerous acute pain conditions treated in the outpatient setting require no more than a few days of an opioid pain medicine
- It is recommended to reserve ER/LA opioid pain medicines for severe and persistent pain that requires an extended treatment period with a daily opioid pain medicine and for which alternative treatment options are inadequate
Opioid analgesic risk evaluation and mitigation strategy (REMS)
- To ensure that benefits of opioid analgesics outweigh risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed; use the following link to obtain the Patient Counseling Guide (PCG): www.fda.gov/OpioidAnalgesicREMSPCG
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities
- To obtain further information on opioid analgesic REMS and for a list of accredited REMS CME/CE, call 1-800-503-0784, or log on to www.opioidanalgesicrems.com; the FDA Blueprint can be found at www.fda.gov/OpioidAnalgesicREMSBlueprint
Concomitant use with benzodiazepines or other CNS depressants
- If concomitant use with benzodiazepines is warranted, strongly consider prescribing naloxone for emergency treatment of opioid overdose, as is recommended for all patients in methadone treatment for opioid use disorder
- Concomitant use of methadone and benzodiazepines or other CNS depressants increases risk of adverse reactions including overdose and death; medication-assisted treatment of opioid use disorder, however, should not be categorically denied to patients taking these drugs; prohibiting or creating barriers to treatment can pose an even greater risk of morbidity and mortality due to opioid use disorder alone
- Educate patients about risks of concomitant use of benzodiazepines, sedatives, opioid analgesics, or alcohol
- Develop strategies to manage use of prescribed or illicit benzodiazepines or other CNS depressants at admission to methadone treatment, or if it emerges as a concern during treatment; adjustments to induction procedures and additional monitoring may be required
- There is no evidence to support dose limitations or arbitrary caps of methadone as a strategy to address benzodiazepine use in methadone-treated patients; if a patient is sedated at time of methadone dosing, ensure that a medically-trained healthcare provider evaluates the cause of sedation, and delays or omits the methadone dose if appropriate
- Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use; in some cases monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off a prescribed benzodiazepine or other CNS depressant or decreasing to lowest effective dose may be appropriate .
- For patients in methadone treatment, benzodiazepines are not the treatment of choice for anxiety or insomnia; before co- prescribing benzodiazepines, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments to address anxiety or insomnia
- Ensure that other healthcare providers prescribing benzodiazepines or other CNS depressants are aware of the patient’s methadone treatment and coordinate care to minimize the risks associated with concomitant use
- In addition, take measures to confirm that patients are taking the medications prescribed and not diverting or supplementing with illicit drugs; toxicology screening should test for prescribed and illicit benzodiazepines
Patient access to naloxone for emergency treatment of opioid overdose
- Discuss availability of naloxone for emergency treatment of opioid overdose with patient and caregiver
- Assess potential need for naloxone; consider prescribing for emergency treatment of opioid overdose
- Because patients being treated for opioid use disorder have potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for emergency treatment of opioid overdose, both when initiating and renewing treatment
- Also consider prescribing naloxone if patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose
- Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with therapy
- Consult on availability and ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (eg, by prescription, directly from a pharmacist, or as part of a community-based program)
- Educate patients regarding the signs and symptoms of respiratory depression and to call 911 or seek immediate emergency medical help in the event of a known or suspected overdose
Pregnancy & Lactation
Pregnancy
There are no adequate and well-controlled studies in pregnant women; untreated opioid addiction is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death; in addition, untreated opioid addiction often results in continued or relapsing illicit opioid use; neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy
Pregnant women in methadone maintenance programs may have reduced incidence of obstetric and fetal complications and neonatal morbidity and mortality when compared to women using illicit drugs; untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes and risk of continued or relapsing illicit opioid use; these risks should be considered in women receiving maintenance treatment of opioid addiction; for women treated with pain severe enough to require daily, around-the-clock, long-term opioid treatment, therapy should be administered during pregnancy only if potential benefit justifies potential risk to fetus
Infertility
- Chronic use of opioids may cause reduced fertility in females and males of reproductive potential; it is not known whether effects on fertility are reversible; reproductive function in human males may be decreased by methadone treatment; reductions in ejaculate volume and seminal vesicle and prostate secretions have been reported in methadone-treated individuals; in addition, reductions in serum testosterone levels and sperm motility, and abnormalities in sperm morphology have been reported
Lactation
Methadone present in low levels in human milk; did not show adverse reactions in breastfed infants; developmental and health benefits of breastfeeding should be considered along with mother’s clinical need for methadone and any potential adverse effects on the breastfed child from drug or from underlying maternal condition; advise breastfeeding women taking methadone to monitor the infant for increased drowsiness and breathing difficulties
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Narcotic agonist-analgesic of opiate receptors; inhibits ascending pain pathways, thus altering perception and response to pain; produces analgesia, respiratory depression, and sedation
Absorption
Bioavailability: 36-100%
Onset: PO, 0.5-1 hr; parenteral, 10-20 min
Duration: 4-8 hr; repeated administration, 22-48 hr; overdosage, 36-48 hr
Peak plasma time: 1-7.5 hr
Distribution
Protein bound: 85-90%
Vd: 1-8 L/kg
Metabolism
Metabolized in liver via N-demethylation
Elimination
Half-life: 8-59 hr
Excretion: Urine
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| Methadose oral
-
|
40 mg tablet |  |
|
| Methadone Intensol oral
-
|
10 mg/mL liquid |  |
|
| Diskets oral
-
|
40 mg tablet |  |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
5 mg tablet |  |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
5 mg/5 mL solution |  |
|
| methadone oral
-
|
10 mg tablet | 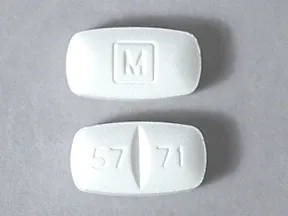 |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
5 mg tablet |  |
|
| methadone oral
-
|
5 mg tablet | 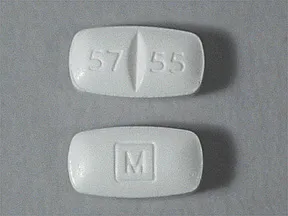 |
|
| methadone oral
-
|
40 mg tablet |  |
|
| methadone oral
-
|
10 mg/5 mL solution | 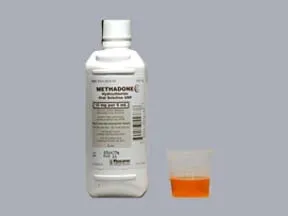 |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
5 mg tablet |  |
|
| methadone oral
-
|
5 mg/5 mL solution |  |
|
| methadone oral
-
|
10 mg tablet |  |
|
| methadone oral
-
|
5 mg tablet |  |
|
| methadone oral
-
|
10 mg/mL liquid |  |
|
| methadone oral
-
|
10 mg/mL liquid |  |
|
| methadone injection
-
|
10 mg/mL vial |  |
|
| methadone injection
-
|
10 mg/mL vial |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
methadone intravenous
METHADONE - INJECTION
(METH-a-done)
COMMON BRAND NAME(S): Dolophine
WARNING: Methadone has a risk for abuse and addiction, which can lead to overdose and death. Methadone may also cause severe, possibly fatal, breathing problems and heartbeat problems. To lower your risk, your doctor should have you use the smallest dose of methadone that works, and use it for the shortest possible time. Do not increase your dose or use this medication more often than directed. See also How to Use section for more information about addiction.Ask your doctor or pharmacist if you should have naloxone available to treat opioid overdose. Teach your family or household members about the signs of an opioid overdose and how to treat it.The risk for severe breathing problems or heartbeat problems is higher when you start this medication, when you are switching from another opioid to methadone, after a dose increase, or if you use the wrong dose/strength. Breathing problems from methadone may not happen right away after using a dose. Most heartbeat problems have happened in people using large doses of methadone for pain relief, but this problem can also occur in people getting smaller doses to treat opioid addiction. Using this medication with alcohol or other drugs that can cause drowsiness or breathing problems may cause very serious side effects, including death. Also, other medications can affect the removal of methadone from your body, which may affect how methadone works. Be sure you know how to use methadone and what other drugs you should avoid taking with it. See also Drug Interactions section. Get medical help right away if any of these very serious side effects occur: slow/shallow breathing, unusual lightheadedness, severe drowsiness/dizziness, difficulty waking up, fast/irregular heartbeat, fainting.Keep this medicine in a safe place to prevent theft, misuse, or abuse. If someone accidentally uses or swallows this drug, get medical help right away.Before using this medication, women of childbearing age should talk with their doctor(s) about the risks and benefits. Pregnancy may affect the amount of this drug in your body, so tell your doctor if you are pregnant or if you plan to become pregnant. During pregnancy, this medication should be used only when clearly needed. It may slightly increase the risk of birth defects if used during the first two months of pregnancy. Also, using it for a long time or in high doses near the expected delivery date may harm the unborn baby. To lessen the risk, use the smallest effective dose for the shortest possible time. Babies born to mothers who use this drug for a long time may develop severe (possibly fatal) withdrawal symptoms. Tell the doctor right away if you notice any symptoms in your newborn baby such as crying that doesn't stop, slow/shallow breathing, irritability, shaking, vomiting, diarrhea, poor feeding, or difficulty gaining weight.
USES: This medication is used to treat moderate to severe pain. Methadone is an opioid medication. It acts on certain centers in the brain to relieve pain.This medication is also used to treat opioid use disorder as part of an approved treatment program. It helps prevent withdrawal symptoms caused by stopping other opioids.
HOW TO USE: See also Warning section.Read the Patient Information Leaflet if available from your pharmacist before you start using methadone and each time you get a refill. If you have any questions, ask your doctor or pharmacist.Depending on your product, this medication is given by injection into a vein, into a muscle, or under the skin. Use it exactly as directed by your doctor. Read and learn all of the manufacturer's instructions for preparation and use. If you have any questions about using this medication properly, consult your doctor or pharmacist.The dosage is based on your medical condition and response to treatment.Before using, check this product visually for particles or discoloration. If either is present, do not use the liquid. Before injecting each dose, clean the injection site with rubbing alcohol. If this medication is given into a muscle or under the skin, it is important to change the location of the injection site with each dose to avoid problem areas under the skin.Learn how to store and discard needles and medical supplies safely. Consult your pharmacist for more details.If nausea occurs, consult your doctor or pharmacist for ways to decrease it (such as lying down for 1 to 2 hours with as little head movement as possible).If you are using this medication for pain, remember that pain medications work best if they are used as the first signs of pain occur. If you wait until the pain has worsened, the medication may not work as well.Suddenly stopping this medication may cause withdrawal, especially if you have used it for a long time or in high doses. To prevent withdrawal, your doctor may lower your dose slowly. Tell your doctor or pharmacist right away if you have any withdrawal symptoms such as restlessness, mental/mood changes (including anxiety, trouble sleeping, thoughts of suicide), watering eyes, runny nose, nausea, diarrhea, sweating, muscle aches, or sudden changes in behavior.When this medication is used for a long time, it may not work as well. Your doctor may need to increase your dose or change your medication. Talk with your doctor if this medication stops working well.Though it helps many people, this medication may sometimes cause addiction. This risk may be higher if you have a substance use disorder (such as overuse of or addiction to drugs/alcohol). Use this medication exactly as prescribed to lower the risk of addiction. Ask your doctor or pharmacist for more details.Tell your doctor if your condition does not get better or if it gets worse, or if you have any new pain.
SIDE EFFECTS: See also Warning section.Nausea, vomiting, constipation, lightheadedness, dizziness, dry mouth, drowsiness, and increased sweating may occur. Pain, redness, or swelling at the injection site may occur if this medication is given into a muscle or under the skin. If any of these effects last or get worse, tell your doctor or pharmacist promptly.To prevent constipation, eat dietary fiber, drink enough water, and exercise. You may also need to take a laxative. Ask your pharmacist which type of laxative is right for you.To reduce the risk of dizziness and lightheadedness, get up slowly when rising from a sitting or lying position.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.Tell your doctor right away if you have any serious side effects, including: interrupted breathing during sleep (sleep apnea), mental/mood changes (such as agitation, confusion, hallucinations), stomach/abdominal pain, difficulty urinating, signs of your adrenal glands not working well (such as loss of appetite, unusual tiredness, weight loss).Get medical help right away if you have any very serious side effects, including: seizure, severe drowsiness/difficulty waking up.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: Before using methadone, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: brain disorders (such as head injury, tumor, seizures), breathing problems (such as asthma, sleep apnea, chronic obstructive pulmonary disease-COPD), kidney disease, liver disease, mental/mood disorders (such as confusion, depression, thoughts of suicide), personal or family history of a substance use disorder (such as overuse of or addiction to drugs/alcohol), stomach/intestinal problems (such as blockage, constipation, diarrhea due to infection, paralytic ileus), difficulty urinating (such as due to enlarged prostate), disease of the pancreas (pancreatitis), gallbladder disease.Methadone may cause a condition that affects the heart rhythm (QT prolongation). QT prolongation can rarely cause serious (rarely fatal) fast/irregular heartbeat and other symptoms (such as severe dizziness, fainting) that need medical attention right away.The risk of QT prolongation may be increased if you have certain medical conditions or are taking other drugs that may cause QT prolongation. Before using methadone, tell your doctor or pharmacist of all the drugs you take and if you have any of the following conditions: certain heart problems (heart failure, slow heartbeat, QT prolongation in the EKG), family history of certain heart problems (QT prolongation in the EKG, sudden cardiac death).Low levels of potassium or magnesium in the blood may also increase your risk of QT prolongation. This risk may increase if you use certain drugs (such as diuretics/"water pills") or if you have conditions such as severe sweating, diarrhea, or vomiting. Talk to your doctor about using methadone safely.This drug may make you dizzy or drowsy. Alcohol or marijuana (cannabis) can make you more dizzy or drowsy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Avoid alcoholic beverages. Talk to your doctor if you are using marijuana (cannabis).Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).Older adults may be more sensitive to the side effects of this drug, especially confusion, dizziness, drowsiness, slow/shallow breathing, and QT prolongation (see above).During pregnancy, this medication should be used only when clearly needed. It may harm an unborn baby. Discuss the risks and benefits with your doctor. (See also Warning section.)This drug passes into breast milk and may have undesirable effects on a nursing infant. Tell the doctor right away if your baby develops unusual sleepiness, difficulty feeding, or trouble breathing. Consult your doctor before breastfeeding or if you plan to stop breastfeeding.
DRUG INTERACTIONS: See also Warning section.Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug include: certain pain medications (mixed opioid agonist-antagonists such as butorphanol, nalbuphine, pentazocine), naltrexone, samidorphan.Taking MAO inhibitors with this medication may cause a serious (possibly fatal) drug interaction. Do not take any MAO inhibitors (isocarboxazid, linezolid, metaxalone, methylene blue, moclobemide, phenelzine, procarbazine, rasagiline, safinamide, selegiline, tranylcypromine) during treatment with this medication. Most MAO inhibitors should also not be taken for two weeks before and after treatment with this medication. Ask your doctor when to start or stop taking this medication.Other medications can affect the removal of methadone from your body, which may affect how methadone works. Examples include St. John's wort, azole antifungals (such as itraconazole), macrolide antibiotics (such as erythromycin), rifamycins (such as rifampin), drugs used to treat seizures (such as carbamazepine), among others.The risk of serious side effects (such as slow/shallow breathing, severe drowsiness/dizziness) may be increased if this medication is used with other products that may also cause drowsiness or breathing problems. Tell your doctor or pharmacist if you are using other products such as other opioid pain or cough relievers (such as codeine, hydrocodone), alcohol, marijuana (cannabis), drugs for sleep or anxiety (such as alprazolam, lorazepam, zolpidem), muscle relaxants (such as carisoprodol, cyclobenzaprine), or antihistamines (such as cetirizine, diphenhydramine).Check the labels on all your medicines (such as allergy or cough-and-cold products) because they may contain ingredients that cause drowsiness. Ask your pharmacist about using those products safely.This medication may interfere with certain lab tests (such as amylase and lipase levels), possibly causing false test results. Make sure lab personnel and all your doctors know you use this drug.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, give them naloxone if available, then call 911. If the person is awake and has no symptoms, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: slow/shallow breathing, extreme drowsiness, slow heartbeat, severe dizziness, pinpoint pupils, coma.
NOTES: Do not share this medication with others. Sharing it is against the law.This medication has been prescribed for your current condition only. Do not use it later for another condition unless told to do so by your doctor. A different medication may be necessary in those cases.
MISSED DOSE: If you miss a dose, use it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Use your next dose at the regular time. Do not double the dose to catch up.
STORAGE: Store at room temperature away from light. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Information last revised March 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.




