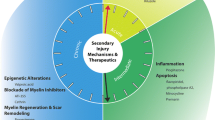
Abstract
Pre-clinical studies place tumor necrosis factor (TNF) as a central player in the inflammatory response after spinal cord injury (SCI), and blocking its production and/or activity has been proposed as a possible treatment option after SCI. This systematic review provides an overview of the literature on the temporal and cellular expression of TNF after SCI and clarifies the potential for its therapeutic manipulation in SCI. A systematic search was performed in EMBASE (Ovid), MEDLINE (Ovid), and Web of Science (Core Collection). The search terms were the MeSH forms of tumor necrosis factor and spinal cord injury in the different databases, and the last search was performed on February 3, 2021. We found twenty-four articles examining the expression of TNF, with most using a thoracic contusive SCI model in rodents. Two articles described the expression of TNF receptors in the acute phase after SCI. Twenty-one articles described the manipulation of TNF signaling using genetic knock-out, pharmaceutical inhibition, or gain-of-function approaches. Overall, TNF expression increased rapidly after SCI, within the first hours, in resident cells (neurons, astrocytes, oligodendrocytes, and microglia) and again in macrophages in the chronic phase after injury. The review underscores the complexity of TNF’s role after SCI and indicates that TNF inhibition is a promising therapeutic option. This review concludes that TNF plays a significant role in the inflammatory response after SCI and suggests that targeting TNF signaling is a feasible therapeutic approach.




Image is created with BioRender.com


Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- AIS:
-
American spinal injury association (ASIA) impairment scale
- Arg 1:
-
Arginase-1
- CD:
-
Cluster of differentiation
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CXCL1:
-
C-X-C motif chemokine ligand 1
- GFAP:
-
Glial fibrillary acidic protein
- i.m.:
-
Intramuscular
- i.p.:
-
Intraperitoneal
- Iba1:
-
Ionized calcium-binding adaptor molecule 1
- IL:
-
Interleukin
- KO:
-
Knock-out
- MAP2:
-
Microtubule-associated protein 2
- MDA:
-
Malondialdehyde
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- qPCR:
-
Quantitative polymerase chain reaction
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- s.c.:
-
Subcutaneous
- SCI:
-
Spinal cord injury
- solTNF:
-
Soluble tumor necrosis factor
- TACE:
-
Tumor necrosis factor-alpha converting enzyme
- tmTNF:
-
Transmembrane tumor necrosis factor
- TNF:
-
Tumor necrosis factor
- TNFR:
-
Tumor necrosis factor receptor
References
Alexander JK, Popovich PG (2009) Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Neurotherapy 175:125–137
Assas MB, Levison SE, Little M, England H, Battrick L, Bagnall J et al (2017) Anti-inflammatory effects of infliximab in mice are independent of tumour necrosis factor alpha neutralization. Clin Exp Immunol 187(2):225–233
Atretkhany KN, Gogoleva VS, Drutskaya MS, Nedospasov SA (2020) Distinct modes of TNF signaling through its two receptors in health and disease. J Leukoc Biol 107(6):893–905
Bartholdi D, Schwab ME (1997) Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci 9(7):1422–1438
Bastien D, Lacroix S (2014) Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol 258:62–77
Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H (2008) Cognitive dysfunction in mice deficient for TNF- and its receptors. Am J Med Genet B 147B(7):1056–1064
Bayrakli F, Balaban H, Ozum U, Duger C, Topaktas S, Kars HZ (2012) Etanercept treatment enhances clinical and neuroelectrophysiological recovery in partial spinal cord injury. Eur Spine J 21(12):2588–2593
Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M et al (2002) Control of synaptic strength by glial TNFalpha. Science 295(5563):2282–2285
Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR et al (2005) Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci 25(29):6734–6744
Biglari B, Swing T, Child C, Buchler A, Westhauser F, Bruckner T et al (2015) A pilot study on temporal changes in IL-1beta and TNF-alpha serum levels after spinal cord injury: the serum level of TNF-alpha in acute SCI patients as a possible marker for neurological remission. Spinal Cord 53(7):510–514
Borcek AO, Civi S, Ocal O, Gulbahar O (2015) Effects of tumor necrosis factor alpha blocker adalimumab in experimental spinal cord injury. J Korean Neurosurg Soc 57(2):73–76
Celik H, Karatay M, Erdem Y, Yildirim AE, Sertbas I, Karatay E et al (2016) The biochemical, histopathological and clinical comparison of the neuroprotective effects of subcutaneous adalimumab and intravenous methylprednisolone in an experimental compressive spinal cord trauma model. Turk Neurosurg 26(4):622–631
Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Watanabe S et al (2011) Tumor necrosis factor-alpha antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine 36(17):1350–1358
Chi LY, Yu J, Zhu H, Li XG, Zhu SG, Kindy MS (2008) The dual role of tumor necrosis factor-alpha in the pathophysiology of spinal cord injury. Neurosci Lett 438(2):174–179
Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B et al (2019) Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis 10(2):367–382
Clausen BH, Degn M, Sivasaravanaparan M, Fogtmann T, Andersen MG, Trojanowsky MD et al (2016) Conditional ablation of myeloid TNF increases lesion volume after experimental stroke in mice, possibly via altered ERK1/2 signaling. Sci Rep 6:29291
Clausen BH, Wirenfeldt M, Hogedal SS, Frich LH, Nielsen HH, Schroder HD et al (2020) Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol Commun 8(1):81
Davies AL, Hayes KC, Dekaban GA (2007) Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 88(11):1384–1393
Dinomais M, Stana L, Egon G, Richard I, Menei P (2009) Significant recovery of motor function in a patient with complete T7 paraplegia receiving etanercept. J Rehabil Med 41(4):286–288
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209(2):378–388
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB et al (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264
Ellman DG, Degn M, Lund MC, Clausen BH, Novrup HG, Flaeng SB et al (2016) Genetic ablation of soluble TNF does not affect lesion size and functional recovery after moderate spinal cord injury in mice. Mediators Inflamm 2016:2684098
Ellman DG, Novrup HG, Jorgensen LH, Lund MC, Yli-Karjanmaa M, Madsen PM et al (2017) Neuronal ablation of IKK2 decreases lesion size and improves functional outcome after spinal cord injury in mice. JSM Neurosurg. 5(3):1090
Ellman DG, Lund MC, Nissen M, Nielsen PS, Sorensen C, Lester EB et al (2020) Conditional ablation of myeloid TNF improves functional outcome and decreases lesion size after spinal cord injury in mice. Cells 9(11):1–23
Esposito E, Cuzzocrea S (2009) TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem 16(24):3152–3167
Esposito E, Cuzzocrea S (2011) Anti-TNF therapy in the injured spinal cord. Trends Pharmacol Sci 32(2):107–115
Eugster HP, Frei K, Bachmann R, Bluethmann H, Lassmann H, Fontana A (1999) Severity of symptoms and demyelination in MOG-induced EAE depends on TNFR1. Eur J Immunol 29(2):626–632
Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM et al (2014) High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol 254:109–120
Farooque M, Isaksson J, Olsson Y (2001) Improved recovery after spinal cord injury in neuronal nitric oxide synthase-deficient mice but not in TNF-alpha-deficient mice. J Neurotrauma 18(1):105–114
Farrell K, Houle JD (2019) Systemic inhibition of soluble tumor necrosis factor with XPro1595 exacerbates a post-spinal cord injury depressive phenotype in female rats. J Neurotrauma 36(21):2964–2976
Fillit H, Ding WH, Buee L, Kalman J, Altstiel L, Lawlor B et al (1991) Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci Lett 129(2):318–320
Fischer R, Kontermann RE, Pfizenmaier K (2020) Selective targeting of TNF receptors as a novel therapeutic approach. Front Cell Dev Biol 8:401
Fraidakis MJ, Kiyotani T, Pernold K, Bergstrom J, Olson L (2007) Recovery from spinal cord injury in tumor necrosis factor-alpha, signal transducers and activators of transcription 4 and signal transducers and activators of transcription 6 null mice. NeuroReport 18(2):185–189
Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG et al (2017) Opposing functions of microglial and macrophagic TNFR2 in the pathogenesis of experimental autoimmune encephalomyelitis. Cell Rep 18(1):198–212
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Bramanti P et al (2006) Immunomodulatory effects of etanercept in an experimental model of spinal cord injury. J Pharmacol Exp Ther 316(3):1006–1016
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E et al (2008) TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock 29(1):32–41
Gong LL, Lv YH, Li SL, Feng T, Zhou Y, Sun YY et al (2020) Changes in transcriptome profiling during the acute/subacute phases of contusional spinal cord injury in rats. Ann Trans Med 8(24):15
Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B et al (1995) The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83(5):793–802
Grell M, Wajant H, Zimmermann G, Scheurich P (1998) The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA 95(2):570–575
Harrington JF, Messier AA, Levine A, Szmydynger-Chodobska J, Chodobski A (2005) Shedding of tumor necrosis factor type 1 receptor after experimental spinal cord injury. J Neurotrauma 22(8):919–928
Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K et al (2002) Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma 19(6):753–761
Hermann GE, Rogers RC, Bresnahan JC, Beattie MS (2001) Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis 8(4):590–599
Huie JR, Ferguson AR, Kyritsis N, Pan JZ, Irvine KA, Nielson JL et al (2021) Machine intelligence identifies soluble TNFa as a therapeutic target for spinal cord injury. Sci Rep 11(1):3442
Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z et al (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 26(38):9703–9712
Jones TB, McDaniel EE, Popovich PG (2005) Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des 11(10):1223–1236
Kigerl KA, McGaughy VM, Popovich PG (2006) Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol 494(4):578–594
Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G et al (2001) Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci 21(17):6617–6625
Klusman I, Schwab ME (1997) Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain Res 762(1–2):173–184
Kong X, Gao J (2017) Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med 21(5):941–954
Kregel KC, Zhang HJ (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol 292(1):R18-36
Kroner A, Greenhalgh AD, Zarruk JG, PassosdosSantos R, Gaestel M, David S (2014) TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83(5):1098–1116
Kumamaru H, Saiwai H, Ohkawa Y, Yamada H, Iwamoto Y, Okada S (2012) Age-related differences in cellular and molecular profiles of inflammatory responses after spinal cord injury. J Cell Physiol 227(4):1335–1346
Kwon BK, Stammers AMT, Belanger LM, Bernardo A, Chan D, Bishop CM et al (2010) Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J Neurotrauma 27(4):669–682
Kwon BK, Bloom O, Wanner IB, Curt A, Schwab JM, Fawcett J et al (2019) Neurochemical biomarkers in spinal cord injury. Spinal Cord 57(10):819–831
Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH et al (2009) Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci 29(5):1319–1330
Lambertsen KL, Finsen B, Clausen BH (2019) Post-stroke inflammation-target or tool for therapy? Acta Neuropathol 137(5):693–714
Lee YL, Shih K, Bao P, Ghirnikar RS, Eng LF (2000a) Cytokine chemokine expression in contused rat spinal cord. Neurochem Int 36(4–5):417–425
Lee YB, Yune TY, Baik SY, Shin YH, Du S, Rhim H et al (2000b) Role of tumor necrosis factor-alpha in neuronal and glial apoptosis after spinal cord injury. Exp Neurol 166(1):190–195
Li Y, Cao T, Ritzel RM, He J, Faden AI, Wu J (2020) Dementia, depression, and associated brain inflammatory mechanisms after spinal cord injury. Cells 9(6):1420
Lin S, Zhou Z, Zhao H, Xu C, Guo Y, Gao S et al (2021) TNF promotes M1 polarization through mitochondrial metabolism in injured spinal cord. Free Radic Biol Med 172:622–632
Madsen PM, Motti D, Karmally S, Szymkowski DE, Lambertsen KL, Bethea JR et al (2016) Oligodendroglial TNFR2 mediates membrane TNF-dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J Neurosci 36(18):5128–5143
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm 5:45
Mironets E, Osei-Owusu P, Bracchi-Ricard V, Fischer R, Owens EA, Ricard J et al (2018) Soluble TNF alpha signaling within the spinal cord contributes to the development of autonomic dysreflexia and ensuing vascular and immune dysfunction after spinal cord injury. J Neurosci 38(17):4146–4162
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Moore SA, Oglesbee MJ (2012) Involvement of the choroid plexus in the inflammatory response after acute spinal cord injury in dogs: an immunohistochemical study. Vet Immunol Immunopathol 148(3–4):348–352
Mukhamedshina YO, Akhmetzyanova ER, Martynova EV, Khaiboullina SF, Galieva LR, Rizvanov AA (2017) Systemic and local cytokine profile following spinal cord injury in rats: a multiplex analysis. Front Neurol 8:581
Naruo S, Okajima K, Taoka Y, Uchiba M, Nakamura T, Okabe H et al (2003) Prostaglandin E1 reduces compression trauma-induced spinal cord injury in rats mainly by inhibiting neutrophil activation. J Neurotrauma 20(2):221–228
Novrup HG, Bracchi-Ricard V, Ellman DG, Ricard J, Jain A, Runko E et al (2014) Central but not systemic administration of XPro1595 is therapeutic following moderate spinal cord injury in mice. J Neuroinflamm 11:14
Ogurcov S, Shulman I, Garanina E, Sabirov D, Baichurina I, Kuznetcov M et al (2021) Blood serum cytokines in patients with subacute spinal cord injury: a pilot study to search for biomarkers of injury severity. Brain Sci 11(3):1–12
Olmos G, Llado J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014:861231
O’Reilly ML, Mironets E, Shapiro TM, Crowther K, Collyer E, Bethea JR et al (2021) Pharmacological inhibition of soluble tumor necrosis factor-alpha two weeks after high thoracic spinal cord injury does not affect sympathetic hyperreflexia. J Neurotrauma 01:01
Oshima T, Lee S, Sato A, Oda S, Hirasawa H, Yamashita T (2009) TNF-alpha contributes to axonal sprouting and functional recovery following traumatic brain injury. Brain Res 1290:102–110
Pan JZ, Ni L, Sodhi A, Aguanno A, Young W, Hart RP (2002) Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J Neurosci Res 68(3):315–322
Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M (2006) Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol 59(5):843–851
Pineau I, Lacroix S (2007) Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 500(2):267–285
Popovich PG, Wei P, Stokes BT (1997) Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol 377(3):443–464
Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT (1999) Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158(2):351–365
Probert L (2015) TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302:2–22
Scivoletto G, Morganti B, Ditunno P, Ditunno JF, Molinari M (2003) Effects on age on spinal cord lesion patients’ rehabilitation. Spinal Cord 41(8):457–464
Sharief MK, Hentges R (1991) Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med 325(7):467–472
Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM et al (2017) Animal models of spinal cord injury: a systematic review. Spinal Cord 55(8):714–721
Shi LL, Zhang N, Xie XM, Chen YJ, Wang R, Shen L et al (2017) Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genomics 18:14
Shi ZJ, Ning GZ, Zhang B, Yuan SY, Zhou HX, Pan B et al (2019) Signatures of altered long noncoding RNAs and messenger RNAs expression in the early acute phase of spinal cord injury. J Cell Physiol 234(6):8918–8927
Stammers AT, Liu J, Kwon BK (2012) Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J Neurosci Res 90(4):782–790
Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440(7087):1054–1059
Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT (1998) Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 152(1):74–87
Sweis R, Biller J (2017) Systemic complications of spinal cord injury. Curr Neurol Neurosci Rep 17(2):8
Taoka Y, Okajima K, Murakami K, Johno M, Naruo M (1998) Role of neutrophil elastase in compression-induced spinal cord injury in rats. Brain Res 799(2):264–269
Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA Jr, Goeddel DV (1991) The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA 88(20):9292–9296
Tsarouchas TM, Wehner D, Cavone L, Munir T, Keatinge M, Lambertus M et al (2018) Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nature Commun 9(1):4670
Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL (2002) Treatment of spinal cord impact injury in the rat with transforming growth factor-beta. J Neurol Sci 200(1–2):33–41
van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J (2010) Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 34(3):184–192 (Discussion 92)
Vidal PM, Lemmens E, Geboes L, Vangansewinkel T, Nelissen S, Hendrix S (2013) Late blocking of peripheral TNF-alpha is ineffective after spinal cord injury in mice. Immunobiology 218(2):281–284
von Leden RE, Khayrullina G, Moritz KE, Byrnes KR (2017) Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury. J Neuroinflamm 14:14
Wang CX, Nuttin B, Heremans H, Dom R, Gybels J (1996) Production of tumor necrosis factor in spinal cord following traumatic injury in rats. J Neuroimmunol 69(1–2):151–156
Wang CX, Reece C, Wrathall JR, Shuaib A, Olschowka JA, Hao CH (2002) Expression of tumor necrosis factor alpha and its mRNA in the spinal cord following a weight-drop injury. NeuroReport 13(11):1391–1393
Xu J, Xiaoqiang E, Liu H, Li F, Cao Y, Tian J, Yan J (2015) Tumor necrosis factor-alpha is a potential diagnostic biomarker for chronic neuropathic pain after spinal cord injury. Neurosci Lett 595:30–34
Yakovlev AG, Faden AI (1994) Sequential expression of c-fos protooncogene, TNF-alpha, and dynorphin genes in spinal cord following experimental traumatic injury. Mol Chem Neuropathol 23(2–3):179–190
Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM (2001) Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma 18(5):563–568
Yan P, Liu NK, Kim GM, Xu JM, Xu J, Li Q et al (2003) Expression of the type 1 and type 2 receptors for tumor necrosis factor after traumatic spinal cord injury in adult rats. Exp Neurol 183(2):286–297
Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN (2004) Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine 29(9):966–971
Yang LQ, Jones NR, Blumbergs PC, Van Den Heuvel C, Moore EJ, Manavis J et al (2005) Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci 12(3):276–284
Yli-Karjanmaa M, Larsen KS, Fenger CD, Kristensen LK, Martin NA, Jensen PT et al (2019) TNF deficiency causes alterations in the spatial organization of neurogenic zones and alters the number of microglia and neurons in the cerebral cortex. Brain Behav Immun 82:279–297
Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H et al (2003) Increased production of tumor necrosis factor-alpha induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma 20(2):207–219
Zhang X, Shi LL, Gao X, Jiang D, Zhong ZQ, Zeng X et al (2015) Lentivirus-mediated inhibition of tumour necrosis factor-alpha improves motor function associated with PRDX6 in spinal cord contusion rats. Sci Rep 5:7
Zhou X, He XJ, Ren Y (2014) Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res 9(20):1787–1795
Zhou HX, Shi ZJ, Kang Y, Wang Y, Lu L, Pan B et al (2018) Investigation of candidate long noncoding RNAs and messenger RNAs in the immediate phase of spinal cord injury based on gene expression profiles. Gene 661:119–125
Acknowledgements
Silas Arlt Tvingsholm is acknowledged for help with graphical designs and Ulla Damgaard Munk is acknowledged for the technical assistance. Claire Gudex is acknowledged for proofreading.
Funding
The funding source for this work was from the Lundbeck Foundation (Grant No. R230-2016-3019); Faculty of Health Science, SDU, Denmark; Desirée and Niels Yde’s Foundation, Denmark; Fonden for Lægevidenskabens Fremme; Overlægerådets legatudvalg, Odense University Hospital, Denmark; Auguststinus Fonden, Denmark; Kong Christian den Tiendes Fond, Denmark.
Author information
Authors and Affiliations
Contributions
MCL and KLL performed the search and analyzed and interpreted the data. RB contributed with human postmortem spinal cord tissue sections. MCL, KLL, and BHC wrote the manuscript and all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Paraffin-embedded postmortem human spinal cord samples are obtained from The Miami Project Human Core Bank at the University of Miami Miller School of Medicine managed by Alexander Marcillo, MD, and Yan Shi, MSc.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lund, M.C., Clausen, B.H., Brambilla, R. et al. The Role of Tumor Necrosis Factor Following Spinal Cord Injury: A Systematic Review. Cell Mol Neurobiol 43, 925–950 (2023). https://doi.org/10.1007/s10571-022-01229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01229-0