Abstract
Purpose of Review
To synthesize the existing literature regarding the complex interplay between sleep disturbance, obesity, and diabetes. The review emphasizes the three pillars of health being diet, exercise, and sleep, with the notion that if one is ignored, then the other two could suffer.
Recent Findings
Sleep deprivation is associated with incident obesity, perhaps mediated by dysregulation in leptin and ghrelin — hormones important in regulation of appetite. Sleep apnea is very common particularly among obese people with type 2 diabetes mellitus. Treatment of sleep apnea has clear symptomatic benefits although its impact on long-term cardiometabolic health is less clear.
Summary
Sleep disturbance may be an important modifiable risk for patients at risk of cardiometabolic disease. An assessment of sleep health may be an important component of the comprehensive care of patients with obesity and diabetes mellitus.
Similar content being viewed by others
Introduction
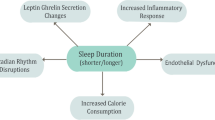
Obesity has been increasingly pervasive over the past several decades, in part due an intersection of genetic and behavioral factors, specifically increased consumption of high caloric processed foods, and more sedentary lifestyles [1,2,3]. Current estimates suggest that 1/3 of people in the USA have normal weight, 1/3 are overweight (defined by BMI 25–30 kg/m2), and 1/3 are obese (BMI > 30 kg/m2) [4]. Recent studies have suggested that based on the prevalence of obesity, life expectancy may start to decrease over time as a result [5]. The mechanisms underlying obesity related complications are complex, but type 2 diabetes mellitus (T2DM) and obstructive sleep apnea (OSA) are common comorbidities [6, 7]. Sleep deprivation is a common problem in today’s 24/7 society, with some evidence suggesting an important impact of sleep loss on overall health [8, 9]. OSA is estimated to affect almost 1 billion people worldwide [10, 11]. OSA is defined by repetitive collapse of the pharyngeal airway during sleep leading to derangements in gas exchange (hypoxemia and hypercapnia), as well as surges in catecholamines and other counter-regulatory hormones [12, 13]. Data from the SLEEP-AHEAD study (a sub-study under the LOOK-AHEAD obesity study) [6, 13] suggested that OSA (apnea hypopnea index — AHI>5.0/h) was present in 86.6% of obese patients with T2DM [7]. Of note, clinically important OSA (defined by AHI > 15/h) was present in 53.1% of this cohort. However, despite general acknowledgement that diet, exercise, and sleep are the three pillars of health [14, 15], most OSA remains undiagnosed and untreated [16, 17]. The interactions between obesity, OSA, and T2DM have been reviewed extensively and are summarized in Fig. 1. Although obesity is a major risk factor for OSA, as will be discussed further, treatment of OSA itself has been demonstrated in instances to lead to weight gain due to behavioral changes [18, 19]. Of note, OSA is characterized by sleep fragmentation whereas sleep deprivation refers to inadequate duration of sleep. The term sleep disturbance is used more generally to refer to various sleep pathologies including sleep fragmentation as well as sleep deprivation. We review here four specific topics in this context worthy of further consideration:
Proposed interactions between T2DM, obesity, OSA, and NAFLD. Obesity is a known risk factor for T2DM, OSA, and NAFLD. T2DM is linked bidirectionally with OSA with some evidence that each factor may worsen the disease process of the other. OSA may in theory directly impact NAFLD. There is some evidence that treatment of OSA itself may lead to weight gain likely due to behavioral changes
Sleep Deprivation
- 1.
Sleep deprivation. Sleep deprivation (defined by habitual sleep less than 7–9 h per night) has been clearly associated with incident obesity in many different epidemiological cohorts [20,21,22]. Patel et al. analyzed data from the Nurses’ Health Study and showed that women sleeping 5 h per night experienced more weight gain than those reporting 7 h of sleep per night [14]. Studies from the same cohort showed increased incident T2DM associated with short sleep as compared to people reporting adequate sleep duration [23]. Mechanistic studies have been performed by Spiegel et al. examining the impact of sleep deprivation on hormones that regulate appetite [24,25,26,27]. The authors randomized patients to obtain either inadequate sleep or adequate sleep during an in laboratory study in which constant glucose infusion was provided. They observed that sleep deprivation led to suppression of leptin levels and increase in ghrelin levels as compared to those sleeping adequately. Both hormones changed with sleep deprivation in a direction that would be predicted to stimulate appetite [26]. Thus, there is evidence that inadequate sleep may drive weight gain via dysregulation in satiety [28]. Of note, these hormonal changes have been somewhat inconsistent in various studies, amplifying the need for further research [29, 30]. However, despite reasonably compelling data regarding metabolic risk of sleep deprivation, OSA itself as a disease process may not increase body weight. In fact, several meta-analyses have suggested that treatment of OSA with CPAP (continuous positive airway pressure) is associated with weight gain, although the mechanisms are unclear [31,32,33,34].
OSA and Type 2 Diabetes Mellitus
- 2.
OSA and T2DM. Obesity is a common risk factor for both OSA and T2DM [35]. However, there are several proposed connections between OSA and T2DM independent of obesity. First, OSA may worsen T2DM and cause hyperglycemia with repetitive apnea inciting surges in catecholamines and other counter-regulatory hormones [36,37,38]. However, studies assessing the impact of OSA treatment with CPAP have shown mixed results without clear improvement of glycemic control [37, 39,40,41]. The reasons for this discrepancy are unclear but may relate to poor CPAP adherence, dietary indiscretion after symptomatic improvement with CPAP therapy, or patient selection in these studies. Second, T2DM may worsen OSA via neuromyopathic effects [38, 42]. Some data suggest that in patients with T2DM, OSA progresses in severity over time even without major weight gain [6, 43]. A theoretical explanation could be that T2DM is associated with abnormalities in control of breathing as well as neuromuscular effects impacting pharyngeal mechanics and worsening OSA. Third, T2DM and OSA may have synergistic effects on atherosclerotic and vascular disease risk. T2DM is known to impact the microcirculation and vascular smooth muscle, whereas OSA is thought to impact the endothelium preferentially [44, 45]. These findings support treatment of OSA especially in patients with T2DM to improve glycemic control and potentially reduce cardiometabolic risk. However, definitive randomized controlled trials regarding CPAP intervention to prevent cardiovascular disease are lacking [46, 47].
Despite the numerous connections between OSA and T2DM, a major portion of patients with T2DM continue to be undertreated for OSA [48]. The Sleep-Ahead study was a sub-study under the Look-Ahead study which showed 86.6% of obese T2DM patients had clinically important OSA [13]. One year after the diagnosis was given to both patients and their physicians, > 95% of patients with OSA remained untreated [6, 7]. As a result, there is a need for increased awareness to promote OSA as a valuable therapeutic target with implications on cardiometabolic health for diabetic patients.
OSA and Non-Alcoholic Fatty Liver Disease
- 3.
OSA and non-alcoholic fatty liver disease (NAFLD): OSA and NAFLD are both associated with insulin resistance, although mechanistic research in this context is sparse. There are a number of studies showing a potential association between OSA and NAFLD [49]. NAFLD is sometimes regarded as a cardiovascular risk factor although the causal pathways are unclear and may well involve insulin resistance, obesity, and OSA at least to some extent [50]. A study by Corey et al. suggested that among patients undergoing bariatric surgery, the presence of OSA was a strong predictor of hepatic fibrosis [51]. In theory, OSA could contribute to NAFLD but may also increase risk of hepatic fibrosis and possibly hepatoma [52, 53]. A number of basic studies in mice have suggested the importance of intermittent hypoxia in contributing to liver abnormalities [54, 55]. Kallwitz et al. showed in obese patients with NAFLD that OSA was associated with elevated ALT levels and a possible link to histological evidence of liver disease [56]. These findings were supported by Mishra et al. who observed that OSA may be a risk factor for progression of NAFLD to NASH (non-alcoholic steatohepatitis) [57]. However, clinical data showing improvement in liver structure and function with OSA treatment are still evolving [58, 59].
Treatment of OSA, Obesity, and T2DM
- 4.
Treatment: The three pillars of health are often referred to as diet, exercise, and sleep [28]—with the dogma that if one ignores one, the other two will be compromised [20]. Thus, optimal health may require addressing all three factors [60]. However, sleep health is often ignored despite its potential contributions to treatment of OSA, obesity, and T2DM [61]. We focus on four treatments in this section:
- (a)
Exercise: Aerobic exercise is known to have benefits for overall health. Contracting muscle takes up glucose independently of the presence of insulin and potentiates insulin metabolic action after exercise, suggesting a major benefit to exercise in reducing hyperglycemia. Exercise has also been associated with improvement in daytime sleepiness and OSA severity even in the context of minimal weight change [62]. As a result, exercise training and rehabilitation may have a beneficial role as adjunctive treatment of OSA. Aerobic exercise is established as a vital component of weight management with additional benefit noted with high-intensity interval training, but likely works more effectively with concurrent diet and sleep interventions [60, 63,64,65,66].
- (b)
Weight loss medications: Weight loss medications developed over the past decade have become more efficacious in weight management and glycemic control. Previously, agents such as phentermine/topiramate, bupropion-naltrexone, and orlistat were commonly used and noted to have weight loss benefit, though use was limited by adverse effects, such as diarrhea with orlistat [67]. GLP1 (glucagon-like peptide 1) receptor agonists have emerged as promising treatments with daily liraglutide and weekly semaglutide injections which are FDA approved for both treatment of T2DM and obesity. Tirzepatide is a newer agent approved for T2DM therapy but with findings already suggestive of major weight loss in the diabetic population. However, this medication has not been adequately studied in nondiabetic patients at this time and is pending approval for use for obesity. The impact of these weight loss medications on OSA is less clear and is a topic of ongoing investigation (NCT05412004). One such study was the SCALE Sleep Apnea trial which evaluated the effects of liraglutide 3.0 mg daily in nondiabetic patients with diagnosed OSA but not on CPAP, which found a statistically significant reduction in AHI compared to placebo (− 12.2 events vs − 6.1 events, p = 0.0150), likely mediated through weight loss (− 5.7% compared to placebo group − 1.6%) [68]. Treatment of OSA and T2DM via weight loss medications may be a viable approach to minimize cardiometabolic risk [69,70,71].
- (c)
Bariatric surgery: Metabolic and bariatric surgery is increasingly used to facilitate major weight loss mostly via sleeve gastrectomy and Roux-en-Y bypass procedures which have shown the greatest efficacy. Over time, surgical techniques have evolved to help these processes become minimally invasive. Several studies confirm a durable weight loss following bariatric surgery as well as significant improvement (and sometimes resolution) of both T2DM and OSA. In some studies, OSA can recur over time, in part mediated by weight re-gain with aging and redistribution of body fat. Thus, long-term follow-up of these patients is recommended to optimize cardiometabolic risk following bariatric surgery [72,73,74,75].
- (d)
CPAP: Nasal CPAP has been shown to improve daytime symptoms of OSA, including sleepiness and daily functioning, and blood pressure [76, 77]. Although CPAP is frequently regarded as poorly tolerated, recent data suggest that adherence can be achieved in most patients given modern therapy, education, and troubleshooting of the mask interface. Patient engagement tools have been shown to achieve 87% adherence based on US Medicare criteria, suggesting that treatment of OSA can be achieved consistently with CPAP therapy [78]. However, there is general acknowledgement that alternative therapies will be required to optimize OSA treatment in addition to CPAP [79]. OSA is now recognized as having varying endophenotypes that need to be recognized rather than employing the current “one-size-fits-all” approach [80]. Despite suggestive observational data, definitive data from randomized trials are not available to show that CPAP leads to reductions in stroke or myocardial infarction [81]. As mentioned, data are equivocal regarding the benefits of CPAP related to glycemic control.
Of note, CPAP has been associated with weight gain in some studies although the mechanisms are unclear. Some authors have measured extracellular fluid volume and have suggested that CPAP may cause fluid accumulation leading to weight gain [34]. Other authors have measured respiratory work associated with repetitive obstructive apnea and suggested that CPAP may reduce caloric expenditure by reducing the work of breathing in patients with OSA [82]. One concept that has been observed clinically is that some OSA patients when treated with CPAP have improvement in energy and quality of life and resume normal social activities leading to increased dietary indiscretion (i.e., eating out more often or having a beer with friends) and weight gain. Some hormonal theories have also been proposed regarding CPAP-induced weight gain, although the exact mechanism remains unclear [83].
Conclusions
In summary, there are numerous interactions between obesity, OSA, and T2DM that impact cardiovascular and metabolic health. Sleep disturbance is an important and underrecognized risk factor which can be targeted via treatment of OSA or improving sleep hygiene to prevent progression of T2DM and obesity. Although treatment of OSA with CPAP does not clearly improve glycemic control, OSA remains an important therapeutic target for patients with T2DM to reduce cardiometabolic risk. OSA remains an underdiagnosed and undertreated condition in patients with T2DM, likely due to lack of awareness or systematic screening protocols. By integrating sleep health as a consideration in the treatment in all patients, there is potential for prevention of obesity, OSA, and T2DM and an improved quality of life.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McTigue K, Kuller L. Cardiovascular risk factors, mortality, and overweight. JAMA. 2008;299(11):1260–1. https://doi.org/10.1001/jama.299.11.1260-c.
Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176–85.
Apovian CM. The obesity epidemic--understanding the disease and the treatment. N Engl J Med. 2016;374:177–9. https://doi.org/10.1056/NEJMe1514957.
McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, Stefanick ML, Van Horn L, Kuller L. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. https://doi.org/10.1001/jama.296.1.79.
Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. https://doi.org/10.1056/NEJMsr043743.
Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. https://doi.org/10.1001/archinternmed.2009.266.
Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–9. https://doi.org/10.2337/dc08-1776.
Dinges DF. The state of sleep deprivation: from functional biology to functional consequences. Sleep Med Rev. 2006;10:303–5. https://doi.org/10.1016/j.smrv.2006.07.001.
Ayas N, White D, Manson J, Stampfer M, Speizer F, Malhotra A, Hu F. A prospective study of sleep duration and coronary artery disease in women. Arch Intern Med. 2002;163(2):205–9.
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pepin JL, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98. https://doi.org/10.1016/S2213-2600(19)30198-5.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. https://doi.org/10.1093/aje/kws342.
Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. https://doi.org/10.1016/S0140-6736(13)60734-5.
Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. https://doi.org/10.1056/NEJMoa1212914.
Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. https://doi.org/10.1093/aje/kwj280.
Malhotra CK, Gunge D, Advani I, Boddu S, Nilaad S, Crotty Alexander LE. Assessing the potential impact of age and inhalant use on sleep in adolescents. J Clin Sleep Med. 2021;17:2233–9. https://doi.org/10.5664/jcsm.9414.
Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–400. https://doi.org/10.1001/jama.2020.3514.
Veasey SC, Rosen IM. Obstructive sleep apnea in adults. N Engl J Med. 2019;380:1442–9. https://doi.org/10.1056/NEJMcp1816152.
Tasali E, Chapotot F, Leproult R, Whitmore H, Ehrmann DA. Treatment of obstructive sleep apnea improves cardiometabolic function in young obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:365–74. https://doi.org/10.1210/jc.2010-1187.
Patel SR, Mehra R. The weighty issue of obesity management in sleep apnea. Chest. 2015;148:1127–9. https://doi.org/10.1378/chest.15-1010.
Mukherjee S, Patel SR, Kales SN, Ayas NT, Strohl KP, Gozal D, Malhotra A. American Thoracic Society ad hoc Committee on Healthy S. An Official American Thoracic Society statement: the importance of healthy sleep. Recommendations and Future Priorities. Am J Respir Crit Care Med. 2015;191:1450–8. https://doi.org/10.1164/rccm.201504-0767ST.
Ogilvie RP, Patel SR. The epidemiology of sleep and diabetes. Curr Diab Rep. 2018;18:82. https://doi.org/10.1007/s11892-018-1055-8.
Knutson KL, Wu D, Patel SR, Loredo JS, Redline S, Cai J, Gallo LC, Mossavar-Rahmani Y, Ramos AR, Teng Y, et al. Association between sleep timing, obesity, diabetes: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep. 2017;40:4. https://doi.org/10.1093/sleep/zsx014.
Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. https://doi.org/10.2337/diacare.26.2.380.
Spiegel K, Leproult R, Copinschi G, Van Cauter E. Impact of sleep length on the 24-h leptin profile. Sleep. 2001;24:A74.
Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–2. https://doi.org/10.1001/jama.288.12.1471-a.
Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50.
Spiegel K, lR, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;23:1435–9.
Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–41. https://doi.org/10.1059/0003-4819-153-7-201010050-00006.
van Egmond LT, Meth EMS, Engstrom J, Ilemosoglou M, Keller JA, Vogel H, Benedict C. Effects of acute sleep loss on leptin, ghrelin, and adiponectin in adults with healthy weight and obesity: a laboratory study. Obesity (Silver Spring). 2023;31:635–41. https://doi.org/10.1002/oby.23616.
Zhu B, Shi C, Park CG, Zhao X, Reutrakul S. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2019;45:18–30. https://doi.org/10.1016/j.smrv.2019.02.002.
Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–64. https://doi.org/10.1136/thoraxjnl-2014-205361.
Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24:843–51. https://doi.org/10.1016/j.beem.2010.08.011.
Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62:569–76. https://doi.org/10.1016/j.jacc.2013.05.045.
Herculano S, Grad GF, Drager LF, de Albuquerque ALP, Melo CM, Lorenzi-Filho G, Genta PR. Weight gain induced by continuous positive airway pressure in patients with obstructive sleep apnea is mediated by fluid accumulation: a randomized crossover controlled trial. Am J Respir Crit Care Med. 2021;203:134–6. https://doi.org/10.1164/rccm.202005-1853LE.
Reutrakul S, Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152:1070–86. https://doi.org/10.1016/j.chest.2017.05.009.
Weinstock TG, Rosen CL, Marcus CL, Garetz S, Mitchell RB, Amin R, Paruthi S, Katz E, Arens R, Weng J, et al. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. Sleep. 2014;37:261–9. https://doi.org/10.5665/sleep.3394.
Weinstock TG, Wang X, Rueschman M, Ismail-Beigi F, Aylor J, Babineau DC, Mehra R, Redline S. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–625B. https://doi.org/10.5665/sleep.1816.
Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. Regulatory Peptides. 1997;71:29–36.
Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–13. https://doi.org/10.1164/rccm.200909-1423OC.
Punjabi NM, Ahmed MM, Polotsky VY, Beamer BA, O'Donnell CP. Sleep-disordered breathing, glucose intolerance, and insulin resistance. Respir Physiol Neurobiol. 2003;136:167–78.
Zhao X, Zhang W, Xin S, Yu X, Zhang X. Effect of CPAP on blood glucose fluctuation in patients with type 2 diabetes mellitus and obstructive sleep apnea. Sleep Breath. 2022;26:1875–83. https://doi.org/10.1007/s11325-021-02556-0.
Saboisky JP, Stashuk DW, Hamilton-Wright A, Carusona AL, Campana LM, Trinder J, Eckert DJ, Jordan AS, McSharry DG, White DP, et al. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:322–9. https://doi.org/10.1164/rccm.201106-1058OC.
Fisher D, Pillar G, Malhotra A, Peled N, Lavie P. Long-term follow-up of untreated patients with sleep apnoea syndrom. Respiratory Medicine. 2002;96:337–43.
Yim-Yeh S, Rahangdale S, Nguyen AT, Stevenson KE, Novack V, Veves A, Malhotra A. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring). 2011;19:17–22. https://doi.org/10.1038/oby.2010.116.
Rahangdale S, Yeh SY, Malhotra A, Veves A. Therapeutic interventions and oxidative stress in diabetes. Front Biosci. 2009;14:192–209.
McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–31. https://doi.org/10.1056/NEJMoa1606599.
Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613–20. https://doi.org/10.1164/rccm.201601-0088OC.
Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 1985;2005(99):1998–2007. https://doi.org/10.1152/japplphysiol.00695.2005.
Ahmed MH, Byrne CD. Obstructive sleep apnea syndrome and fatty liver: association or causal link? World J Gastroenterol. 2010;16:4243–52. https://doi.org/10.3748/wjg.v16.i34.4243.
Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. https://doi.org/10.1056/NEJMoa2029349.
Corey KE, Misdraji J, Gelrud L, King LY, Zheng H, Malhotra A, Chung RT. Obstructive sleep apnea is associated with nonalcoholic steatohepatitis and advanced liver histology. Dig Dis Sci. 2015;60:2523–8. https://doi.org/10.1007/s10620-015-3650-8.
Kendzerska T, Povitz M, Leung RS, Boulos MI, McIsaac DI, Murray BJ, Bryson GL, Talarico R, Hilton JF, Malhotra A, et al. Obstructive sleep apnea and incident cancer: a large retrospective multicenter clinical cohort study. Cancer Epidemiol Biomarkers Prev. 2021;30:295–304. https://doi.org/10.1158/1055-9965.EPI-20-0975.
Gozal D, Almendros I, Hakim F. Sleep apnea awakens cancer: a unifying immunological hypothesis. Oncoimmunology. 2014;3:e28326. https://doi.org/10.4161/onci.28326.
Mesarwi OA, Malhotra A. Putting it together: sleep apnea, the integrated stress response, and metabolic dysfunction. Am J Respir Cell Mol Biol. 2017;57:391–2. https://doi.org/10.1165/rcmb.2017-0204ED.
Mesarwi OA, Moya EA, Zhen X, Gautane M, Zhao H, Wegbrans Giro P, Alshebli M, McCarley KE, Breen EC, Malhotra A. Hepatocyte HIF-1 and intermittent hypoxia independently impact liver fibrosis in murine NAFLD. Am J Respir Cell Mol Biol. 2021;4:390–402. https://doi.org/10.1165/rcmb.2020-0492OC.
Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41:918–21. https://doi.org/10.1097/01.mcg.0000225692.62121.55.
Mishra P, Nugent C, Afendy A, Bai C, Bhatia P, Afendy M, Fang Y, Elariny H, Goodman Z, Younossi ZM. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28:1080–6. https://doi.org/10.1111/j.1478-3231.2008.01822.x.
Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199:830–41. https://doi.org/10.1164/rccm.201806-1109TR.
Kim LJ, Pham LV, Polotsky VY. Sleep apnea, hypoxia inducible factor, and fatty liver: more questions than answers? Am J Respir Cell Mol Biol. 2021;65:337–8. https://doi.org/10.1165/rcmb.2021-0204ED.
Vallat R, Berry SE, Tsereteli N, Capdevila J, Khatib HA, Valdes AM, Delahanty LM, Drew DA, Chan AT, Wolf J, et al. How people wake up is associated with previous night's sleep together with physical activity and food intake. Nat Commun. 2022;13:7116. https://doi.org/10.1038/s41467-022-34503-2.
Singh P, Beyl RA, Stephens JM, Noland RC, Richard AJ, Boudreau A, Hebert RC, Ravussin E, Broussard JL, St-Onge MP, et al. Effect of sleep restriction on insulin sensitivity and energy metabolism in postmenopausal women: a randomized crossover trial. Obesity (Silver Spring). 2023;1:1. https://doi.org/10.1002/oby.23739.
Awad KM, Malhotra A, Barnet JH, Quan SF, Peppard PE. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125:485–90. https://doi.org/10.1016/j.amjmed.2011.11.025.
Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol. 2001;535:313–22. https://doi.org/10.1111/j.1469-7793.2001.t01-2-00313.x.
Iftikhar IH, Bittencourt L, Youngstedt SD, Ayas N, Cistulli P, Schwab R, Durkin MW, Magalang UJ. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7–14. https://doi.org/10.1016/j.sleep.2016.06.001.
Berge J, Hjelmesaeth J, Hertel JK, Gjevestad E, Smastuen MC, Johnson LK, Martins C, Andersen E, Helgerud J, Storen O. Effect of aerobic exercise intensity on energy expenditure and weight loss in severe obesity-a randomized controlled trial. Obesity (Silver Spring). 2021;29:359–69. https://doi.org/10.1002/oby.23078.
Park I, Diaz J, Matsumoto S, Iwayama K, Nabekura Y, Ogata H, Kayaba M, Aoyagi A, Yajima K, Satoh M, et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci Rep. 2021;11:4410. https://doi.org/10.1038/s41598-021-83817-6.
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. https://doi.org/10.1056/NEJMoa1200225.
Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310–9. https://doi.org/10.1038/ijo.2016.52.
Aaseth J, Ellefsen S, Alehagen U, Sundfor TM, Alexander J. Diets and drugs for weight loss and health in obesity - an update. Biomed Pharmacother. 2021;140:111789. https://doi.org/10.1016/j.biopha.2021.111789.
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. https://doi.org/10.1056/NEJMoa2206038.
Frias JP, Fernandez Lando L, Brown K. Tirzepatide versus semaglutide once weekly in type 2 diabetes. Reply. N Engl J Med. 2022;386:e17. https://doi.org/10.1056/NEJMc2114590.
Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, de Luca M, Faria SL, Goodpaster KPS, Haddad A, et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. 2023;33:3–14. https://doi.org/10.1007/s11695-022-06332-1.
Raphelson JR, Schmickl CN, Sonners C, Kreitinger K, Grunvald E, Horgan S, Malhotra A. Obesity hypoventilation syndrome and postsurgical outcomes in a bariatric surgery cohort. Obes Surg. 2022;32:1–7. https://doi.org/10.1007/s11695-022-06073-1.
Kreitinger KY, Lui MMS, Owens RL, Schmickl CN, Grunvald E, Horgan S, Raphelson JR, Malhotra A. Screening for obstructive sleep apnea in a diverse bariatric surgery population. Obesity (Silver Spring). 2020;28:2028–34. https://doi.org/10.1002/oby.23021.
Pillar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight increase 7.5 years after weight reduction surgery. Chest. 1994;106:1702–4.
Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–5.
Pepperell J, Ramdassingh-Dow S, Crosthwaite N, Mullins R, Jenkinson C, Stradling J, Davies R. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10.
Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest. 2018;153:843–50. https://doi.org/10.1016/j.chest.2017.11.005.
Jen R, Grandner MA, Malhotra A. Future of sleep-disordered breathing therapy using a mechanistic approach. Can J Cardiol. 2015;31:880–8. https://doi.org/10.1016/j.cjca.2015.02.007.
Malhotra A, Mesarwi O, Pepin JL, Owens RL. Endotypes and phenotypes in obstructive sleep apnea. Curr Opin Pulm Med. 2020;26:609–14. https://doi.org/10.1097/MCP.0000000000000724.
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. https://doi.org/10.1016/S0140-6736(05)71141-7.
Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. American Journal of Physiology. 1996;271:E1036–43.
O'Donnell CP. Metabolic consequences of intermittent hypoxia. Adv Exp Med Biol. 2007;618:41–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human/Animal Studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
Dr. Malhotra is funded by the NIH. He reports income related to medical education from Livanova, Eli Lilly, Zoll, and Jazz. ResMed provided a philanthropic donation to UCSD. All the other authors report no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurnool, S., McCowen, K.C., Bernstein, N.A. et al. Sleep Apnea, Obesity, and Diabetes — an Intertwined Trio. Curr Diab Rep 23, 165–171 (2023). https://doi.org/10.1007/s11892-023-01510-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-023-01510-6