Pro-Oxidant/Antioxidant Balance during a Prolonged Exposure to Moderate Altitude in Athletes Exhibiting Exercise-Induced Hypoxemia at Sea-Level
Abstract
:Highlights
1. Introduction
2. Materials and Methods
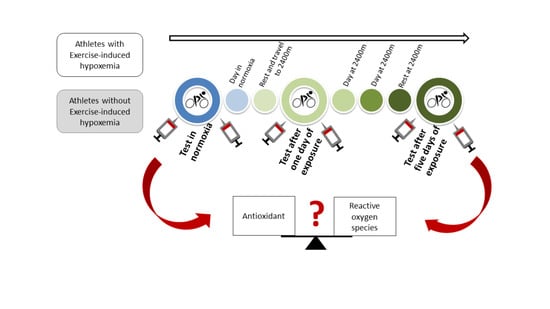
2.1. Subjects
2.2. Protocol
2.3. Maximal Exercise Test and Gas Exchange Measurements
2.4. Arterial O2 Saturation and Determination of EIH
2.5. Blood Sampling, Oxidative Stress and Antioxidant Assays
2.6. Statistical Analysis
3. Results
3.1. Anthropometric Data
3.2. Arterial O2 Saturation, Oxygen Consumption and Performance
3.3. Oxidative Stress Markers
4. Discussion
4.1. Oxidative Stress at Sea Level
4.2. Oxidative Stress during Exposure to Moderate Altitude
4.3. Putative Physiological Effects of Oxidative Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joanny, P.; Steinberg, J.; Robach, P.; Richalet, J.P.; Gortan, C.; Gardette, B.; Jammes, Y. Operation Everest III (Comex’97): The Effect of Simulated Sever Hypobaric Hypoxia on Lipid Peroxidation and Antioxidant Defence Systems in Human Blood at Rest and after Maximal Exercise. Resuscitation 2001, 49, 307–314. [Google Scholar] [CrossRef]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.W.; Radak, Z. High Altitude and Oxidative Stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Oxydative Stress; Elsevier: Amsterdam, The Netherland, 2007. [Google Scholar]
- Pialoux, V.; Mounier, R.; Rock, E.; Mazur, A.; Schmitt, L.; Richalet, J.-P.; Robach, P.; Coudert, J.; Fellmann, N. Effects of Acute Hypoxic Exposure on Prooxidant/Antioxidant Balance in Elite Endurance Athletes. Int. J. Sports Med. 2009, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Faiss, R.; Pialoux, V.; Sartori, C.; Faes, C.; Dériaz, O.; Millet, G.P. Ventilation, Oxidative Stress, and Nitric Oxide in Hypobaric versus Normobaric Hypoxia. Med. Sci. Sports Exerc. 2013, 45, 253–260. [Google Scholar] [CrossRef]
- Debevec, T.; Pialoux, V.; Saugy, J.; Schmitt, L.; Cejuela, R.; Mury, P.; Ehrström, S.; Faiss, R.; Millet, G.P. Prooxidant/Antioxidant Balance in Hypoxia: A Cross-Over Study on Normobaric vs. Hypobaric “Live High-Train Low”. PLoS ONE 2015, 10, e0137957. [Google Scholar] [CrossRef] [Green Version]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial Complex III Is Required for Hypoxia-Induced ROS Production and Cellular Oxygen Sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Choi, D.-K. Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.A.; Schumacker, P.T. Sensors and Signals: The Role of Reactive Oxygen Species in Hypoxic Pulmonary Vasoconstriction. J. Physiol. 2019, 597, 1033–1043. [Google Scholar] [CrossRef]
- Pialoux, V.; Brugniaux, J.V.; Fellmann, N.; Richalet, J.-P.; Robach, P.; Schmitt, L.; Coudert, J.; Mounier, R. Oxidative Stress and HIF-1 Alpha Modulate Hypoxic Ventilatory Responses after Hypoxic Training on Athletes. Respir. Physiol. Neurobiol. 2009, 167, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Hanly, P.J.; Foster, G.E.; Brugniaux, J.V.; Beaudin, A.E.; Hartmann, S.E.; Pun, M.; Duggan, C.T.; Poulin, M.J. Effects of Exposure to Intermittent Hypoxia on Oxidative Stress and Acute Hypoxic Ventilatory Response in Humans. Am. J. Respir. Crit. Care Med. 2009, 180, 1002–1009. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Hanson, P.G.; Henderson, K.S. Exercise-Induced Arterial Hypoxaemia in Healthy Human Subjects at Sea Level. J. Physiol. 1984, 355, 161–175. [Google Scholar] [CrossRef]
- Constantini, K.; Tanner, D.A.; Gavin, T.P.; Harms, C.A.; Stager, J.M.; Chapman, R.F. Prevalence of Exercise-Induced Arterial Hypoxemia in Distance Runners at Sea Level. Med. Sci. Sports Exerc. 2017, 49, 948–954. [Google Scholar] [CrossRef]
- Stewart, I.B.; Pickering, R.L. Effect of Prolonged Exercise on Arterial Oxygen Saturation in Athletes Susceptible to Exercise-Induced Hypoxemia. Scand. J. Med. Sci. Sports 2007, 17, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Davies, B.; Young, I.S. Intermittent Hypoxic Training: Implications for Lipid Peroxidation Induced by Acute Normoxic Exercise in Active Men. Clin. Sci. 2001, 101, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Kyparos, A.; Riganas, C.; Nikolaidis, M.G.; Sampanis, M.; Koskolou, M.D.; Grivas, G.V.; Kouretas, D.; Vrabas, I.S. The Effect of Exercise-Induced Hypoxemia on Blood Redox Status in Well-Trained Rowers. Eur. J. Appl. Physiol. 2012, 112, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B. Arterial Desaturation during Exercise in Man: Implication for O2 Uptake and Work Capacity. Scand. J. Med. Sci. Sports 2003, 13, 339–358. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Mathieu-Costello, O. Stress Failure of Pulmonary Capillaries as a Limiting Factor for Maximal Exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-Induced Oxidative Stress in Humans: Cause and Consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-Induced Oxidative Stress: Past, Present and Future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, C.; Higashi, Y.; Kimura, M.; Noma, K.; Hara, K.; Nakagawa, K.; Kawamura, M.; Chayama, K.; Yoshizumi, M.; Nara, I. Effect of Different Intensities of Exercise on Endothelium-Dependent Vasodilation in Humans: Role of Endothelium-Dependent Nitric Oxide and Oxidative Stress. Circulation 2003, 108, 530–535. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, G.; Kliszczewiscz, B.; Barberio, M.; Ballmann, C.; Peters, B.; Slivka, D.; Dumke, C.; Cuddy, J.; Hailes, W.; Ruby, B.; et al. Acute Hypoxia and Exercise-Induced Blood Oxidative Stress. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Quindry, J.; Dumke, C.; Slivka, D.; Ruby, B. Impact of Extreme Exercise at High Altitude on Oxidative Stress in Humans. J. Physiol. 2016, 594, 5093–5104. [Google Scholar] [CrossRef] [Green Version]
- Debevec, T.; Millet, G.P.; Pialoux, V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front. Physiol. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.F.; Emery, M.; Stager, J.M. Degree of Arterial Desaturation in Normoxia Influences VO2max Decline in Mild Hypoxia. Med. Sci. Sports Exerc. 1999, 31, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Benoit, H.; Busso, T.; Castells, J.; Geyssant, A.; Denis, C. Decrease in Peak Heart Rate with Acute Hypoxia in Relation to Sea Level VO(2max). Eur. J. Appl. Physiol. 2003, 90, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Grataloup, O.; Busso, T.; Castells, J.; Denis, C.; Benoit, H. Evidence of Decrease in Peak Heart Rate in Acute Hypoxia: Effect of Exercise-Induced Arterial Hypoxemia. Int. J. Sports Med. 2007, 28, 181–185. [Google Scholar] [CrossRef]
- Raberin, A.; Meric, H.; Mucci, P.; Ayerbe, J.L.; Durand, F. Muscle and Cerebral Oxygenation during Exercise in Athletes with Exercise-Induced Hypoxemia: A Comparison between Sea Level and Acute Moderate Hypoxia. Eur. J. Sport Sci. 2020, 20, 803–812. [Google Scholar] [CrossRef]
- Legrand, R.; Ahmaidi, S.; Moalla, W.; Chocquet, D.; Marles, A.; Prieur, F.; Mucci, P. O2 Arterial Desaturation in Endurance Athletes Increases Muscle Deoxygenation. Med. Sci. Sports Exerc. 2005, 37, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Gaston, A.-F.; Durand, F.; Roca, E.; Doucende, G.; Hapkova, I.; Subirats, E. Exercise-Induced Hypoxaemia Developed at Sea-Level Influences Responses to Exercise at Moderate Altitude. PLoS ONE 2016, 11, e0161819. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Powers, S.; Cicale, M.; Collop, N.; Huang, D.; Criswell, D. Validity of Pulse Oximetry during Exercise in Elite Endurance Athletes. J. Appl. Physiol. 1992, 72, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Wagner, P.D. Exercise-Induced Arterial Hypoxemia. J. Appl. Physiol. 1999, 87, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Klimova, T.; Chandel, N.S. Mitochondrial Complex III Regulates Hypoxic Activation of HIF. Cell Death Differ. 2008, 15, 660–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, F.; Guillot, M.; Gosselin, N. Augmentation de La Fréquence d’apparition de l’hypoxémie Induite Par l’exercice Chez Des Rameurs de Haut-Niveau. Sci. Sports 2004, 19, 296–300. [Google Scholar] [CrossRef]
- Cunningham, D.A.; Goode, P.B.; Critz, J.B. Cardiorespiratory Response to Exercise on a Rowing and Bicycle Ergometer. Med. Sci. Sports 1975, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Szal, S.E.; Schoene, R.B. Ventilatory Response to Rowing and Cycling in Elite Oarswomen. J. Appl. Physiol. 1989, 67, 264–269. [Google Scholar] [CrossRef]
- West, J.B.; Tsukimoto, K.; Mathieu-Costello, O.; Prediletto, R. Stress Failure in Pulmonary Capillaries. J. Appl. Physiol. 1991, 70, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Dodd, S.; Lawler, J.; Landry, G.; Kirtley, M.; McKnight, T.; Grinton, S. Incidence of Exercise Induced Hypoxemia in Elite Endurance Athletes at Sea Level. Eur. J. Appl. Physiol. Occup. Physiol. 1988, 58, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.; Weber, D.; Kochlik, B.; Stuetz, W.; Toussaint, O.; Debacq-Chainiaux, F.; Dollé, M.E.T.; Jansen, E.H.J.M.; Gonos, E.S.; Sikora, E.; et al. Gender- and Age-Dependencies of Oxidative Stress, as Detected Based on the Steady State Concentrations of Different Biomarkers in the MARK-AGE Study. Redox Biol. 2019, 24, 101204. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C.; Zhang, H.J. An Integrated View of Oxidative Stress in Aging: Basic Mechanisms, Functional Effects, and Pathological Considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef] [PubMed]
- Ribon, A.; Pialoux, V.; Saugy, J.J.; Rupp, T.; Faiss, R.; Debevec, T.; Millet, G.P. Exposure to Hypobaric Hypoxia Results in Higher Oxidative Stress Compared to Normobaric Hypoxia. Respir. Physiol. Neurobiol. 2016, 223, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Debevec, T.; Pialoux, V.; Mekjavic, I.B.; Eiken, O.; Mury, P.; Millet, G.P. Moderate Exercise Blunts Oxidative Stress Induced by Normobaric Hypoxic Confinement. Med. Sci. Sports Exerc. 2014, 46, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Khawli, F.A.; Moody, M.R. Reactive Oxygen in Skeletal Muscle. III. Contractility of Unfatigued Muscle. J. Appl. Physiol. 1993, 75, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.D.; Westerblad, H. Acute Effects of Reactive Oxygen and Nitrogen Species on the Contractile Function of Skeletal Muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef] [PubMed]

| EIH (n = 9) | NEIH (n = 10) | |
|---|---|---|
| Age (years) | 25.5 ± 1.5 * | 33.8 ± 2.3 |
| Height (cm) | 181 ± 2 | 176 ± 2 |
| Body mass (kg) | 71.7 ± 2.3 | 69.6 ± 2.3 |
| Body mass index (m kg−2) | 21.7 ± 0.6 | 22.2 ± 0.4 |
| Training volume (h week−1) | 13.7 ± 1.3 | 13.5 ± 1.8 |
| Training history (years) | 7.3 ± 0.5 | 8.6 ± 1.9 |
| Group | SL | H1 | H2 | |
|---|---|---|---|---|
| SpO2rest (%) | EIH | 98.7 ± 0.2 | 94.3 ± 0.4 *† | 94.3 ± 0.4 *† |
| NEIH | 99.1 ± 0.1 | 96 ± 0.4 † | 97.4 ± 0.3 †‡ | |
| SpO2max (%) | EIH | 93.2 ± 0.3 * | 79.7 ± 1.0 *† | 81.2 ± 1.1 *† |
| NEIH | 96.8 ± 0.4 | 84.7 ± 0.6 † | 87.1 ± 0.8 †‡ | |
| VErest (L min−1) | EIH | 16.0 ± 0.7 | 18.1 ± 0.7 † | 20.0 ± 0.7 †‡ |
| NEIH | 13.8 ± 0.6 | 15.6 ± 1.4 † | 18.1 ± 1.0 †‡ | |
| VEmax (L min−1) | EIH | 172 ± 4.9 | 184 ± 5.5 | 191 ± 4.9 † |
| NEIH | 175 ± 7.9 | 181 ± 6.9 | 183 ± 7.3 | |
| VO2max (mL min−1 kg−1) | EIH | 69.2 ± 1.8 | 58.4 ± 2.0 † | 59.3 ± 1.5 † |
| NEIH | 65.3 ± 3.0 | 52.7 ± 1.9 † | 55.8 ± 2.2 † | |
| Wattmax (Watt) | EIH | 465 ± 12 * | 416 ± 11 *† | 406 ± 10 *† |
| NEIH | 409 ± 13 | 366 ± 11 † | 362 ± 10 † |
| Condition | Sea-Level | H1 | H2 | |||
|---|---|---|---|---|---|---|
| Exercise | Pre | Post | Pre | Post | Pre | Post |
| GPX (µmol L−1 min−1) | ||||||
| EIH | 45.4 ± 2.9 | 46.5 ± 3.9 | 53 ± 2.6 † | 48.5 ± 2.2 | 52.5 ± 2.4 † | 43.9 ± 3.3§ |
| NEIH | 45.5 ± 2.7 | 51.3 ± 3.7 | 50.8 ± 2.5 † | 48.8 ± 2 | 49.9 ± 2.3 † | 44.9 ± 3.2§ |
| Condition × Exercise (p = 0.040) | ||||||
| Catalase (µmol L−1 min−1) | ||||||
| EIH | 95 ± 19 | 176 ± 37§ | 37.1 ± 4.0 † | 53.9 ± 4.8§ † | 50.8 ± 8.4 | 61.5 ± 9.9§ † |
| NEIH | 110 ± 38 | 152 ± 40§ | 28.8 ± 3.8 † | 40.9 ± 4.2§ † | 19 ± 2.3 | 45.9 ± 5.2§ † |
| Condition (p < 0.001); Exercise (p = 0.009) | ||||||
| FRAP (µmol L−1) | ||||||
| EIH | 532 ± 40 | 422 ± 38§ | 340 ± 40 † | 355 ± 50 | 485 ± 60 | 638 ± 80§‡ |
| NEIH | 479 ± 38 | 436 ± 36 | 321 ± 38 † | 381 ± 48 | 361 ± 57 | 450 ± 76 |
| Condition (p < 0.001); Group × Condition (p = 0.073); Condition × Exercise (p = 0.002) | ||||||
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raberin, A.; Nader, E.; Lopez Ayerbe, J.; Alfonsi, G.; Mucci, P.; Rytz, C.L.; Pialoux, V.; Durand, F. Pro-Oxidant/Antioxidant Balance during a Prolonged Exposure to Moderate Altitude in Athletes Exhibiting Exercise-Induced Hypoxemia at Sea-Level. Life 2021, 11, 228. https://doi.org/10.3390/life11030228
Raberin A, Nader E, Lopez Ayerbe J, Alfonsi G, Mucci P, Rytz CL, Pialoux V, Durand F. Pro-Oxidant/Antioxidant Balance during a Prolonged Exposure to Moderate Altitude in Athletes Exhibiting Exercise-Induced Hypoxemia at Sea-Level. Life. 2021; 11(3):228. https://doi.org/10.3390/life11030228
Chicago/Turabian StyleRaberin, Antoine, Elie Nader, Jorge Lopez Ayerbe, Gauthier Alfonsi, Patrick Mucci, Chantal L. Rytz, Vincent Pialoux, and Fabienne Durand. 2021. "Pro-Oxidant/Antioxidant Balance during a Prolonged Exposure to Moderate Altitude in Athletes Exhibiting Exercise-Induced Hypoxemia at Sea-Level" Life 11, no. 3: 228. https://doi.org/10.3390/life11030228