Today the Association of American Physicians & Surgeons filed its motion for a preliminary injunction to compel release to the public of hydroxychloroquine by the Food & Drug Administration (FDA) and the Department of Health & Human Services (HHS), in AAPS v. HHS, No. 1:20-cv-00493-RJJ-SJB (W.D. Mich.). Nearly 100 million doses of hydroxychloroquine (HCQ) were donated to these agencies, and yet they have not released virtually any of it to the public.
Millions of Americans fear attending political gatherings, religious services, and even large family get-togethers without the availability of early treatment if they were to contract COVID-19. Why should Americans have to wait until they or a loved one is on a ventilator before they gain access to medication to overcome this virus?
“Why does the government continue to withhold more than 60 million doses of HCQ from the public?” asks Jane Orient, M.D., the Executive Director of AAPS. “This potentially life-saving medication is wasting away in government warehouses while Americans are dying from COVID-19.”
Today AAPS files its motion for a preliminary injunction to compel the government to release HCQ from its stockpile to the public, which could then immediately benefit from it. Reports of an uptick in COVID-19 in Arizona and elsewhere could then be handled without irrational, unjustified limitations on this medication imposed by the FDA.
AAPS agrees with President Trump’s adviser Peter Navarro, Ph.D., who decries the obstruction by officials within the FDA to making this medication available to the public. President Trump himself has successfully taken this medication as a preventative measure, so why can’t ordinary Americans?
“A perfect storm of politics in this presidential election year, along with conflicts of interest at the Defendant federal agencies, has resulted in unjustified obstacles to access to HCQ, an inexpensive medication having a track record of more than 75 years of safety,” AAPS writes in its brief being filed today in federal court.
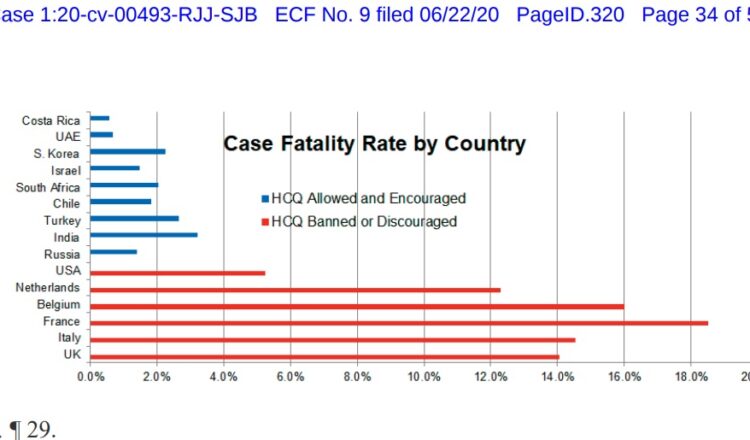
AAPS files with the court a chart showing how countries that encourage HCQ use, such as South Korea, India, Turkey, Russia, and Israel, have been far more successful in combatting COVID-19 than countries that have banned or discouraged early HCQ use, as the FDA has. Last week the FDA even misled the public by falsely stating that HCQ should not be used to treat COVID-19, when multiple studies show its benefits, and thousands of patients have been successfully treated worldwide.
“The interference with public access to hydroxychloroquine is disrupting our political processes,” notes AAPS General Counsel Andrew Schlafly. “Perhaps that is what some want, in order to deter Americans from attending political conventions and even voting, but it is unconstitutional for the FDA to infringe on these constitutional rights by blocking access to this safe medication.”
The Association of American Physicians and Surgeons (AAPS) has represented physicians of all specialties in all states since 1943. The AAPS motto is omnia pro aegroto, meaning everything for the patient.
Documents:
Memorandum in support of motion: https://aapsonline.org/judicial/aaps-v-fda-hcq-6-22-2020.pdf
Exhibit 1: Declaration of Jane M. Orient, M.D.: https://drive.google.com/file/d/1cxEESr-y0ckqRn81uV1zuF_r0A_faItq/view?usp=sharing
Exhibit 2: Declaration of Jeremy Snavely: https://drive.google.com/file/d/1ziyzFbeEPLv8_HnJxDeMkqb21s8j4MTx/view?usp=sharing
Original complaint filed June 2: http://aapsonline.org/judicial/aaps-v-fda-hcq-6-2-2020.pdf
For PDFs of all Motions, Exhibits, and Supporting Documents see: https://drive.google.com/drive/folders/1oUeDIaqxodyaY68ABFoBv1n0xZBF-nLT?usp=sharing
AAPS reply brief filed 7/20/2020: https://aapsonline.org/more-evidence-presented-for-why-hydroxychloroquine-should-be-made-available-in-a-new-court-filing-by-aaps/
AAPS Motion to Expedite 7/30/2020: https://aapsonline.org/judicial/aaps-v-fda-hcq-7-30-2020.pdf