Abstract
Key message
Treatment with methyl jasmonate can slow down the healing of stem bark wounds in Norway spruce seedlings.
Abstract
In woody plants, healing of bark wounds is a tolerance trait involved in recovery from stem damage. Yet, little is known on how wound healing may be affected by plant protection treatments such as methyl jasmonate application (MeJA, a plant hormone triggering increased resistance to pests). Here, we examined if MeJA can affect healing of an existing and a subsequently inflicted stem wound on Norway spruce (Picea abies) seedlings, the effect of treatment on plant growth, and potential trade-offs between healing and resistance to insect damage. Seedlings from 18 full-sib families were mechanically wounded (or not) on the lower stem and treated with MeJA (or water) one week after. Two months later, another wound was inflicted and wound area was measured during six months. Growth of non-wounded and wounded seedlings were compared, and correlations between family estimates of healing rates and field insect damage were examined. We found that MeJA slowed down wound healing. For the first and second wound, respectively, MeJA-treated seedlings experienced 15% and 9% slower healing rates, and wounds remained 58% and 69% larger in size compared to water-treated seedlings. Stem wounding and MeJA together were more detrimental to seedling diameter than height growth, relative to each treatment alone. Finally, resistance to field insect damage and wound healing rates were not significantly correlated. We conclude that MeJA-mediated seedling protection may trade-off with bark wound healing, which may be negative for seedling vigor. However, further studies are needed to evaluate if such effects outweigh the benefits that MeJA provides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on plant resistance to pests have provided a broad knowledge base from which sustainable alternatives to reduce damage have been or can be developed (Smith 2005; Walters et al. 2014; Stenberg et al. 2015; Mitchell et al. 2016). Resistance encompasses those traits that enable plants to stop or reduce damage by an attacker (e.g., herbivores and pathogens). For instance, resistance traits such as secondary chemicals can reduce plant palatability, while leaf waxes and trichomes can pose physical barriers to insect pests; such traits can decrease pest performance and damage to crops (e.g., Dalin and Björkman 2003; Hariprasad and Emden 2010; Tian et al. 2012; Santolamazza-Carbone et al. 2016). Relative to resistance, traits mediating tolerance to pests have been less studied (Moreira et al. 2012; Mitchell et al. 2016; Peterson et al. 2017; Karlsson Green et al. 2020). Tolerance describes the ability of plants to withstand and recover from damage by attackers. To evaluate tolerance to damage, plant growth and reproduction traits are often measured and compared before and after attack occurs (Strauss and Agrawal 1999). One of the drawbacks of using resistance in plant protection is that pests may evolve countermeasures to overcome these traits (e.g., Yates and Michel 2018). On the other hand, if plant tolerance is enhanced, little to no selective pressure is imposed on the pests (Koch et al. 2016; Peterson et al. 2017). This is because tolerance involves plant compensatory responses that occur after damage, which do not directly affect the attackers’ physiology or behavior. Yet, the underlying mechanisms behind what plant responses increase tolerance to pests, and the factors that can affect it, remain less understood and mostly under-exploited within plant protection (Koch et al. 2016; Mitchell et al. 2016).
One important tolerance trait for tree species is the ability to heal bark wounds or injuries caused by insects, fire, and trampling (Romero 2014; Chano et al. 2015). Stem bark wounds can damage or destroy vascular tissue, interrupt nutrient and water transport (Bansal et al. 2013; Romero 2014), or can even facilitate infection by pathogens (Klepzig et al. 1991; Savatin et al. 2014). A few studies have investigated wound healing in trees and the factors that can affect it, e.g., in peach trees (Prunus persica) (Biggs 1986a, b), and in the conifers Abies alba, Picea abies, Pinus sylvestris and Larix decidua (Oven and Torelli 1994, 1999; Schneuwly-Bollschweiler and Schneuwly 2012). These studies have mostly focused on older trees (> 20 years old), yet it is known that wound healing rates can vary with tree age (Tavankar et al. 2019). Moreover, stem damage can greatly impact tree survival, especially in juvenile plants (Stephens and Westoby 2015). Wound healing may be particularly important for young plants of Norway spruce (P. abies) and Scots pine (P. sylvestris) in European forest regeneration sites, given that these seedlings are attacked by the bark-chewing insect pest the pine weevil (Hylobius abietis) (Nilsson et al. 2010). Pine weevils are attracted to areas with freshly-felled conifer trees since they oviposit in and around the stump roots (Nordlander et al. 1997). Adults can feed on the thin stem bark of young and mature trees (Nordlander et al. 1986; Örlander et al. 2000), but detrimental effects arise from adults feeding on the stem bark of planted seedlings. Seedling herbivory by weevils can lead to girdling (entire ring of bark removed around the stem circumference), which disconnects the phloem above and below the wound, and can negatively affect seedling performance and survival (Bansal et al. 2013). The ability to rapidly recover from weevil damage may, thus, be crucial for successful seedling establishment in the field.
Currently, one of the alternatives being investigated to protect conifer seedlings from pine weevil damage is the use of plant-induced resistance. Exogenous application of the plant hormone methyl jasmonate (MeJA) can activate induced resistance mechanisms and reduce pine weevil damage to Picea and Pinus spp. seedlings (Zas et al. 2014; Chen et al. 2021a; Puentes et al. 2021; Berggren et al. 2023). However, following MeJA treatment, a reduction in plant growth can occur (Heijari et al. 2005; Gould et al. 2009; Zas et al. 2014; Chen et al. 2021b). Plants have limited resources to allocate to different activities. Thus, trade-offs are usually expected when multiple activities (e.g., defense, growth and reproduction) compete for plant resources (Herms and Mattson 1992; Endara and Coley 2011). Treatment with MeJA is expected to cause a diversion of resources into processes involved in resistance. For example, MeJA application results in increased phenolic production and phloem lignification, and reprogramming of the cambial zone to form traumatic resin ducts in the conifers Pseudotsuga menziesii, Sequoiadendron giganteum and P. abies (Martin et al. 2002; Hudgins et al. 2004). Such morphological and chemical defensive responses can be costly for plants (Cipollini and Heil 2010). Despite these expected changes in resource allocation, the effects of MeJA have not been examined from a plant tolerance perspective.
Considering the role that bark wound healing can play in conifer seedling survival, and the emerging potential of MeJA as a plant protection tool, we conducted an experiment to investigate the effects of MeJA on the rate of stem wound healing as a tolerance trait. Studies on conifers have revealed that the first response to stem bark wounding is abundant resin production; moreover, closure of the wound occurs from the remaining vascular cambium along the margins of the wound (Chano et al. 2015, 2017). Vascular cambium is the growth tissue found in the stem, which produces secondary xylem inwards and secondary phloem outwards. From the lateral edges of the stem wound, where intact bark remains, proliferation of the cambium occurs in a way that the cells ‘bend’ inward, and reach the wound surface (Chano et al. 2015). Cambial cells divide forming new vascular tissue, and the cambium grows towards the center of the wound. Eventually, the healing tissue mass from both lateral edges meets, merges and covers the entire wound surface (Chano et al. 2015). These responses likely entail a diversion of resources to the wounded site. Since MeJA may also change resource allocation, treatment with this hormone could potentially interfere with processes involved in bark wound healing. Using Norway spruce (Picea abies) seedlings, we aimed to answer the following questions:
- (1)
Can treatment with MeJA affect the healing of an existing stem wound inflicted prior to treatment, and a subsequent wound inflicted after treatment on the same plant?
- (2)
How does wounding by itself and together with MeJA treatment affect plant growth?
- (3)
Is there a correlation between wound healing rate (estimated in the present experiment) and pine weevil damage in the field for plants that have not been treated with MeJA?
We first examined the effect of MeJA on the healing of a stem bark wound that was mechanically inflicted one week before MeJA treatment, and its effects on a subsequently inflicted second wound eight weeks after MeJA treatment occurred. After wound infliction, we recorded wound size (exposed xylem) every other week to estimate wound healing rate, and measured seedling height growth and diameter growth for a total of four months. The Norway spruce seedlings used in the experiment originated from 18 different full-sib families, which are part of the Swedish national spruce breeding program. Families from the breeding program are of practical interest since breeding for resistance may also be an alternative to protect seedlings from pine weevil damage (Zas et al. 2017). Seedlings from 14 of the Norway spruce families used in this laboratory experiment were also planted in the field in a separate experiment (see Chen 2021c), and damage inflicted by pine weevils at the fresh clear-cut was assessed. To answer question 3, we used the field data to examine the correlations per family between wound healing rate from the present experiment (considered a trait that mediates tolerance to pest damage) and pine weevil damage (considered a resistance trait, measured as the inverse of total pine weevil damage) in non-MeJA-treated plants.
Materials and methods
Plant material
Norway spruce seedlings from 18 full-sib families were obtained from the research station of the Forestry Research Institute of Sweden (Skogforsk) located in Ekebo, Sweden. Each family included 6–10 seedlings. This material came from the clonal archive of the Swedish Norway spruce national breeding program. The seedlings were sown in plug trays (BCC Plant the Planet, HIKO V-150, item code: 21-22135) in late February 2017 with standard peat substrate for one year of cultivation. To prevent bud set, supplemental light was provided until the beginning of May. In August 2017, the seedlings were transferred outdoors where they remained until December when they were packaged and stored in a freezer room for overwintering. This is a standard nursery practice used to simulate winter conditions for conifer plants, which are then thawed the following spring/summer for planting.
In early June 2018, 177 frozen stored seedlings arrived at the Swedish University of Agricultural Sciences in Uppsala, Sweden. After thawing, seedlings were transplanted on June 26 (day 1, used as a reference time point in the methods and Results sections). We took the entire soil plug from the plug tray (without damaging the roots), placed it in a plastic pot (diameter = 14 cm) pot and surrounded it with soil up to the top (gardening soil; P-jord, Hasselfors garden, Sweden). The larger pots allow plants to further grow their roots compared to when they are in the plug trays. Plants were kept in a greenhouse (16 hL/8hD; temperature: 20/16 °C) during the duration of the experiment.
Wounding and methyl jasmonate treatments
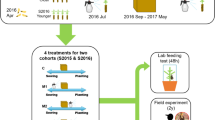
Four different treatments including exogenous MeJA application and mechanical wounding were conducted as depicted in Fig. 1 and as follows:
Schematic diagram of the treatments included in the present study. Day 1 represents the first experimental day, when seedlings were planted in pots after thawing. On day 14, the first large wound was inflicted. One week after inflicting the first wound (day 21), MeJA or water was exogenously applied to Norway spruce (Picea abies) seedlings (MW and CW treatments, respectively). Two months later (day 77), a second smaller wound was inflicted. For non-wounded seedlings, MeJA or water was sprayed (M and C treatments, respectively) at the same time point as for wounded seedlings. Wound area and plant growth were measured every other week until December (day 175)
- (1)
MeJA and wounding treatment (MW): A rectangular wound (height: 40, width: 5 mm) was inflicted on the lower part of the seedling stem using a scalpel on July 10 (day 14, i.e., 14 days after the seedlings were transplanted). We avoided including side branches in the wound area, and the height of most wounds was about 1–2 cm from the soil. If there were many branches around the lower stem, we inflicted the wound higher up or lower down to avoid cutting the branches. In the rectangular area, bark, phloem and vascular cambium were removed until the xylem was exposed (see Fig. S1). The average wound area (± standard error) in this treatment was 147.3 ± 47.5 mm2 and the width of the wound (5 mm) spanned about 30% of the stem circumference (see Fig. S1). One week later, on July 17 (day 21), seedlings were sprayed once with MeJA (10 mM) following the procedure described in Chen et al. (2021b). Two months after MeJA treatment, on September 11 (day 77), a second and smaller rectangular wound, 15 mm high and 5 mm wide, was inflicted on seedlings. The effects of MeJA on resistance to pine weevil damage have been shown to persist up to two years after application (Zas et al. 2014). Thus, we expect that any changes caused by MeJA should still be present in the plants two months after treatment. We inflicted a smaller wound than the first one to minimize the likelihood of mortality. The average wound area (± standard error) was 51.4 ± 7.0 mm2. The lower end of this wound was located about 1 cm above the upper end of the first wound, and on the opposite side of the stem.
- (2)
Control and wounding treatment (CW): Two wounds were inflicted on seedlings as described for the MW treatment, but water was used instead of MeJA to treat seedlings on day 21. The average wound areas (± standard error) for the first and second wound (on day 14 and day 77) were, respectively, 145.0 ± 39.6 and 49.3 ± 6.9 mm2.
- (3)
MeJA control treatment (M): No wounds were inflicted on seedlings, but seedlings were treated with MeJA (10 mM) on day 21.
- (4)
Control treatment (C): No wounds were inflicted, but seedlings were sprayed with water on day 21.
A total of 40 seedlings were assigned to each treatment, with 16–18 families being represented in each treatment due to the uneven number of individuals per family received from the nursery (see Table S1 for family names, and actual sample sizes per family in each treatment). Note that for three seedlings (1 in the CW treatment, and 2 in the MW treatment), the second wound was not inflicted by mistake and these plants were excluded from all statistical analyses (see total sample sizes per treatment in Figs. 2 and 3).
Estimated mean healing rate (amount of bark formed per day, mm2/day) (± 95% confidence intervals) for the first wound (filled circles) and the second wound (filled triangles) inflicted on Norway spruce (Picea abies) seedlings treated with MeJA (MW) or not (CW). The first wound was inflicted on day 14 (day 1: transplanted into pots); seedlings were treated with water (CW) or MeJA (MW) on day 21, and the second wound was inflicted on day 77. Sample sizes (n) used in the statistical analyses are shown. The interaction between wound order × treatment was not statistically significant (Table 1), indicating that MeJA affected the healing rate of both wounds to a similar extent. Note that the y-axis does not start at 0
Average wound area (exposed xylem area, mm2) ± standard error of an existing (first) wound and a subsequently inflicted (second) wound in Norway spruce (Picea abies) seedlings, treated with MeJA (MW) or not (CW) over the experimental period (days 1–175). The first wound was inflicted on day 14 after seedlings were transplanted into pots on day 1 (June 26th ). Wounded seedlings were sprayed with water (CW) or MeJA (MW) on day 21, and the second wound was inflicted on day 77. Filled green circles connected by dashed or solid lines show the average wound size of seedlings in the CW treatment for the first and second wound, respectively. Filled orange triangles connected by dashed or solid lines show the average wound size of seedlings in the MW treatment for the first and second wound, respectively. Arithmetic means ± standard error for wound areas can be found in Table S2
Wound closure and plant growth measurements
To examine the effects of treatment on wound healing and plant growth, wound closure and seedling growth were measured regularly. The first growth measurements were conducted on June 26, 2018 (day 1). From that point onwards, the aboveground height and stem basal diameter were measured every other week until December 18, 2018 (day 175). From the day that the first wound was inflicted (July 10, day 14), wound area (exposed xylem) was measured with the same frequency as the growth measurements (every other week). A digital caliper was used to measure the wound width on the upper (Wup) and lower sides (Wdown), as well as the vertical wound height (H). The wound area was estimated by using the equation for calculating the area of a trapezoid: wound area Awound = (Wup + Wdown)/2*H. If the wound healed to an irregular shape, it was divided into several trapezoids and the sum of all areas was used. The extent of newly formed bark per day was described as wound healing rate (final measurement of the exposed xylem subtracted from the initial measurement = amount of healed bark, divided by the number of experimental days). Wound healing measurements continued until December 18 (day 175), unless the wound had completely healed before this date.
Seedling resistance to pine weevil damage
Seedlings from 14 of the 18 full-sib families used in this experiment were also planted in the field as part of a parallel experiment (see Chen 2021c). The field experiment aimed to explore genetic variation in constitutive resistance to pine weevil damage in Norway spruce seedlings. Thus, seedlings were not treated with MeJA prior to planting. For these 14 families, the number of replicates per family ranged from 3 to 13 plants in the field. Briefly, a field trial was established in a one-year-old clear-cut during the spring of 2018 in southern Sweden (Remningstorp, 58°28′ N, 13°34′ E). Seedlings were attacked by pine weevils naturally present at the site, and pine weevil damage was recorded in June and September of 2018. We estimated stem area debarked (mm2) by pine weevils as described in Chen et al. (2021a). We visually estimated the percentage of the stem that was debarked by the pine weevil, and the height from the root collar to where the last feeding scar could be found on the stem. Then, using this percentage, the equation for the circumference of a circle, and the height up to the last scar, we calculated area debarked (cm2) for each plant as: Stem circumference (π·d) × (debarked height up to the last feeding scar) × (percentage debarked).
Statistical analyses
All data were analyzed using R software v. 3.6.3 (R Core team 2020) with R studio 1.2.5042 (R Core team 2020), and graphs were generated using the ggplot2 (Wickham 2016) and the ggpubr (Kassambara 2020) packages. Significance of main effects and interactions was tested with analysis of deviance using the Anova command from the car package (Fox and Weisberg 2019). Differences among treatment levels were pairwise-compared using the emmeans command in the emmeans package (Lenth 2020).
First, to examine how wound healing rate (average amount of bark formed per day, mm2/day, see Sect. 5 for equation) was affected by MeJA treatment, and whether the effect varied depending on if it was an existing or a subsequent wound, we fitted a linear mixed-effects model [lmer command from lme4 package, (Bates et al. 2015)]. The explanatory variables included treatment (CW and MW) and wound order (the first wound and the second wound) as fixed variables. Seedling identity nested in family (n = 18) was included as a random variable, and seedling initial basal diameter (mm, measured on day 1 after seedlings were transplanted in pots) as a continuous covariate. In addition, we included the interaction between wound order and treatment, and the interaction of wound order and seedling initial basal diameter. The relationship between diameter and healing rates for each wound were also explored. Pearson’s product-moment correlations were estimated using the cor.test command from the base R stats package (R Core team 2020).
Similarly, to examine the effects of treatment on changes in actual wound size (exposed xylem area, mm2) across time, we fitted separate linear mixed-effects models [lme command from the nlme package (Pinheiro et al. 2020)] for each wound. Wound size was log-transformed for the first wound, and square-root transformed for the second wound, to meet model assumptions. The model examining changes in size for the first wound included treatment (CW and MW), time (number of days after transplanting) as a linear and quadratic term, and the interaction of these two as fixed variables. The random variables were seedling identity nested within family (n = 18), and time as a linear and quadratic term since it was a repeated-measurements analysis. To examine changes in size for the second wound, the model included the same variables as the one for the first wound; however, time was included only as a linear term in the fixed and random variables since the quadratic term did not provide better model fit. Initial seedling basal diameter (mm, measured on day 1 when seedlings were transplanted to pots) was included in both models as a covariate.
In addition, we examined the effect of wounding and MeJA treatment on seedling growth. We fitted models with height increment and basal diameter increment as response variables. Increments here were defined as the difference between the plant growth measurements taken on day 1 and day 175 for the first wound, and the growth difference between day 73 and day 175 for the second wound. We fitted two linear mixed models [lmer command from lme4 package (Bates et al. 2015)]. These models included the fixed variable of treatment (C, M, CW and MW), the random variable of family (n = 18), and the continuous covariate of initial seedling size [height (cm) or diameter (mm), measured on day 1 after seedlings were transplanted into pots] for height and diameter increment, respectively.
Lastly, to investigate the potential relationship between resistance to pine weevil damage in the field and wound healing of Norway spruce seedlings in the laboratory experiment, we correlated family estimates of wound healing rate (mm2/day) for non-MeJA (water)-treated plants and area debarked (cm2) by pine weevils. The estimates of weevil damage per family were obtained from the analyses conducted for the parallel field experiment (see Chen 2021c). Estimated means of wound healing rate for the first and second wound for each family were obtained by fitting a linear model [lm command from base R stats package (R Core team 2020)]. The model included family and wound order as fixed variables, and seedling initial basal diameter (mm, measured on day 1 after seedlings were transplanted in pots) as a continuous covariate, and the interaction of family and initial basal diameter. The interaction of wound order and initial basal diameter was added to allow for different slopes of initial basal diameter for each wound and family. Estimated means of wound healing rate for each family were then obtained by the emmeans command. Pearson’s product-moment correlations between family estimates of area debarked (measured in June and September 2018) and wound healing rates (for the first and second wound) were conducted separately using the cor.test command from the base R stats package (R Core team 2020).
Results
Effects of MeJA on wound healing
We found that the healing of stem bark wounds inflicted on Norway spruce seedlings differed among treatments. MeJA slowed down the healing rate (average amount of bark formed per day, mm2/day) of both the first and second wound to a similar extent (non-significant wound order × treatment interaction, but significant treatment effect; Table 1). Seedlings treated with MeJA (MW) experienced healing rates that were 15% and 9% lower for the first and second wound, respectively, relative to that of seedlings treated with water (CW) (Fig. 2). Regardless of treatment, the overall average healing rate of the first wound was significantly greater than that of the second wound (Table 1; Fig. 2). Moreover, the healing rate of the first wound was positively correlated with initial basal diameter for plants in both treatments (Fig. S2a, S2b). On the other hand, the healing rate of the second wound was positively correlated with seedling diameter measured right before wounding, but only for MeJA-treated plants (Fig. S2c, S2d).
Given that MeJA slowed down the rate of bark wound healing, changes in wound area (exposed xylem, mm2) were different between treatments over time (significant time × treatment interaction for both wounds; Table 2). The extent of exposed xylem was significantly greater for MeJA-treated seedlings over the entire experimental period (Fig. 4). Therefore, by the end of the experiment, the first and second wounds of MeJA-treated seedlings (MW) were 58% and 69% larger in size, respectively, than those of water-treated seedlings (CW) (Fig. 4). Overall, little change in wound size was observed the first two weeks after infliction of the first wound for both treatments (Fig. 4; CW and MW, from days 14 to 28). Four to six weeks after damage infliction, a steep decrease in wound area occurred for both treatments (Fig. 4; from day 28 to 73); however, this decrease was more pronounced for water-treated (CW) seedlings (Fig. 4; specifically between days 28 and 42). After this period, wound area continued to decrease but at a slower pace up to about 8–10 weeks post-wounding. After that, little to no change occurred for the remainder of the measurement period (Fig. 4; day 73 to day 175). The same pattern was observed for the second wound, with a steep change in size occurring between days 96 and 119, and little change occurring between days 140 and 175 (Fig. 4).
Growth increment for Norway spruce (Picea abies) seedlings receiving different treatments. Non-wounded seedlings were sprayed with water (C) or MeJA (M). Wounded seedlings were sprayed with water (CW) or MeJA (MW) after an existing (first) wound, but before a subsequently inflicted (second) wound. Estimated means of (a) seedling height increment (cm) (± 95% confidence intervals) and (b) basal diameter increment (mm) (± 95% confidence intervals) are shown. Sample sizes (n) used in the statistical analyses are shown, and letters indicate significantly different means. Arithmetic means ± standard error for each measured time point can be found in Table S3. Note that the y-axes do not start at 0
We also observed that a few seedlings in both the MeJA- and water-treated groups had completely closed their wounds. For the first wound, 2 seedlings (number of seedlings in each treatment; CW = 1 seedling, MW = 1 seedling) had completely healed 82 days post-wounding. By the end of experiment (162 days after inflicting the first wound), 69 seedlings had healed their first wound to more than half of the original wound size (CW = 37 seedlings, MW = 32 seedlings), and 8 seedlings had completely healed their wounds (CW = 4 seedlings, MW = 4 seedlings). For the second wound, 3 seedlings in the control group had completely healed 63 days post-wounding, and 1 seedling in the MeJA-treated group had healed completely 77 days after wounding. By the end of the experiment (96 days after inflicting the second wound), 12 seedlings had completely closed their second wound (CW = 8 seedlings, MW = 4 seedlings). No seedlings died during the duration of the experiment.
Effects of wounding and MeJA on plant growth
Even though seedlings were of similar size at the start of the experiment, we found some differences in height and diameter increment among plants receiving different treatments (Table 3, Fig. S3). For seedlings treated with water, the height increments of wounded seedlings (CW) and intact seedlings (C) were not significantly different (Fig. 3a). Similarly, for MeJA-treated seedlings, the height increment of wounded seedlings (MW) did not differ from that of intact seedlings (M) (Fig. 3a). These non-significant results indicate that wounding alone did not have a negative effect on height increment. However, MeJA had an overall significant negative effect on height increment. Seedlings treated with MeJA (M and MW) had significantly less height growth than non-treated seedlings (C and CW) (Fig. 3a). On the other hand, diameter increment exhibited a different pattern than that of height increment. Only seedlings that were both wounded and treated with MeJA (MW) had significantly less diameter growth compared to intact control seedlings (C) (Fig. 3b). Seedlings that were only treated with MeJA (M) and seedlings that were only wounded (CW) did not have significantly different diameter growth compared to intact control seedlings (C) (Fig. 3b). Thus, MeJA alone or wounding alone did not have a significant negative effect on diameter increment, but wounding and MeJA together were detrimental for seedling basal diameter growth.
Correlation between plant resistance to insect damage and healing rate
We also examined correlations between family estimates of pine weevil damage in the field from a parallel study, with wound healing rates from the present study. We used estimates of wound healing rates from plants not treated with MeJA, given that seedlings in the field were also untreated. Regardless of whether we examined pine weevil damage early or late in the season or the healing of the first or second wound, we found no significant correlation between seedling resistance to pine weevil damage and wound healing rate (Fig. 5). Nonetheless, we observed some trends when comparing healing rates and pine weevil damage among families. A few families that received little pine weevil damage later in the growing season exhibited slower wound healing rates for the first wound (e.g., family 7; Fig. 5a and c). On the other hand, some pine weevil-resistant families showed faster-healing rates for the second wound (e.g., family 12; Fig. 5b and d). Moreover, some of the families that healed both wounds faster tended to be more resistant to pine weevil damage early in the season but not later in the season (e.g., family 8; Fig. 5).
Relationship between mean estimates of wound healing rates (average amount of bark formed per day, mm2/day) and pine weevil (Hylobius abietis) damage (stem debarked area, cm2) for non-MeJA-treated plants in 14 Norway spruce (Picea abies) full-sib families. The relationship between field damage from the first assessment (June, 2018) with (a) the first wound, and with (b) the second wound, and the relationship between field damage from the second assessment (September, 2018) with (c) the first wound, and with (d) the second wound are shown. Each family was given a number from 1 to 14, but their original names can be found in Table S4. The regression line (blue), correlation coefficient (R) with the P-value (p) and confidence interval were estimated using Pearson-moment correlations. Estimated means ± standard error for field debarked area and wound healing rate per family can be found in Table S4. Note that the y-axes do not start at 0 and the x-axes vary among the plots
Discussion
We found that exogenous application of the plant hormone methyl jasmonate (MeJA) can affect bark wound healing in Norway spruce seedlings. Healing of an existing and a subsequent wound were negatively affected by treatment, with each wound healing 15% and 9% slower and wounds being 58% and 69% larger in size, respectively, for MeJA vs. water-treated seedlings by the end of the experiment. Thus, MeJA-mediated seedling protection (i.e., induced resistance) may entail a trade-off with wound healing, an important tolerance trait for recovering from stem bark damage. As previously reported, treatment with MeJA resulted in height growth reductions, but when combined with wounding, it reduced diameter growth to a greater extent relative to intact, non-treated seedlings. Lastly, we did not detect a significant correlation between family estimates of pine weevil damage in the field and wound healing rates (for non-MeJA-treated plants) from the present experiment. However, a few notable Norway spruce families may be of practical interest, since they exhibited both higher wound healing rates and lower levels of pine weevil damage in the field.
To our knowledge, no previous studies have directly examined how MeJA treatment may affect healing of stem bark wounds in conifers. Yet, from previous studies there are indications that MeJA could indirectly affect wound healing processes by, e.g., having an impact on plant resource and nutrient allocation, photosynthesis, and stem anatomical structures. For instance, it has been reported that MeJA treatment resulted in remobilization of nitrogen from leaves to roots in rice (Oryza sativa) and tomato (Solanum lycopersicum) (Gomez et al. 2010; Wu et al. 2019). Moreover, it has been shown that jasmonic acid (JA, the free acid form of MeJA) treatment can cause a loss of chlorophyll from leaves or cell cultures (Weidhase et al. 1987). Significantly reduced chlorophyll content and decreased levels of photosynthesis-related proteins were also found in rice plants after MeJA treatment (Wu et al. 2019). In conifers, a study on Maritime pine (Pinus pinaster) found increased fine root biomass following MeJA treatment, indicating increased carbon allocation to roots (Moreira et al. 2012). In Monterey pine (P. radiata), a temporal reduction in CO2 uptake and photosynthesis was observed following MeJA treatment, but these changes had no long-term effects on the plants (Gould et al. 2008). Moreover, it is well established that MeJA induces traumatic resin duct formation and increases levels of chemical defenses in various conifers (Franceschi et al. 2002; Martin et al. 2002; Hudgins et al. 2004; Miller et al. 2005; López-Villamor et al. 2021), which entails a cost in terms of resources invested in secondary chemistry and stem structural changes. Moreover, these changes are associated with increased resistance to insects and pathogens (e.g., Erbilgin et al. 2006; Gould et al. 2009; Zas et al. 2014; Puentes et al. 2021). Thus, MeJA treatment may diminish or divert resources away from wound healing processes, which could help explain the negative effect observed on bark wound closure of Norway spruce seedlings.
Treatment with MeJA could have also interfered directly with tissue re-growth activity in the stem bark. In conifers, the first response after a stem bark wound is lignification and suberization (i.e., hardening) of the cortical parenchyma cells (undifferentiated cells) that line the lateral edges of the wound and connect to the remaining bark around the stem (Oven and Torelli 1999; Chano et al. 2015). This provides a first barrier to pathogen entry and reduces water loss. Secondly, the remaining vascular cambium along the edges of the wound divides and grows towards the exposed surface of the wound (Oven and Torelli 1999; Chano et al. 2015). Parenchyma cells arise from a special type of cell division, and build what is known as the protective callus (lignified and suberized) around the growing cambium mass (Oven and Torelli 1999; Chano et al. 2015). Newly formed periderm develops from the callus, and this growing mass of cambium and periderm continues to proliferate and differentiate (Oven and Torelli 1999; Chano et al. 2015). These tissue masses from the wound edges continue to grow until they meet, merge and close the wound (Oven and Torelli 1999; Chano et al. 2015). In our study, we observed light green cell masses along the edge of the wound two weeks after inflicting the first wound (Fig. S4a), which we suspect to be the cambium + callus growing mass according to Chano et al. (2015). Eventually, the growing green tissue met, merged and sealed the wound as expected (Fig. S4b and c).
It has been shown that callus induction may be inhibited by JA (Ikeuchi et al. 2017). For example, JA retarded callus formation in potato (Solanum tuberosum) (Ravnikar and Gogala 1990), and JA and MeJA inhibited callus growth in soybean (Glycine max) (Ueda and Kato 1982). MeJA treatment has also been shown to limit organ growth by reducing cell number and size in Arabidopsis thaliana mutants (Noir et al. 2013), and JA treatment can negatively affect mitosis during callus formation in cell cultures (Świa̧tek et al. 2002; Świątek et al. 2004). Given the negative effect of JA and MeJA on callus and cell growth reported in other plants, it is possible that MeJA treatment interfered directly with the healing of bark wounds in Norway spruce seedlings. However, our study does not allow us to pinpoint the specific mechanisms underlying the observed negative effects. Thus, further studies will be needed that examine changes in plant photosynthesis, resource mobilization and callus/tissue formation following wounding and MeJA treatment.
From a plant protection perspective, our findings indicate that MeJA-mediated seedling protection may entail a reduction in seedlings’ bark healing capabilities. To understand the consequences of these findings, we need to consider the advantages conferred by MeJA. Treatment with MeJA provides protective effects by increasing conifer seedling resistance to pine weevil damage. MeJA induces chemical defenses, resulting in qualitative and quantitative changes that make treated seedlings less palatable for pine weevils (Zas et al. 2014; Lundborg et al. 2016a, b; Nybakken et al. 2021). Hence, treated seedlings often receive less damage overall, but they are also damaged in a way that is more spread out across the stem (Fedderwitz et al. 2016). In turn, this results in lower girdling rates and greater survival rates for MeJA-treated relative to untreated seedlings. Because of these benefits, MeJA has been put forward as a tool for protecting planted conifer seedlings during forest regeneration (Zas et al. 2014; Chen et al. 2021a; Puentes et al. 2021; Berggren et al. 2023). However, if MeJA slows down stem bark wound healing and closure, this would constitute an undesired effect of treatment. Slower healing of bark wounds would entail exposed areas that can act as entry point for pathogens, and reduced phloem connection along the stem which interferes with nutrient transport (e.g., Romero 2014; Rademacher et al. 2019). These effects could be detrimental for seedling vigor and may, thus, entail economic costs by diminishing the extent and quality of forest regeneration.
Nonetheless, from our study, we are not able to determine if a trade-off with bark wound healing outweighs the benefits of using MeJA as a plant protection tool. Several aspects need to be considered. First, since MeJA treatment results in less area debarked by pine weevils and reduced likelihood of girdling (e.g., Zas et al. 2014), treated seedlings may have less bark damage or smaller wounds to heal compared to untreated ones. Untreated seedlings may suffer even greater disconnection along the stem phloem relative to treated seedlings, or have wounds that cannot be fully closed if girdling occurs. Hence, seedlings with fewer but ‘slower-healing’ wounds may be preferred over seedlings with larger but ‘faster-healing’ wounds. Second, it is possible that the negative effects of MeJA on wound healing are not long-lasting. From a nursery perspective, MeJA treatment can be applied to Norway spruce seedlings before winter storage, and it will effectively provide protection against pine weevil damage when seedlings are planted the following spring (Chen et al. 2021a). In our study, the negative effects of MeJA on wound healing remained for roughly 3–4 months after treatment. Yet, these effects may or may not persist for several growing seasons. Hence, studies examining the effects of MeJA on wound healing for a longer period are required to conclusively evaluate if and how they fade with time. Lastly, the effects of MeJA on wound healing should also be assessed in the field, where plants receive multiple pine weevil wounds simultaneously. Overall, our findings provide a key factor to consider when further evaluating the consequences and practical implementation of using MeJA in conifer seedling protection.
In addition to effects on wound healing, we also examined the effect of treatment on Norway spruce seedling growth. We found that MeJA had an overall negative effect on seedling height regardless of whether plants were wounded or not (Fig. 3a). However, for seedling basal diameter, MeJA and wounding together resulted in a greater reduction in growth (Fig. 3b). The negative effects of MeJA on plant growth are in line with those previously reported in other studies (Heijari et al. 2005; Gould et al. 2008; Krokene et al. 2008; Chen et al. 2021a). The growth reduction may, for example, be due to the negative effects of MeJA on cell mitosis, and these inhibitory effects are often more prominent for leaf rather than root growth (Zhang and Turner 2008). In contrast to seedling height elongation, which occurs from existing buds, basal diameter growth may be more impaired by disconnection of the bark phloem. Injuries along the stem may partially or completely hinder resource transport from the photosynthesizing needles to lower parts (e.g., Rademacher et al. 2019). Hence, resource diversion due to MeJA treatment and physical impairment of resource transport due to wounding, may together result in a stronger effect on basal diameter than each factor alone.
Interestingly, we also found a positive correlation between healing rate of the first wound and initial stem basal diameter of Norway spruce seedlings (before any treatment was inflicted; Fig. S2). Tree vigor, often measured as diameter growth, has been previously reported to positively affect wound healing (Neely 1988; Boyes et al. 2019; Jones et al. 2019; Tavankar et al. 2019). A thicker stem diameter may imply a greater number of cells (or cell layers) from which callus formation and/or centripetal cambium growth can rapidly occur to cover the exposed area. We also found that the first and larger wound healed faster overall (regardless of treatment) relative to the second and smaller wound (Fig. 4; Table 2). A previous study has found, for example, that larger wounds healed or closed to a greater extent than smaller wounds within one growing season (Neely 1988). Since stem bark wounds heal from the edges, a larger wound perimeter may also entail a greater number of cells from which cambium growth can occur. It would be of interest to further explore not only stem diameter, but also the relationship between bark layer thickness and the rate of wound healing in seedlings.
Lastly, we examined the relationship between Norway spruce family estimates of pine weevil damage in the field (considered a resistance trait; stem area debarked) with wound healing rates for non-MeJA-treated plants in our experiment (considered a tolerance to damage trait; average amount of bark formed per day). We found no significant correlation between these two traits for the families examined (Fig. 5). Theoretically, a trade-off (negative correlation) between resistance and tolerance to insect damage is expected, but experimental evidence has shown that this is not always the case (Leimu and Koricheva 2006). In some herbs, e.g., tall morning glory (Ipomoea purpurea), wild radish (Raphanus raphanistrum) and turnip greens (Brassica rapa), trade-offs between resistance and tolerance have been reported (Fineblum and Rausher 1995; Stowe 1998; Strauss et al. 2003). In other studies involving woody plants, e.g., willow (Salix cordata), silver birch (Betula pendula), aspen (Populus tremuloides) and Douglas fir (Pseudotsuga menziesii), no trade-off between resistance and tolerance to attackers was found (Shen and Bach 1997; Prittinen et al. 2003; Stevens et al. 2007; Cruickshank et al. 2018). Thus, resistance and tolerance do not always correlate negatively with each other, and plants can possess both high resistance and high tolerance to damage (Leimu and Koricheva 2006; Nuñez-Farfán et al. 2007). In line with this, we found that some families exhibited both greater resistance (received less pine weevil damage) and higher tolerance (faster wound healing rates). Other families showed both low resistance (received more pine weevil damage) and low tolerance (slower wound healing rates), but most families were found between the two extremes (Fig. 5). From a plant protection perspective, a lack of trade-off is desirable since greater resistance and tolerance to insect damage can be beneficial to seedling vigor, and thus, forest regeneration. In addition, families exhibiting both greater resistance and tolerance can be of interest for further developing the breeding program of Norway spruce in Sweden. Our results suggest that breeding for greater resistance may not necessarily result in seedlings that are less tolerant to bark damage. However, our study included few families and replicates per family. Thus, to evaluate the generality of this lack of a trade-off, future studies should examine a greater set of families from the breeding population and even examine this correlation for plants that have been induced by MeJA.
Conclusion
Exogenous application of the plant hormone methyl jasmonate (MeJA) may negatively affect the healing of existing and subsequent stem bark wounds in Norway spruce seedlings. Our findings provide a key factor to consider when evaluating the benefits and drawbacks of using MeJA in conifer seedling protection against pest damage. We also found that stem wounding and MeJA together may be more detrimental to seedling diameter than height growth, relative to when these factors occur alone. Finally, we did not detect a significant correlation between family estimates of pine weevil damage in the field and wound healing rates from the present experiment in non-MeJA-treated plants. This indicates a lack of a trade-off between resistance to pine weevil damage and tolerance to bark damage, which is desirable from a plant protection and plant breeding perspective, but more families should be examined.
Author contribution statement
AP, CB, NB and HB conceived and designed the original experiment. The research questions were further improved following discussions between NB and YC. YC conducted the experiment and carried out the data analyses. K-AH produced the plant material and carried out the analysis from the field experiment from which Norway spruce family estimates of pine weevil damage were obtained. YC wrote the draft with input from AP, CB and HB. All authors contributed to further revisions of the paper, and approved the submitted version.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bansal S, Hallsby G, Lofvenius MO, Nilsson MC (2013) Synergistic, additive and antagonistic impacts of drought and herbivory on Pinus sylvestris: leaf, tissue and whole-plant responses and recovery. Tree Physiol 33(5):451–463. https://doi.org/10.1093/treephys/tpt019
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Berggren K, Nordkvist M, Björkman C, Bylund H, Klapwijk MJ, Puentes A (2023) Synergistic effects of methyl jasmonate treatment and propagation method on Norway spruce resistance against a bark-feeding insect. Front Plant Sci. https://doi.org/10.3389/fpls.2023.1165156
Biggs AR (1986a) Wound age and infection of peach bark by Cytospora leucostoma. Can J Bot 64(10):2319–2321. https://doi.org/10.1139/b86-303
Biggs AR (1986) Phellogen regeneration in injured peach tree bark. Ann Bot 57(4):463–470. https://doi.org/10.1093/oxfordjournals.aob.a087128
Boyes KN, Hietala-Henschell KG, Barton AP, Storer AJ, Marshall JM (2019) Linking tree growth rate, damage repair, and susceptibility to a genus-specific pest infestation. J For Res 30(5):1935–1941. https://doi.org/10.1007/s11676-019-00896-y
Chano V, López R, Pita P, Collada C, Soto Á (2015) Proliferation of axial parenchymatic xylem cells is a key step in wound closure of girdled stems in Pinus canariensis. BMC Plant Biol 15(1):64. https://doi.org/10.1186/s12870-015-0447-z
Chano V, Collada C, Soto A (2017) Transcriptomic analysis of wound xylem formation in Pinus canariensis. BMC Plant Biol 17(1):234. https://doi.org/10.1186/s12870-017-1183-3
Chen Y (2021c) Exploiting Plant Defenses to Protect Conifer Seedlings against Pine Weevils (Doctoral dissertation, Swedish University of Agricultural Sciences, Sweden). Retrieved from https://pub.epsilon.slu.se/25949/
Chen Y, Bylund H, Björkman C, Fedderwitz F, Puentes A (2021) Seasonal timing and recurrence of methyl jasmonate treatment influence pine weevil damage to Norway spruce seedlings. New For 52(3):431–448. https://doi.org/10.1007/s11056-020-09803-4
Chen Y, Puentes A, Björkman C, Brosset A, Bylund H (2021) Comparing exogenous methods to induce plant-resistance against a bark-feeding insect. Front Plant Sci 12:1504. https://doi.org/10.3389/fpls.2021.695867
Cipollini D, Heil M (2010) Costs and benefits of induced resistance to herbivores and pathogens in plants. CABI Rev. https://doi.org/10.1079/PAVSNNR20105005
Cruickshank MG, Bleiker KP, Sturrock RN, Becker E, Leal I (2018) Resistance and tolerance of Douglas-fir seedlings to artificial inoculation with the fungus Ophiostoma pseudotsugae. For Pathol 48(5):e12437. https://doi.org/10.1111/efp.12437
Dalin P, Björkman C (2003) Adult beetle grazing induces willow trichome defence against subsequent larval feeding. Oecologia 134(1):112–118. https://doi.org/10.1007/s00442-002-1093-3
Endara M-J, Coley PD (2011) The resource availability hypothesis revisited: a meta-analysis. Funct Ecol 25:389–398. https://doi.org/10.1111/j.1365-2435.2010.01803.x
Erbilgin N, Krokene P, Christiansen E, Zeneli G, Gershenzon J (2006) Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 148:426–436. https://doi.org/10.1007/s00442-006-0394-3
Fedderwitz F, Nordlander G, Ninkovic V, Björklund N (2016) Effects of jasmonate-induced resistance in conifer plants on the feeding behaviour of a bark-chewing insect, Hylobius abietis. J Pest Sci 89(1):97–105. https://doi.org/10.1007/s10340-015-0684-9
Fineblum WL, Rausher MD (1995) Tradeoff between resistance and tolerance to herbivore damage in a morning glory. Nature 377(6549):517–520. https://doi.org/10.1038/377517a0
Fox J, Weisberg S (2019) An R companion to applied regression. Sage, Thousand Oaks CA
Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89(4):578–586. https://doi.org/10.3732/ajb.89.4.578
Gomez S, Ferrieri RA, Schueller M, Orians CM (2010) Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol 188(3):835–844. https://doi.org/10.1111/j.1469-8137.2010.03414.x
Gould N, Reglinski T, Spiers M, Taylor JT (2008) Physiological trade-offs associated with methyl jasmonate—induced resistance in Pinus radiata. Can J For Res 38(4):677–684. https://doi.org/10.1139/x07-193
Gould N, Reglinski T, Northcott GL, Spiers M, Taylor JT (2009) Physiological and biochemical responses in Pinus radiata seedlings associated with methyl jasmonate-induced resistance to Diplodia pinea. Physiol Mol Plant Pathol 74(2):121–128. https://doi.org/10.1016/j.pmpp.2009.10.002
Hariprasad KV, van Emden HF (2010) Mechanisms of partial plant resistance to diamondback moth (Plutella xylostella) in brassicas. Int J pest Manag 56(1):15–22. https://doi.org/10.1080/09670870902980834
Heijari J, Nerg AM, Kainulainen P, Viiri H, Vuorinen M, Holopainen JK (2005) Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomol Exp Appl 115(1):117–124. https://doi.org/10.1111/j.1570-7458.2005.00263.x
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67(3):283–335. https://doi.org/10.1086/417659
Hudgins JW, Franceschi VR (2004) Methyl Jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol 135(4):2134–2149. https://doi.org/10.1104/pp.103.037929
Ikeuchi M, Iwase A, Rymen B, Lambolez A, Kojima M, Takebayashi Y, Heyman J, Watanabe S, Seo M, De Veylder L, Sakakibara H, Sugimoto K (2017) Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol 175(3):1158–1174. https://doi.org/10.1104/pp.17.01035
Jones DA, Harrington CA, Marshall D (2019) Modeling wound-closure response over time in Douglas-fir trees. For Sci 65(2):156–163. https://doi.org/10.1093/forsci/fxy049
Karlsson Green K, Stenberg JA, Lankinen à (2020) Making sense of Integrated Pest Management (IPM) in the light of evolution. Evol Appl 13(8):1791–1805. https://doi.org/10.1111/eva.13067
Kassambara A (2020) ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.3.0: https://CRAN.R-project.org/package=ggpubr
Klepzig KD, Raffa KF, Smalley EB (1991) Association of an insect-fungal complex with red pine decline in Wisconsin. For Sci 37(4):1119–1139. https://doi.org/10.1093/forestscience/37.4.1119
Koch KG, Chapman K, Louis J, Heng-Moss T, Sarath G (2016) Plant tolerance: a unique approach to control hemipteran pests. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01363
Krokene P, Nagy NE, Solheim H (2008) Methyl jasmonate and oxalic acid treatment of Norway spruce: anatomically based defense responses and increased resistance against fungal infection. Tree Physiol 28(1):29–35. https://doi.org/10.1093/treephys/28.1.29
Leimu R, Koricheva J (2006) A meta-analysis of tradeoffs between plant tolerance and resistance to herbivores: combining the evidence from ecological and agricultural studies. Oikos 112(1):1–9. https://doi.org/10.1111/j.0030-1299.2006.41023.x
Lenth R emmeans: Estimated marginal means, aka least-squares means. R package version 1.4.6. 2020 [cited. Available from https://CRAN.R-project.org/package=emmeans
López-Villamor A, Zas R, Pérez A, Cáceres Y, Nunes da Silva M, Vasconcelos M, Vázquez-González C, Sampedro L, Solla A (2021) Traumatic resin ducts induced by methyl jasmonate in Pinus spp. Trees 35(2):557–567. https://doi.org/10.1007/s00468-020-02057-9
Lundborg L, Fedderwitz F, Björklund N, Nordlander G, Borg-Karlson AK (2016a) Induced defenses change the chemical composition of pine seedlings and influence meal properties of the pine weevil Hylobius abietis. Phytochemistry 130:99–105. https://doi.org/10.1016/j.phytochem.2016.06.002
Lundborg L, Nordlander G, Björklund N, Nordenhem H, Borg-Karlson AK (2016b) Methyl jasmonate-induced monoterpenes in Scots pine and Norway spruce tissues affect pine weevil orientation. J Chem Ecol 42(12):1237–1246. https://doi.org/10.1007/s10886-016-0790-z
Martin D, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129(3):1003–1018. https://doi.org/10.1104/pp.011001
Miller B, Madilao LL, Ralph S, Bohlmann J (2005) Insect-induced conifer defense. White pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol 137(1):369–382. https://doi.org/10.1104/pp.104.050187
Mitchell C, Brennan RM, Graham J, Karley AJ (2016) Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Front Plant Sci 7:1132. https://doi.org/10.3389/fpls.2016.01132
Moreira X, Zas R, Sampedro L (2012) Genetic variation and phenotypic plasticity of nutrient re-allocation and increased fine root production as putative tolerance mechanisms inducible by methyl jasmonate in pine trees. J Ecol 100(3):810–820. https://doi.org/10.1111/j.1365-2745.2011.01938.x
Neely D (1988) Wound closure rates on trees. J Arboric 14(10):250–254
Nilsson U, Luoranen J, Kolström T, Örlander G, Puttonen P (2010) Reforestation with planting in northern Europe. Scand J For Res 25(4):283–294. https://doi.org/10.1080/02827581.2010.498384
Noir S, Bömer M, Takahashi N, Ishida T, Tsui T-L, Balbi V, Shanahan H, Sugimoto K, Devoto A (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161(4):1930–1951. https://doi.org/10.1104/pp.113.214908
Nordlander G, Eidmann HH, Jacobsson U, Nordenhem H, Sjödin K (1986) Orientation of the pine weevil Hylobius abietis to underground sources of host volatiles. Entomol Exp Appl 41(1):91–100. https://doi.org/10.1111/j.1570-7458.1986.tb02177.x
Nordlander G, Nordenhem H, Bylund H (1997) Oviposition patterns of the pine weevil Hylobius abietis. Entomol Exp Appl 85(1):1–9. https://doi.org/10.1046/j.1570-7458.1997.00229.x
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38(1):541–566. https://doi.org/10.1146/annurev.ecolsys.38.091206.095822
Nybakken L, Fløistad IS, Magerøy M, Lomsdal M, Strålberg S, Krokene P, Asplund J (2021) Constitutive and inducible chemical defences in nursery-grown and naturally regenerated Norway spruce (Picea abies) plants. For Ecol Manag 491:119180. https://doi.org/10.1016/j.foreco.2021.119180
Örlander G, Nordlander G, Wallertz K, Nordenhem H (2000) Feeding in the crowns of Scots pine trees by the pine weevil Hylobius abietis. Scand J For Res 15(2):194–201. https://doi.org/10.1080/028275800750015000
Oven P, Torelli N (1994) Wound response of the bark in healthy and declining silver firs (Abies Alba). IAWA J 15(4):407–415. https://doi.org/10.1163/22941932-90001375
Oven P, Torelli N (1999) Response of the cambial zone in conifers to wounding. Phyton Ann Rei Bot Horn 39(3):133–137
Peterson RK, D. AC, Varella, Higley LG (2017) Tolerance: the forgotten child of plant resistance. PeerJ 5:e3934. https://doi.org/10.7717/peerj.3934
Pinheiro J, Bates D, DebRoy S, S. D, and, Team RC (2020) nlme: Linear and nonlinear mixed effects models. R package version 3.1–144: https://CRAN.R-project.org/package=nlme
Prittinen K, Pusenius J, Koivunoro K, Roininen H (2003) Genotypic variation in growth and resistance to insect herbivory in silver birch (Betula pendula) seedlings. Oecologia 137(4):572–577. https://doi.org/10.1007/s00442-003-1384-3
Puentes A, Zhao T, Lundborg L, Björklund N, Borg-Karlson AK (2021) Variation in methyl jasmonate-induced defense among Norway spruce clones and trade-offs in resistance against a fungal and an insect pest. Front Plant Sci 12:962. https://doi.org/10.3389/fpls.2021.678959
R Core team (2020) R: a language and environment for statistical computing. Vienna, Austria. R Foundation for Statistical Computing
Rademacher TT, Basler D, Eckes-Shephard AH, Fonti P, Friend AD, Moine JL, Richardson AD (2019) Using direct phloem transport manipulation to advance understanding of carbon dynamics in forest trees. Front For Global Change 2:11. https://doi.org/10.3389/ffgc.2019.00011
Ravnikar M, Gogala N (1990) Regulation of potato meristem development by jasmonic acid in vitro. J Plant Growth Regul 9(1):233–236. https://doi.org/10.1007/BF02041968
Romero C (2014) Bark: structure and functional ecology. Adv econ Bot 17:5–25
Santolamazza-Carbone S, Sotelo T, Velasco P, Cartea ME (2016) Antibiotic properties of the glucosinolates of Brassica Oleracea Var. Acephala similarly affect generalist and specialist larvae of two Lepidopteran pests. J Pest Sci 89(1):195–206. https://doi.org/10.1007/s10340-015-0658-y
Savatin DV, Gramegna G, Modesti V, Cervone F (2014) Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci 5:470. https://doi.org/10.3389/fpls.2014.00470
Schneuwly-Bollschweiler M, Schneuwly DM (2012) How fast do european conifers overgrow wounds inflicted by rockfall? Tree Physiol 32(8):968–975. https://doi.org/10.1093/treephys/tps059
Shen CS, Bach CE (1997) Genetic variation in resistance and tolerance to insect herbivory in Salix Cordata. Ecol Entomol. https://doi.org/10.1046/j.1365-2311.1997.00078.x
Smith CM (2005) Plant resistance to arthropods: molecular and conventional approaches. Springer Science & Business Media, Netherlands
Stenberg JA, Heil M, Åhman I, Björkman C (2015) Optimizing crops for biocontrol of pests and disease. Trends Plant Sci 20(11):698–712. https://doi.org/10.1016/j.tplants.2015.08.007
Stephens AEA, Westoby M (2015) Effects of insect attack to stems on plant survival, growth, reproduction and photosynthesis. Oikos 124(3):266–273. https://doi.org/10.1111/oik.01809
Stevens MT, Waller DM, Lindroth RL (2007) Resistance and tolerance in Populus tremuloides: genetic variation, costs, and environmental dependency. Evol Ecol 21(6):829–847. https://doi.org/10.1007/s10682-006-9154-4
Stowe KA (1998) Experimental evolution of resistance in Brassica rapa: correlated response of tolerance in lines selected for glucosinolate content. Evolution 52(3):703–712. https://doi.org/10.1111/j.1558-5646.1998.tb03695.x
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14(5):179–185. https://doi.org/10.1016/S0169-5347(98)01576-6
Strauss SY, Watson W, Allen MT (2003) Predictors of male and female tolerance to insect herbivory in Raphanus raphanistrum. Ecology 84(8):2074–2082. https://doi.org/10.1890/02-0267
Świa̧tek A, Lenjou M, Van Bockstaele D, Inzé D, Van Onckelen H (2002) Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol 128(1):201–211. https://doi.org/10.1104/pp.010592
Świątek A, Azmi A, Stals H, Inzé D, Van Onckelen H (2004) Jasmonic acid prevents the accumulation of cyclin B1;1 and CDK-B in synchronized tobacco BY-2 cells. FEBS Lett 572(1):118–122. https://doi.org/10.1016/j.febslet.2004.07.018
Tavankar F, Picchio R, Lo Monaco A, Nikooy M, Venanzi R, Bonyad AE (2019) Wound healing rate in oriental beech trees following logging damage. Drewno: prace naukowe, doniesienia, komunikaty 62. https://doi.org/10.12841/wood.1644-3985.294.07
Tian D, Tooker J, Peiffer M, Chung SH, Felton GW (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum Lycopersicum). Planta 236(4):1053–1066. https://doi.org/10.1007/s00425-012-1651-9
Ueda J, Kato J (1982) Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol Plant 54(3):249–252. https://doi.org/10.1111/j.1399-3054.1982.tb00255.x
Walters DR, Newton AC, Lyon GD (2014) Induced resistance for plant defense: a sustainable approach to crop protection. Wiley, Hoboken
Weidhase RA, Kramell H-M, Lehmann J, Liebisch H-W, Lerbs W, Parthier B (1987) Methyl jasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Sci 51(2):177–186. https://doi.org/10.1016/0168-9452(87)90191-9
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wu X, Ding C, Baerson SR, Lian F, Lin X, Zhang L, Wu C, Hwang S-Y, Zeng R, Song Y (2019) The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L). Plant Cell Environ 42(2):659–672. https://doi.org/10.1111/pce.13451
Yates AD, Michel A (2018) Mechanisms of aphid adaptation to host plant resistance. Curr Opin insect Sci 26:41–49. https://doi.org/10.1016/j.cois.2018.01.003
Zas R, Björklund N, Nordlander G, Cendán C, Hellqvist C, Sampedro L (2014) Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest, Hylobius abietis. For Ecol Manag 313:212–223. https://doi.org/10.1016/j.foreco.2013.11.014
Zas R, Björklund N, Sampedro L, Hellqvist C, Karlsson B, Jansson S, Nordlander G (2017) Genetic variation in resistance of Norway spruce seedlings to damage by the pine weevil Hylobius abietis. Tree Genet Genomes 13(5):1–12. https://doi.org/10.1007/s11295-017-1193-1
Zhang Y, Turner JG (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 3(11):e3699. https://doi.org/10.1371/journal.pone.0003699
Acknowledgements
We thank Harimurti Buntaran and Adam Flöhr for providing guidance on the statistical analyses and software coding. We also thank Merlin Rensing, Claes and Maria Hellqvist, Henrik Nordenhem, and Paul Eisenblätter for field assistance. This study was funded by the research council for sustainable development FORMAS (project 942-2016-37) and by the Swedish Research Council (project 2018-05389).
Funding
Open access funding provided by Swedish University of Agricultural Sciences. This study was funded by the research council for sustainable development FORMAS (project 942-2016-37) to NB and by the Swedish Research Council (project 2018-05389) to AP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by M. Meijón.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Björkman, C., Bylund, H. et al. Healing of bark wounds in Norway spruce seedlings can be negatively affected by treatment with methyl jasmonate. Trees 37, 1369–1384 (2023). https://doi.org/10.1007/s00468-023-02428-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02428-y