-
PDF
- Split View
-
Views
-
Cite
Cite
Prashant D Tailor, Aoife M Feighery, Bassim El-Sabawi, Abhiram Prasad, Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine, European Heart Journal - Case Reports, Volume 5, Issue 8, August 2021, ytab319, https://doi.org/10.1093/ehjcr/ytab319
Close - Share Icon Share
Abstract
The SARS-CoV-2 pandemic has led to the development of the first mRNA vaccines used in humans. The vaccines are well tolerated, safe, and highly efficacious; however, post-marketing surveillance is revealing potential rare adverse effects. We report a case of symptomatic acute myocarditis following administration of the second dose of mRNA-1273 SARS-CoV-2 vaccine.
A 44-year-old man presented with chest pain and ST-segment elevation 4 days after receiving the second dose of mRNA-1273 SARS-CoV-2 Vaccine. Emergent coronary angiogram showed minimal coronary artery disease. Cardiac magnetic resonance imaging confirmed acute myocarditis. Diagnosis of vaccine-associated myocarditis was made given the temporal relationship and supportive treatment initiated. Follow-up at 1 month confirmed complete symptomatic recovery and echocardiogram demonstrated normalization of cardiac function.
Acute myocarditis should be considered in patients who present with chest pain or dyspnoea within days of receiving mRNA-1273 SARS-CoV-2 vaccination, especially after the second dose. This may be managed successfully with supportive therapies with complete recovery of cardiac function and symptoms. Further research is warranted to determine the mechanisms by which mRNA vaccines may cause myocarditis and for potential long-term cardiovascular injury.
Learning points
To understand the clinical presentation, diagnostic evaluation, and early clinical outcomes of mRNA COVID vaccine-associated acute myocarditis.
To consider mRNA-1273 SARS-CoV-2 vaccination as a cause of acute myocarditis in patients with acute chest pain or heart failure that can be successfully managed with conservative treatment.
Introduction
The BNT162b2 (Pfizer, New York) and mRNA-1273 (Moderna, Cambridge) vaccines are the first two mRNA vaccines approved for human use and have had widespread adoption worldwide with great efficacy and an extremely well-tolerated side effect profile.1–3 As millions of individuals have received the vaccine, there have been reports of vaccine-associated cardiac side effects. Here, we report a case of mRNA-1273 SARS-CoV-2 vaccine associated myocarditis in a previously healthy young male.
Timeline
| Time . | Events . |
|---|---|
| 1 May 2021 | Patient received 2nd dose of mRNA-1273 vaccine |
| 5 May 2021 | Patient presented with chest pain. Electrocardiogram showed ST-segment elevation in the lateral limb and precordial leads. |
| 5 May 2021 | Cardiac catheterization showed mild-non-obstructive coronary artery disease. |
| 5 May 2021 | Transthoracic echocardiogram showed a decreased left ventricular ejection fraction. Treatment initiated with beta-blocker, angiotensin converting enzyme inhibitor, and a short course of diuretics. |
| 6 May 2021 | Cardiac magnetic resonance imaging showed acute myocarditis. Treatment initiated with colchicine. |
| 9 May 2021 | Discharged on colchicine, angiotensin converting enzyme inhibitor and beta-blocker |
| 4 June 2021 | Repeat Transthoracic echocardiogram showing normalization of ejection fraction, and no regional wall motion abnormalities |
| 6 June 2021 | Office follow-up with the patient reporting his symptoms have resolved. |
| Time . | Events . |
|---|---|
| 1 May 2021 | Patient received 2nd dose of mRNA-1273 vaccine |
| 5 May 2021 | Patient presented with chest pain. Electrocardiogram showed ST-segment elevation in the lateral limb and precordial leads. |
| 5 May 2021 | Cardiac catheterization showed mild-non-obstructive coronary artery disease. |
| 5 May 2021 | Transthoracic echocardiogram showed a decreased left ventricular ejection fraction. Treatment initiated with beta-blocker, angiotensin converting enzyme inhibitor, and a short course of diuretics. |
| 6 May 2021 | Cardiac magnetic resonance imaging showed acute myocarditis. Treatment initiated with colchicine. |
| 9 May 2021 | Discharged on colchicine, angiotensin converting enzyme inhibitor and beta-blocker |
| 4 June 2021 | Repeat Transthoracic echocardiogram showing normalization of ejection fraction, and no regional wall motion abnormalities |
| 6 June 2021 | Office follow-up with the patient reporting his symptoms have resolved. |
| Time . | Events . |
|---|---|
| 1 May 2021 | Patient received 2nd dose of mRNA-1273 vaccine |
| 5 May 2021 | Patient presented with chest pain. Electrocardiogram showed ST-segment elevation in the lateral limb and precordial leads. |
| 5 May 2021 | Cardiac catheterization showed mild-non-obstructive coronary artery disease. |
| 5 May 2021 | Transthoracic echocardiogram showed a decreased left ventricular ejection fraction. Treatment initiated with beta-blocker, angiotensin converting enzyme inhibitor, and a short course of diuretics. |
| 6 May 2021 | Cardiac magnetic resonance imaging showed acute myocarditis. Treatment initiated with colchicine. |
| 9 May 2021 | Discharged on colchicine, angiotensin converting enzyme inhibitor and beta-blocker |
| 4 June 2021 | Repeat Transthoracic echocardiogram showing normalization of ejection fraction, and no regional wall motion abnormalities |
| 6 June 2021 | Office follow-up with the patient reporting his symptoms have resolved. |
| Time . | Events . |
|---|---|
| 1 May 2021 | Patient received 2nd dose of mRNA-1273 vaccine |
| 5 May 2021 | Patient presented with chest pain. Electrocardiogram showed ST-segment elevation in the lateral limb and precordial leads. |
| 5 May 2021 | Cardiac catheterization showed mild-non-obstructive coronary artery disease. |
| 5 May 2021 | Transthoracic echocardiogram showed a decreased left ventricular ejection fraction. Treatment initiated with beta-blocker, angiotensin converting enzyme inhibitor, and a short course of diuretics. |
| 6 May 2021 | Cardiac magnetic resonance imaging showed acute myocarditis. Treatment initiated with colchicine. |
| 9 May 2021 | Discharged on colchicine, angiotensin converting enzyme inhibitor and beta-blocker |
| 4 June 2021 | Repeat Transthoracic echocardiogram showing normalization of ejection fraction, and no regional wall motion abnormalities |
| 6 June 2021 | Office follow-up with the patient reporting his symptoms have resolved. |
Case presentation
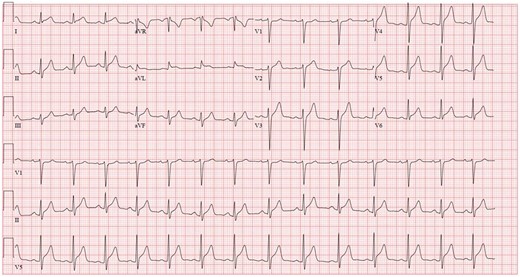
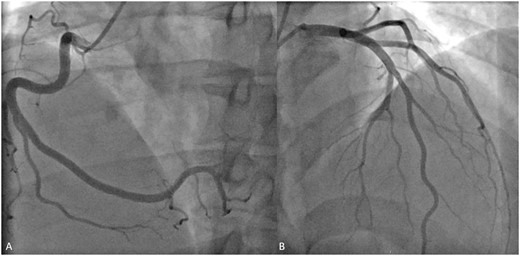
A 44-year-old man with a history of obstructive sleep apnoea, mild asthma, and obesity (BMI 30.9 kg/m2) presented to the hospital with severe chest pain radiating to both arms, associated with acute dyspnoea. The patient was in his usual state of health prior to getting the second dose of mRNA-1273 SARS-CoV-2 Vaccine (Moderna, Cambridge, MA, USA) 4 days earlier. Following vaccine administration, he had experienced malaise, myalgias, headache, and a dry cough. There was no family history of cardiovascular or autoimmune disease, and he was a former smoker. The patient’s home medications were albuterol and fluticasone-salmeterol. In the emergency department, the patient’s electrocardiogram (ECG) showed ST-segment elevation in the lateral limb and precordial leads (Figure 1). Vitals signs in the emergency department were: temperature of 36.9 degrees Celsius, heart rate of 79 beats per minute, blood pressure of 90/72, respiratory rate of 12 breaths per minute, and oxygen saturation of 95%. Physical exam showed minimal lower extremity swelling. The patient was taken for an emergent coronary angiogram which showed very mild coronary artery disease (Figure 2, Videos 1 and 2). Left ventriculogram showed a mildly reduced ejection fraction of 40% with global hypokinesis that was most severe at the apex (Videos 3 and Supplementary material online, Video S1). Left ventricular end-diastolic pressure was markedly elevated at 29 mmHg.
Electrocardiogram at presentation. Initial electrocardiogram in emergency department showing ST-segment elevations in the lateral limb and all precordial leads except V1. These changes were new compared to a prior electrocardiogram done 6 years earlier.
Coronary angiogram. Coronary angiogram of the normal right coronary artery (A) and left coronary artery (B) showing mild focal non-obstructive disease in the mid-left anterior descending artery.
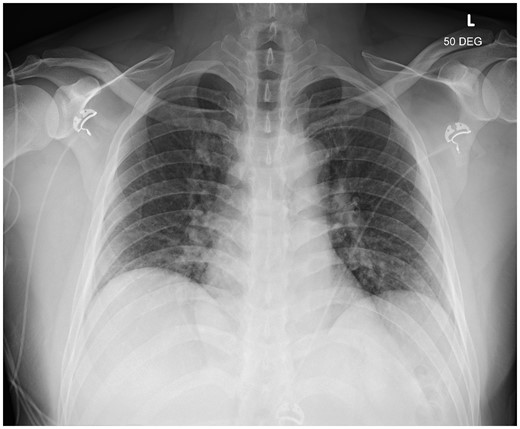
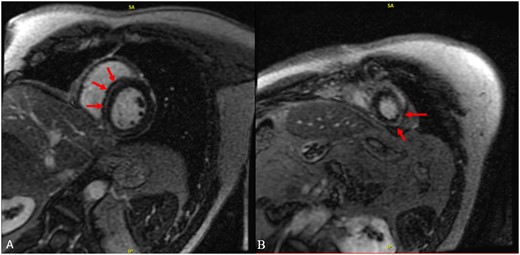
Laboratory blood test confirmed evidence of myocardial injury and systemic inflammation (Table 1). Troponin T levels decreased to 675 ng/L the day after admission. COVID-19 nasal swab testing using polymerase chain reaction was negative. Chest X-ray showed findings consistent with mild pulmonary oedema (Figure 3). Transthoracic Echocardiogram demonstrated borderline enlarged left ventricle with an estimated ejection fraction of 40–45%, mild-moderate generalized left ventricular hypokinesis, slightly worse at the apex, borderline enlarged right ventricle with mildly-moderately decreased systolic function, and no significant valve disease. Cardiac magnetic resonance imaging was compatible with acute myocarditis with patchy linear mid-myocardial enhancement of the septum and inferior walls at the base to mid-ventricle, sub-epicardial/mid-myocardial enhancement of the lateral wall at the mid-ventricle and apical lateral wall (Figure 4). There was associated myocardial oedema involving most of the myocardium and mild pericardial enhancement. Endomyocardial biopsy was not performed as the patient experienced mild symptoms, had no haemodynamic or electrical instability, and responded promptly to initial management.
Chest X-ray. Chest X-ray of the patient showing mild pulmonary oedema.
Cardiac magnetic resonance imaging. Cardiac magnetic resonance imaging showing linear mid-myocardial late gadolinium enhancement of the septum and inferior walls at the base to mid-ventricle (A) and sub-epicardial/mid-myocardial enhancement of the apical lateral wall (B).
Coronary angiography of the right coronary artery. Angiography of the right coronary demonstrating normal vessel with slow flow.
Coronary angiography of the left coronary artery. Angiography of the left coronary demonstrating mild focal non-obstructive disease in the mid-left anterior descending artery with slow flow.
Left ventriculogram Right Anterior Oblique view. Left ventriculogram showing global hypokinesis (apex > base).
Laboratory testing
| Laboratory test . | Patient’s values . | Reference ranges . |
|---|---|---|
| White blood cell count | 9.7 | 3.4–9.6 × 10(9)/L |
| Sedimentation rate | 12 | 2–2 mm/h |
| C-reactive protein | 63.5 | ≤8.0 mg/L |
| Peak troponin T, 5th generation | 847 | ≤15 ng/L |
| NT-proBNP | 1059 | ≤51 pg/mL |
| Eosinophils | 0.04 | 0.03–0.48 × 10(9)/L |
| Laboratory test . | Patient’s values . | Reference ranges . |
|---|---|---|
| White blood cell count | 9.7 | 3.4–9.6 × 10(9)/L |
| Sedimentation rate | 12 | 2–2 mm/h |
| C-reactive protein | 63.5 | ≤8.0 mg/L |
| Peak troponin T, 5th generation | 847 | ≤15 ng/L |
| NT-proBNP | 1059 | ≤51 pg/mL |
| Eosinophils | 0.04 | 0.03–0.48 × 10(9)/L |
Laboratory testing
| Laboratory test . | Patient’s values . | Reference ranges . |
|---|---|---|
| White blood cell count | 9.7 | 3.4–9.6 × 10(9)/L |
| Sedimentation rate | 12 | 2–2 mm/h |
| C-reactive protein | 63.5 | ≤8.0 mg/L |
| Peak troponin T, 5th generation | 847 | ≤15 ng/L |
| NT-proBNP | 1059 | ≤51 pg/mL |
| Eosinophils | 0.04 | 0.03–0.48 × 10(9)/L |
| Laboratory test . | Patient’s values . | Reference ranges . |
|---|---|---|
| White blood cell count | 9.7 | 3.4–9.6 × 10(9)/L |
| Sedimentation rate | 12 | 2–2 mm/h |
| C-reactive protein | 63.5 | ≤8.0 mg/L |
| Peak troponin T, 5th generation | 847 | ≤15 ng/L |
| NT-proBNP | 1059 | ≤51 pg/mL |
| Eosinophils | 0.04 | 0.03–0.48 × 10(9)/L |
The patient was started on supportive therapy for his symptoms, including a brief course of intravenous diuretics for mild symptoms of congestion. Angiotensin-converting enzyme inhibitor and a beta-blocker therapy were commenced to treat systolic dysfunction. He was also initiated on colchicine to treat mild persistent chest pain, which was thought to be related to pericardial inflammation that resolved 4–5 days after presentation. He was discharged home after 5 days of monitoring without evidence of electrical or haemodynamic instability and with NYHA Class I symptoms. One month later, repeat echocardiogram showed normalization of the left ventricular ejection fraction (56%) and size, no regional wall motion abnormalities, and normal longitudinal peak systolic strain. He remained asymptomatic and off diuretics.
Discussion
Vaccine-associated myocarditis is an extremely rare side effect that has been reported in the literature with a variety of vaccines such as tetanus, smallpox, and influenza vaccines.4–7 This, however, has not been reported in the clinical trials of the BNT162b2 and mRNA-1273 vaccines.1,2 It has been postulated that the underlying mechanisms for vaccine-associated myocarditis include a molecular mimicry between viral spike protein or non-specific inflammatory response.5,7 The occurrence after the second vaccine dose raises the possibility of a hypersensitivity response.
Our patient had typical symptoms, biomarkers, ECG profile, and imaging findings of myocarditis. He was diagnosed with vaccine-associated myocarditis because of the strong temporal relationship of systemic inflammatory symptoms and cardiac injury following administration. The patient had no personal or family history of autoimmune disease and did not present with any other symptoms to suggest a rheumatologic disorder. Ischaemic mechanisms were excluded by coronary angiogram. The ECG changes were diffuse and ventricular imaging demonstrating global myocardial dysfunction. Stress (takotsubo) cardiomyopathy was excluded because it is not associated with late gadolinium enhancement on magnetic resonance imaging. Endomyocardial biopsy was deferred as the patient had mild symptoms that improved rapidly, with no haemodynamic or electrical instability, and would have incurred unnecessary procedural risk. Steroids and immunosuppressive medications have no established role in mild cases and are reserved for fulminant myocarditis. Colchicine was utilized as the patient’s predominant symptom was chest pain and there was evidence of pericardial enhancement on magnetic resonance imaging. High-dose NSAID use was considered as a treatment; however, the patient’s hypervolemia and heart failure dissuaded us as this could contribute to an acute worsening.
In the medical literature, there are no reported cases of mRNA-1273 induced myocarditis. There have been three papers detailing myocarditis following administration of BNT162b2 vaccine.8–10 In the journal of Pediatrics, Marshall et al.9 published a multi-institution case series of seven adolescents who developed acute myocarditis following BNT162b2 vaccination. Similar to our patient, all seven patients’ were male, developed symptoms within 4 days of receiving the second dose, and symptoms resolved rapidly.9 There are two adult case reports of acute myocarditis after administration of the 2nd dose of the BNT162b2 vaccine.8,10 Both cases were also in males within four days of vaccination who recovered with supportive care.8,10 Search of the CDC’s Vaccine Adverse Event Reporting System has reported 396 total cases of myocarditis associated with either mRNA SARS-CoV-2 vaccines.11 During its investigation into reports, the CDC has noted there has been a larger than expected number of cases and has posted guidance for doctors to be aware of myocarditis as a possible side effect.12 Similarly, the European Medicines Agency is investigating a possible link between COVID-19 vaccination and myocarditis.13 The American Heart Association/American Stroke Association recommends healthcare professionals be aware of this potential adverse event and should inquire into the timing of any recent COVID vaccination among patients presenting with myocarditis.3 The Drug Safety Research Unit also released a statement identifying 214 cases via the EudraVigilance network.13 It would be unethical to perform a provocative rechallenge to confirm a causal relationship between mRNA-1273 vaccine and acute myocarditis, and there is no other definitive testing that could have been performed in our case to prove causality.
Although expected to be exceedingly rare, this case report, other peer-reviewed papers, and vaccine surveillance networks suggest that the mRNA COVID vaccines may be associated with acute myocarditis that can be successfully managed with supportive care. It is important to note no causal relationship has been proven between mRNA vaccinations and myocarditis and that despite this potentially rare adverse effect the benefits of COVID-19 vaccination greatly exceed the possible risks. Our case demonstrates complete recovery on follow-up in terms of both symptoms and on imaging with conservative treatment. We provide this case report with the aim of highlighting a novel diagnosis for healthcare providers who are likely to it encounter it as the rate of COVID mRNA vaccination rise around the world.
Lead author biography
Prashant Tailor is completing his internal medicine internship at the Mayo Clinic in Rochester, Minnesota. He is interested in machine learning, big data, and personalized medicine.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References





Comments