Abstract
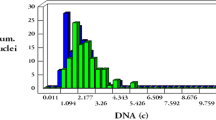
Environmental and occupational exposure to aluminum along with ionizing radiation results in serious health problems. This study was planned to investigate the impact of oxidative stress provoked by exposure to ionizing radiation with aluminum administration upon cellular ultra structure and apoptotic changes in Paneth cells of rat small intestine . Animals received daily aluminum chloride by gastric gavage at a dose 0.5 mg/Kg BW for 4 weeks. Whole body gamma irradiation was applied at a dose 2 Gy/week up to 8 Gy. Ileum malondialdehyde, advanced oxidation protein products, and protein carbonyl were assessed as biomarkers of lipid peroxidation along with superoxide dismutase, catalase, and glutathione peroxidase activities as enzyme antioxidants. Moreover, analyses of cell cycle division and apoptotic changes were evaluated by flow cytometry. Intestinal cellular ultra structure was investigated using transmission electron microscope. Oxidative stress assessment in the ileum of rats revealed that aluminum and ionizing radiation exposure either alone or in combination exhibits a significant effect upon the increase in biomarkers of lipid peroxidation along with tumor necrosis factor-α with concomitant significant decrease of the antioxidant enzyme activities. Flow cytometric analyses showed significant alterations in the percentage of cells during cell cycle division phases along with significant increase in apoptotic cells. Ultra structurally, intestinal cellular alterations with marked injury in Paneth cells at the sites of bacterial translocation in the crypt of lumens were recorded. The results of this study have clearly suggested that aluminum exposure and ionizing either alone or in combination induced apoptosis and oxidative stress in the Paneth cells of rat intestine, which appeared to play a major role in the pathogenesis of cellular damage. Furthermore, the interaction of these two intestinal toxic routes was found to be synergistic.



Similar content being viewed by others
References
Abdel-Wahab WM (2012) AlCl3-induced toxicity and oxidative stress in liver of male rats: protection by melatonin. Life Science Journal 9(4):1173–1183
Aebi H (1984) Catalase in vitro. Methods of Enzymology 105:121–126
Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekant W et al (2008) Safety of aluminium from dietary intake. Scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). The EFSA Journal 754:1–34
Alfrey AC (1986) Aluminum metabolism. Kidney Int 29:8–11
Antebi U, Mathor MB, da Silva AF, Guimarães RP, Honda EK (2016) Effects of ionizing radiation on proteins in lyophilized or frozen demineralized human bone. Revista Brasileira de Ortopedia (English Edition) 51(2):224–230
Aw TY (2005) Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility. Toxicol Appl Pharmacol 204:320–328
Ayabe T, Satchell DP, Pesendorfer P, Tanabe H, Wilson CL, Hagen SJ, Ouellette AJ (2002) Activation of paneth cell α-defensins in mouse small intestine. J Biol Chem 277:5219
Baregamian N, Jun Song JC, Eric Bailey E, Papaconstantinou J, Evers M, Chung DH (2009) Tumor necrosis factor-α and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxidative Medicine and Cell Longevity 2:297–306
Barral M, Dohan A, Allez M, Boudiaf M, Camus M, Laurent V, Hoeffel C, Soyer P (2016) Gastrointestinal cancers in inflammatory bowel disease: an update with emphasis on imaging findings. Crit Rev Oncol Hematol 97:30–46
Bruge F, Tiano L, Cacciamani T, Principi F, Littaru GP (2003) Effect of UV-C mediated oxidative stress in leukemia cell lines and its relation to ubiquinone content. Biofactors 18:15–63
Buraimoh AA (2012) Effects of aluminum chloride exposure on the histology of the small intestine of Wistar rats. International Journal of Applied Science and Technology 2:123–132
Claisse PA (2016) Ionising radiation. Civil Engineering Materials:101–106
Clevers HC, Bevins CL (2013) Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75:289–311
Coronel-León J, López A, Espuny MJ, Beltran MT, Molinos-Gómez A, Rocabayera X et al (2016) Assessment of antimicrobial activity of Nα -lauroyl arginate ethylester (LAE®) against Yersinia enterocolitica and Lactobacillus plantarum by flow cytometry and transmission electron microscopy. Food Control 63:1–10
Davenport A, Roberts NB (1986) Serum aluminum levels in acute renal failure. Lancet 2:1397–1398
Dean PN, Jett JH (1974) Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol 60:523–527
Einor D, Bonisoli-Alquati A, Costantini D, Mousseau TA, Møller AP (2016) Ionizing radiation, antioxidant response and oxidative damage: a meta-analysis. Sci Total Environ 548–549:463–471
Eltahawy NA (2009) Curcumin attenuates gamma radiation induced intestinal damage in rats. Egyptian Journal of Radiation Sciences and Applications 22(2):365–391
Frey B, Hehlgans S, Rödel F, Gaipl US (2015) Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malign diseases. Cancer Lett 368(2):230–237
Glauert AM, Lewis PR (1998) Biological specimen preparation for transmission electron microscopy. Princeton University Press 17:326
Gonzalez MA, Alvarez ML, Pisani GB (2007) Involvement of oxidative stress in the impairment in biliary secretory function induced by intraperitoneal administration of aluminum to rats. Biol Trace Elem Res 116:329–348
Gorbunov NVand Kiang JG (2009) Up-regulation of autophagy in small intestine Paneth cells in response to total-body gamma-irradiation. J Pathol 219:242–252
Gorbunov NV, Garrison BR, Kiang JG (2010) Response of crypt paneth cells in the small intestine following total body γ-irradiation. Int J Immunopathol Pharmacol 23:1111
Harfouche G, Martin MT (2010) Response of normal stem cells to ionizing radiation: a balance between homeostasis and genomic stability. Mutat Res 704(1–3):167–174
Hogenesch H (2013) Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunology 10(3):406
Hyde A, Coughlan B, Naughton C, Hegarty J, Savage E, Grehan J et al (2016) Nurses’, physicians’ and radiographers’ perceptions of the safety of a nurse prescribing of ionising radiation initiative: a cross-sectional survey. Int J Nurs Stud 58:21–30
Hyoh Y, Ishizaka S, Horii T, Fujiwara A, Tegoshi T, Yamada M, Arizono N (2002) Activation of caspases in intestinal villus epithelial cells of normal and nematode infected rats. Gut 50:71
Kong SS, Liochev I, Fridovich I (1992) Aluminum (III) facilitates the oxidation of NADH by the superoxide anion. Free Radic Biol Med 13:9–81
Kregel KC, Zhang HJ (2007) An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American Journal of Physiology and Regulatory Integrative and Comparative 292:18–36
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J et al (2007) Human health risk assessment for aluminum, aluminum oxide and aluminum hydroxide. Journal of Toxicology and Environmental Health B Critical Reviews 10(1):1–269
Kumar V, Gill KD (2009) Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol 83(11):965–978
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods of Enzymology 186:464–478
Linard CR, Ropenga A, Vozenin-Brotons MC, Chaapel A, Mathe D (2003) Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappa B in rat ileal muscularis layer. AJP Gastrointestinal and liver physiology 285:556–565
Lindblad EB (2004) Aluminium compounds for use in vaccines. Immunol Cell Biol 82:497–505
Mathiyazahan DB, Thenmozhi AJ, Manivasagam T (2015) Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: a behavioural, biochemical and molecular approach. J Funct Foods 16:423–435
Meaney E, Vela A, Samaniego V, Meaney A, Asbún J, Zempoalteca JC et al (2008) Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the mefisto study. Clin Exp Pharmacol Physiol 5:895–903
Minami M, Yoshikawa H (1979) A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta 92:37–42
Newairy AS, Salama AF, Hussien HM, Yousef MI (2009) Propolis alleviates aluminum-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol 47:1093–1098
Nicolleti I, Miglorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Orihuela D, Meichtry V, Pregi N, Pizarro M (2005) Short-term oral exposure to aluminum decreases glutathione intestinal levels and changes enzyme activities involved in its metabolism. J Inorg Biochem 99:1871–1878
Osman HM, Shayoub ME, Babiker EM, Osman B, Elhassan AM (2012) Effect of ethanolic leaf extract of Moringa oleifera on aluminum-induced anemia in white albino rats. Jordan Journal of Biological Sciences 5:255–260
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pierre JF, Busch RA, Kudsk KA (2016) The gastrointestinal immune system: implications for the surgical patient. Curr Probl Surg 53:11–47
Porto ML, Lírio LM, Dias AT, Batista AT, Campagnaro BP, Mill JG et al (2015) Increased oxidative stress and apoptosis in peripheral blood mononuclear cells of fructose-fed rats. Toxicol in Vitro 29(8):1977–1981
Potten CS, Chadwick CA (1994) Small intestinal growth regulatory factors extracted by simple diffusion from intact irradiated intestine and tested in vivo. Growth Factors 10(1):63–75
Prakash D, Sudhandiran G (2015) Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. J Nutr Biochem 26(12):1527–1539
Reinke CM, Breitkreutz J, Leuenberger H (2003) Aluminum in over the-counter drugs: risks outweigh benefits. Drug Saf 26:1011–1025
Rishi P, Bhogal A, Arora S, Pandey SK, Verma I, Kaur IP (2015) Improved oral therapeutic potential of nanoencapsulated cryptdin formulation against Salmonella infection. Eur J Pharm Sci 72:27–33
Rouchka EC, Flight RM, Fasciotto BH, Estrada R, Eaton JW, Patibandla PK et al (2016) Transcriptional profile of immediate response to ionizing radiation exposure. Genomics Data 7:82–85
Saada HN, Azab KS (2001) Role of lycopene in recovery of radiation induced injury to mammalian cellular organelles. Pharmazie 56:239–241
Saada HN, Rezk RG, Eltahawy NA (2010) Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother Res 24:204–208
Sazykina TG, Kryshev AI (2016) Simulation of population response to ionizing radiation in an ecosystem with a limiting resource—model and analytical solutions. J Environ Radioact 151(1):50–57
Somosy Z, Horváth G, Telbisz A, Réz G, Pálfia Z (2002) Morphological aspects of ionizing radiation of small intestine. Micron 33:167–178
Smoll NR, Brady Z, Scurrah K, Mathews JD (2016) Exposure to ionizing radiation and brain cancer incidence: the life span study cohort. Cancer Epidemiol 42:60–65
Spitz DR, Azzam EI, Li JJ, Gius D (2004) Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Review 23:311–322
Tao D, Wu J, Feng Y, Qin J, Hu J, Gong J (2004) New method for the analysis of cell cycle–specific apoptosis. Cytometry 57A:70–74
Taylor PC (2001) Anti-TNF therapy for rheumatoid arthritis and other inflammatory diseases. Mol Biotechnol 19:153–168
Tomaszewska E, Winiarska-Mieczan A, Dobrowolski P (2015a) The lack of protective effects of tea supplementation on liver and jejunal epithelium in adult rats exposed to cadmium and lead. Environ Toxicol Pharmacol 40:708–714
Tomaszewska E, Winiarska-Mieczan A, Dobrowolski P (2015b) Hematological and serum biochemical parameters of blood in adolescent rats and histomorphological changes in the jejunal epithelium and liver after chronic exposure to cadmium and lead in the case of supplementation with green tea vs black, red or white tea. Exp Toxicol Pathol 67:331–339
van Es JH, Clevers H (2014) Paneth cells. Curr Biol 24(12):R548
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82(11):789–802
Vasudevaraju P, Govindaraju M, Palanisamy AP, Sambamurti K, Rao KS (2008) Molecular toxicity of aluminium in relation to neurodegeneration. Indian J Med Res 128(4):545–556
Ward RJ, Zhang Y, Crichton RR (2001) Aluminum toxicity and iron homeostasis. J Inorg Biochem 87:9–14
Willhite CC, Ball GL, McLellan CJ (2012) Total allowable concentrations of monomeric inorganic aluminum and hydrated aluminum silicates in drinking water. Critical Review of Toxicology 42(5):358–442
Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM et al (2014) Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Critical Review of Toxicology 44(4):1–80
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J et al (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uraemia. Kidney Int 49:1304–1313
Wu B, Guo H, Cui H, Peng X, Fang J, Zuo Z et al (2016) Pathway underlying small intestine apoptosis by dietary nickel chloride in broiler chickens. Chem Biol Interact 243(5):91–106
Yoshioka T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol 10(5):372–376
Yuan C, Lee Y, Hsu GW (2012) Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci 19:51
Zhu G, Xiang X, Chen X, Wang L, Hu H, Weng S (2009) Renal dysfunction induced by long-term exposure to depleted uranium in rats. Arch Toxicol 83(1):37–46
Zuwała-Jagiełło J, Pazgan-Simon M, Simon K, Warwas M (2011) Advanced oxidation protein products and inflammatory markers in liver cirrhosis: a comparison between alcohol-related and HCV-related cirrhosis. Acta Biochim Pol 58(1):59–65
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors did not receive any financial support for the research, authorship, and publication of this article from any funding agency in the public, commercial, or not-for-profit sectors.
Additional information
Responsible editor: Philippe Garrigues
An erratum to this article is available at http://dx.doi.org/10.1007/s11356-017-9286-9.
Rights and permissions
About this article
Cite this article
Eltahawy, N.A., Elsonbaty, S.M., Abunour, S. et al. Synergistic effect of aluminum and ionizing radiation upon ultrastructure, oxidative stress and apoptotic alterations in Paneth cells of rat intestine. Environ Sci Pollut Res 24, 6657–6666 (2017). https://doi.org/10.1007/s11356-017-8392-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8392-z