Abstract
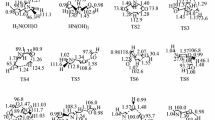
The reaction between the HO radical and (H2O)n (n = 1, 3) clusters has been investigated employing high-level quantum mechanical calculations using DFT-BH&HLYP, QCISD, and CCSD(T) theoretical approaches in connection with the 6-311 + G(2df,2p), aug-cc-pVTZ, and aug-cc-pVQZ basis sets. The rate constants have also been calculated and the tunneling effects have been studied by means of time–dependent wavepacket calculations, performed using the Quantum–Reaction Path Hamiltonian method. According to the findings of previously reported theoretical works, the reaction between HO and H2O begins with the formation of a pre-reactive complex that is formed before the transition state, the formation of a post-reactive complex, and the release of the products. The reaction between HO and (H2O)2 also begins with the formation of a pre-reactive complex, which dissociates into H2O…HO + H2O. The reaction between HO and (H2O)3 is much more complex. The hydroxyl radical adds to the water trimer, and then it occurs a geometrical rearrangement in the pre-reactive hydrogen-bonded complex region, before the transition state. The reaction between hydroxyl radical and water trimer is computed to be much faster than the reaction between hydroxyl radical and a single water molecule, and, in both cases, the tunneling effects are very important mainly at low temperatures. A prediction of the atmospheric concentration of the hydrogen-bonded complexes studied in this work is also reported.






Similar content being viewed by others
Notes
-
The CR3 → CR4 path is very flat and we have failed to find TS5 at BH&HLYP level of theory We have optimized it, and CR3 too, using the B3LYP functional and their corresponding ZPE, entropy and enthalpy corrections have been employed in this case.
References
Wayne RP (2000) Chemistry of atmospheres, 3rd edn. Oxford University Press, Oxford
Wennberg PO, Hanisco TF, Jaeglé L, Jacob DJ, Hintsa EJ, Lanzendorf EJ, Anderson JG, Gao RS, Keim ER, Donnelly SG, Negro LAD, Fahey DW, McKeen SA, Salawitch RJ, Webster CR, May RD, Herman RL, Proffitt MH, Margitan JJ, Atlas EL, Schauffler SM, Flocke F, McElroy CT, Bui TP (1998) Science 279:49–53
Monks PS (2005) Gas-phase radical chemistry in the troposphere. Chem Soc Rev 34:376–395
Jaeglé L, Jacob DJ, Brune WH, Wennberg PO (2001) Atmos Environ 35:469–489
Hofzumahaus A, Rohrer F, Lu KD, Bohn B, Brauers T, Chang CC, Fuchs H, Holland F, Kita K, Kondo Y, Li X, Lou SR, Shao M, Zeng LM, Wahner A, Zhang YH (2009) Science 324(5935):1702–1704. doi:10.1126/science.1164566
Mansergas A, Anglada JM (2007) ChemPhysChem 8:1534–1539
Jacob DJ (2000) Atmos Environ 34(12–14):2131–2159
Chameides WL, Davis DD (1982) J Geophys Res Oceans Atmospheres 87(NC7):4863–4877
Hanson DR, Burkholder JB, Howard CJ, Ravishankara AR (1992) J Phys Chem 96(12):4979–4985
Kregel KC, Zhang HJ (2007) Am J Physiol Regul Integr Comp Physiol 292(1):R18–R36
Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ (2006) Am J Physiol Regul Integr Comp Physiol 291(3):R491–R511
Fridovich I (1998) J Exp Biol 201(Pt 8):1203–1209
Kehrer JP (2000) Toxicology 149(1):43–50
Cooper WJ, Cramer CJ, Martin NH, Mezyk SP, O’Shea KE, von Sonntag C (2009) Chem Rev 109(3):1302–1345. doi:10.1021/cr078024c
Sein MM, Golloch A, Schmidt TC, von Sonntag C (2007) ChemPhysChem 8(14):2065–2067
Lesko TM, Colussi AJ, Hoffmann MR (2004) J Am Chem Soc 126:4432–4436
Sehested K, Corfitzen H, Holcman J, Hart EJ (1998) J Phys Chem A 102:2667–2672
Dubey MK, Mohrschladt R, Donahue NM, Anderson JG (1997) J Phys Chem A 101(8):1494–1500
Masgrau L, Gonzalez-Lafont A, Lluch JM (1999) J Phys Chem A 103(8):1044–1053
Masgrau L, Gonzalez-Lafont A, Lluch JM (1999) J Comput Chem 20(16):1685–1692
Uchimaru T, Chandra AK, Tsuzuki S, Sugie M, Sekiya A (2003) J Comput Chem 24:1538–1548
Hand MR, Rodriquez CF, Williams IH, Balint-Kurti GG (1998) J Phys Chem A 102(29):5958–5966
Basch H, Hoz S (1997) J Phys Chem A 101(24):4416–4431
Deyerl HJ, Luong AK, Clements TG, Continetti RE (2000) Faraday Discuss 115:147–160
Olivella S, Anglada JM, Sole A, Bofill JM (2004) Chem Eur J 10:3404–3410. doi:10.1002/chem.200305714
Vohringer-Martinez E, Hansmann B, Hernandez H, Francisco JS, Troe J, Abel B (2007) Science 315(5811):497–501
Anglada JM, Gonzalez J (2009) ChemPhysChem 10(17):3034–3045. doi:10.1002/cphc.200900387
Luo Y, Maeda S, Ohno K (2009) Chem Phys Lett 469:57–61
Aloisio S, Francisco JS, Friedl RR (2000) J Phys Chem A 104:6597–6601
Zhu RS, Lin MC (2002) Chem Phys Lett 354(3–4):217–226
Anglada JM, Aplincourt P, Bofill JM, Cremer D (2002) ChemPhysChem 2:215–221
Crehuet R, Anglada JM, Bofill JM (2001) Chem Eur J 7(10):2227–2235
Neeb P, Sauer F, Horie O, Moortgat GK (1997) Atmos Environ 31(10):1417–1423
Gonzalez J, Torrent-Sucarrat M, Anglada JM (2010) PhysChemChemPhys 12(9):2116–2125. doi:10.1039/b916659a
Bahnson BJ, Colby TD, Chin JK, Goldstein BM, Klinman JP (1997) Proc Natl Acad Sci USA 94(24):12797–12802
Giese K, Petkovic M, Naundorf H, Kuhn O (2006) Phys Rep Rev Sect Phys Lett 430(4):211–276. doi:10.1016/j.physrep.2006.04.005
Guallar V, Gherman BF, Miller WH, Lippard SJ, Friesner RA (2002) J Am Chem Soc 124(13):3377–3384. doi:10.1021/ja0167248
Makri N (1999) Annual Rev Phys Chem 50:167–191
Pfeilsticker K, Lotter A, Peters C, Bosch H (2003) Science 300(5628):2078–2080
Dunn ME, Pokon EK, Shields GC (2004) J Am Chem Soc 126:2647–2653
Goldman N, Fellers RS, Leforestier C, Saykally RJ (2001) J Phys Chem A 105(3):515–519
Becke AD (1993) J Chem Phys 98:1372
Frisch MJ, Pople JA, Binkley JS (1984) J Chem Phys 80:3265–3269
Hehre WJ, Radom L, Schleyer PvR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York, pp 86–87
Ishida K, Morokuma K, Kormornicki A (1977) J Chem Phys 66:2153
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Fukui K (1970) J Phys Chem 74(23):4161
Cizek J (1969) Adv Chem Phys 14:35
Barlett RJ (1989) J Phys Chem 93:1963
Pople JA, Krishnan R, Schlegel HB, Binkley JS (1978) Int J Quant Chem XIV:545–560
Pople JA, Head-Gordon M, Raghavachari K (1989) J Chem Phys 90(8):4635–4636
Dunning THJ (1989) J Chem Phys 90:1007
Kendall RA, Dunning TH, Harrison RJ (1992) J Chem Phys 96(9):6796–6806
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968
Roos BO (1987) Adv Chem Phys 69:399
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JA Jr, Montgomery J, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ocherski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.01 edn. Gaussian, Inc., Wallingford, CT
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) Gamess 2004. J Comput Chem 14:1347–1363
Gonzalez J, Gimenez X, Bofill JM (2009) J Chem Phys 131(5):054108. doi:10.1063/1.3194135
Gonzalez J, Gimenez X, Bofill JM (2001) J Phys Chem A 105(20):5022–5029. doi:10.1021/jp003793k
Gonzalez J, Gimenez X, Bofill JM (2007) J Comput Chem 28 (13):2111–2121. doi:10.1002/jcc.20729
Gonzalez J, Gimenez X, Bofill JM (2007) J Comput Chem 28(13):2102–2110. doi:10.1002/jcc.20728
Gonzalez J, Gimenez X, Bofill JM (2004) Theor Chem Acc 112(2):75–83. doi:10.1007/s00214-004-0571-6
Askar A, Cakmak AS (1978) J Chem Phys 68(6):2794–2798
Klopper W, de Rijdt J, van Duijneveldt FB (2000) PhysChemChemPhys 2(10):2227–2234
Zhou Z, Qu Y, Fu A, Du B, He F, Gao H (2002) Int J Quant Chem 89:550–558
Cooper PD, Kjaergaard HG, Langford VS, McKinley AJ, Quickenden TI, Schofield DP (2003) J Am Chem Soc 125(20):6048–6049
Engdahl A, Karlstrom G, Nelander B (2003) J Chem Phys 118(17):7797–7802
Ohshima Y, Sato K, Sumiyoshi Y, Endo Y (2005) J Am Chem Soc 127(4):1108–1109
Allodi MA, Dunn ME, Livada J, Kirschner KN, Shields GC (2006) J Phys Chem A 110(49):13283–13289
Soloveichik P, O’Donnell BA, Lester MI, Francisco JS, McCoy AB (2010) J Phys Chem A 114(3):1529–1538. doi:10.1021/jp907885d
Quapp W, Hirsch M, Heidrich D (1998) Theor Chem Acc 100(5–6):285–299
Ess DH, Wheeler SE, Iafe RG, Xu L, Celebi-Olcum N, Houk KN (2008) Angew Chem Int Ed Engl 47(40):7592–7601. doi:10.1002/anie.200800918
Valtazanos P, Ruedenberg K (1986) Theor Chim Acta 69(4):281–307
Tsuji K, Shibuya K (2009) J Phys Chem A 113(37):9945–9951. doi:10.1021/jp903648z
Anglada JM (2004) J Am Chem Soc 126(31):9809–9820
Miller WH (1976) J Chem Phys 65:2216–2223
Acknowledgments
This research has been supported by the Generalitat de Catalunya (Grant 2009SGR01472) and the Spanish Dirección General de Investigación Científica y Técnica (DGYCIT, grants CTQ2008-06536/BQU and CTQ2008-02856/BQU). The calculations described in this work were carried out at the Centre de Supercomputació de Catalunya (CESCA), at the Computational Center of CTI–CSIC, and at the cluster of workstations of our group. Antoni Aguilar-Mogas and Marc Caballero gratefully thank to Ministerio de Ciencia e Innovación for a predoctoral fellowship. Javier González and Miquel Torrent-Sucarrat acknowledge CSIC for a JAE-DOC contract.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published as part of the special issue celebrating theoretical and computational chemistry in Spain.
Rights and permissions
About this article
Cite this article
Gonzalez, J., Caballero, M., Aguilar-Mogas, A. et al. The reaction between HO and (H2O) n (n = 1, 3) clusters: reaction mechanisms and tunneling effects. Theor Chem Acc 128, 579–592 (2011). https://doi.org/10.1007/s00214-010-0824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-010-0824-5