Cardiac manifestations of MIS-C: cardiac magnetic resonance and speckle-tracking data
- 1Service de Pédiatrie, Hôpitaux Pédiatriques de Nice, CHU Lenval, Nice, France
- 2UR2CA, Faculté de Médecine, Equipe CARRES, Université Côte d’Azur, Nice, France
- 3Service de Radiologie, Centre Hospitalier Universitaire de Nice, Nice, France
- 4Faculté de Médecine, Université Côte d’Azur, Nice, France
- 5Service de Cardiologie, Centre Hospitalier Universitaire de Nice, Nice, France
Background: Cardiac involvement is central in MIS-C and represents the main cause of morbidity. In this study, we aimed to assess myocardial damage in patients with MIS-C using cardiac magnetic resonance (CMR) during the acute phase, as well as left ventricular and atrial longitudinal strain on admission, at discharge, and after 3 months.
Methods: We performed a single-center prospective cohort study and case–control study. Between September 2020 and February 2022, we enrolled 39 patients hospitalized for MIS-C at our center. We performed left ventricular and atrial longitudinal 2D strain analysis on admission and during follow-up; echocardiographic data were compared to a matched control population. Patients above 4 years old with increased troponin underwent CMR.
Results: Of 24 patients (mean age: 8.2 ± 4.9 years) who underwent CMR, 14 (58%) presented myocardial edema and 6 (25%) late gadolinium enhancement (LGE). LGE was associated with older age (p < 0.01), increased BMI (p = 0.03), increased ferritin levels (p < 0.001), lower left ventricular (LV) ejection fraction (p < 0.001), LV longitudinal strain (p = 0.004), left atrial (LA) strain (p = 0.05), and prolonged hospital stay (p = 0.02). On admission, LV ejection fraction, LV longitudinal strain, and LA strain were impaired, but each improved gradually over time; LVEF was the fastest to recover, while global LV longitudinal strain was still impaired as compared to controls after 3 months (p = 0.01).
Conclusion: Our study demonstrates that myocardial injury is present in a quarter of MIS-C patients, and impaired LA and LV myocardial deformation persist for at least several weeks after the acute phase. CMR and LV/LA strain could help us to individualize follow-up of MIS-C patients.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a rare but severe complication associated with SARS-CoV-2 infection. It has been reported since April 2020 and it occurs in approximately 0.03% of young people (<21 years old) with SARS-COV2 infection confirmed by nasopharyngeal RT-PCR or by antibody testing (1). It is characterized by a generalized hyperinflammatory response (2), involving the heart in 90% of cases (3–5). Cytokine storm has been observed in MIS-C with a delayed interferon response and slow viral clearance (6). Initial descriptions have pointed out clinical similarities with Kawasaki disease and toxic shock syndrome (7).
Cardiac injury is frequent in children with MIS-C; however, the cardiac phenotype varies hugely among our patients. These patients may require intensive care and inotropic support for several days. Furthermore, cardiac magnetic resonance (CMR) data characterizing the myocardial damage that occurs during the acute phase of MIS-C are lacking. The identification of myocarditis is of crucial importance given the related outcomes (although these are largely dependent on the etiology): approximately one-quarter of patients may present with persistent cardiac dysfunction on follow-up, and 12%–25% of patients may deteriorate and either progress to end-stage heart failure with the need for heart transplant or die. The presence or absence of myocardial injury also determines the restrictions on physical activity and specific cardiovascular follow-up after the acute phase of MIS-C. Thus, it is of critical importance to assess MIS-C patients for the presence or absence of tissue-characterized myocarditis.
On this basis, we aimed to describe the acute and mid-term clinical, CMR, and echocardiographic abnormalities observed in our MIS-C patient cohort.
Materials and methods
Study design and patients
We conducted a prospective observational case–control study including all children with MIS-C hospitalized at our tertiary hospital center (Hôpital pédiatrique universitaire CHU-Lenval) between September 2020 and February 2022. MIS-C was defined according to the CDC/WHO case definition (8, 9), as follows:
- An individual aged <21 years presenting with fever ≥38.0 C and evidence of clinically severe illness requiring hospitalization, with multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological) including severe cardiac illness (myocarditis, pericarditis, coronary artery dilatation, new onset left ventricular dysfunction, 2nd or 3rd degree atrio-ventricular block, or ventricular tachycardia) or rash and non-purulent conjunctivitis; and
- No alternative plausible diagnoses; and
- Laboratory evidence of inflammation and SARS-CoV-2 infection [based on reverse-transcriptase polymerase chain reaction (RT-PCR), serology, or antigen test].
Healthy controls were also enrolled to help determine normal values of 2D global longitudinal LV strain and LA strain. Healthy volunteers were recruited from the outpatient clinic to serve as controls; they were included if they had normal trans-thoracic echocardiography and were in sinus rhythm (children referred for functional heart murmur). They were matched to the study population on age and sex.
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the local research ethics committee. After the provision of written information, guardians' consent was obtained for each participant.
Data collection
Detailed demographic, clinical, and biological information was obtained from the participants' medical records and recorded in an anonymous database. Echocardiographic and CMR data were interpreted by two cardiologists and a radiologist specializing in cardiovascular disease.
Echocardiographic data
Echocardiography was performed using a Philips Affiniti 70 or EPIQ 7 ultrasound machine (Philips Medical Systems, Eindhoven, Netherlands) with an S9-2 or X5-1 probe. 2D imaging included the assessment of left ventricular (LV) segmental and global function: LV ejection fraction (EF), assessed using the Simpson method; presence or absence of segmental LV wall abnormalities; mitral regurgitation; right ventricular dysfunction; pericardial effusion; coronary artery dilatation; and perivascular brightness of the coronary arteries. Left ventricular strain was assessed using speckle-tracking echocardiography of the apical long axis, obtained from the apical four-, two- and three-chamber views, with a frame rate of 60–80/s. The analysis was either performed directly on the ultrasound machine or performed offline using commercially available semi-automated 2D strain software (QLAB, Philips Medical System) (Figure 1A). Left atrial strain was assessed using the apical 4-chamber view (QLAB, Philips Medical System) (Figure 1B). We recorded the peak longitudinal left atrial strain (reservoir strain).
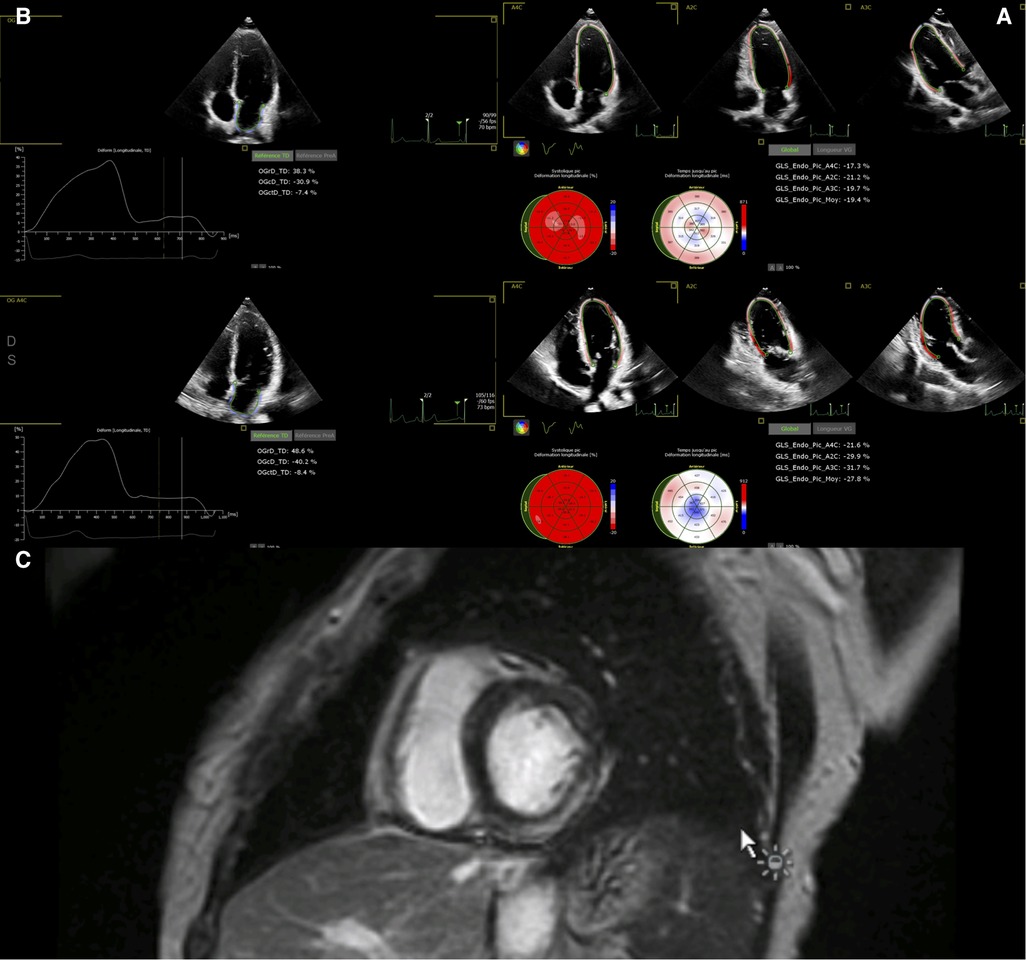
Figure 1. (A) Global left ventricular longitudinal strain in a 12-year-old boy before discharge (upper panel) and after 6 months. (B) Left atrial strain in the same 12-year-old boy before discharge (upper panel) and after 6 months. (C) Cardiac MRI in a 7-year-old boy 3 days after admission, showing late gadolinium enhancement within the inferior and lateral wall.
CMR data
Patient with a significant increase in troponin (or if their LV function was impaired and failed to improve after medical therapy) underwent CMR study.
Studies were performed on a General Electric Optima MR450 1.5 T CMR scanner. We used Lake Louise criteria (10) to determine the presence or absence of myocarditis. The acquisition protocol included conventional balanced steady state free precession cine images in ventricular 2-, 3-, and 4-chamber planes. Following these acquisitions, 0.1 mmol/kg of a gadolinium contrast agent (Dotarem) was administered and flushed with isotonic saline serum. LGE images in three long-axis and a stack of short-axis imaging planes were obtained with a breath-hold phase-sensitive inversion recovery sequence 5–15 min after the contrast injection. The presence and location of LGE were assessed visually (Figure 1C). End-diastolic and end-systolic endocardial borders were drawn semi-automatically for LV volumes and LVEF assessment. The imaging protocol included cine, STIR, and LGE imaging at standardized apical, mid-cavity, and basal short-axis levels. T1 mapping, T2 mapping, and extracellular volume are not available at our center. For purposes of simplicity, patients were classified according to the presence or absence of LGE or myocardial edema on CMR.
Statistical analysis
Data are summarized in the form mean ± standard deviation for continuous variables distributed normally, median [95% confidence interval] for other continuous variables, and number of subjects (%) for categorical variables. Comparisons between patients with and without LGE were performed using the Student's t-test for normally distributed variables or using the Wilcoxon test otherwise. Categorical variables were compared using Fisher's exact test. These analyses were performed using R++ version 1.5.08 (Paris, France). In all analyses, statistical significance was defined as a p-value <0.05.
Results
Demographics and clinical presentation
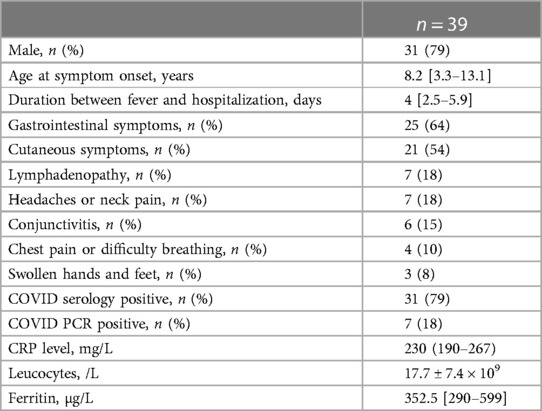
Between September 2020 and February 2022, we enrolled 39 patients with MIS-C. Patient characteristics are presented in Table 1. Our population consisted of a majority of boys (31, 79%); the mean age was 8.2 ± 4.9 years. Most patients had previously been healthy, with the exception of one girl with a history of junctional tachycardia and one boy with Brugada pattern on ECG (which was discovered during the episode of fever related to MIS-C).
All patients presented with persistent fever (median duration before admission: 4 days [2.5–5.9]). Twenty-five patients (64%) had gastrointestinal symptoms (diarrhea, vomiting, or abdominal pain) and 21 (54%) had cutaneous (skin rash) or mucous membrane involvement (red or swollen lips). Less frequently, patients presented with lymphadenopathy (n = 7, 18%); seven patients (18%) suffered from either headaches or neck pain; six (15%) had conjunctivitis; four (10%) presented with chest pain or difficulty breathing; and three (8%) had swollen hands and feet.
All patients presented a marked inflammatory state: their median CRP level was 230 [190–267] mg/L, mean number of leukocytes was 17.7 ± 7.4 × 109/L, and median ferritin level was 352.5 [290–599] µg/L. Previous SARS-CoV-2 infection was confirmed by positive RT-PCR in 20.5% of patients (n = 8) and by positive IgG antibody against SARS-CoV-2 in 79% (n = 31).
Cardiovascular presentation
Nine patients (23%) had a pathologic electrocardiogram (ECG) during the course of their hospital stay: eight presented a first-degree atrio-ventricular block (the PR interval was monitored during the hospital stay, and prolongation appeared after the 3rd day of evolution in five patients), whereas two patients (5%) presented with ST segment abnormalities. No sustained arrythmia was observed.
Three patients (8%) did not present any cardiac involvement; eight (21%) had sub-clinical cardiac injury; and six patients (15%) were admitted with Kawasaki-like presentation (either coronary dilatation or coronary perivascular brightness). Eight patients (21%) presented with clinical pericarditis, whereas seven (18%) had evidence of severe heart failure. “Kawasaki-like” patients were younger than others (4.4 [1.5–8.4] vs. 9.1 [7.6–13.2] years old; p = 0.03).
Cardiac biomarkers (BNP and/or troponin) were elevated in 31 patients (79%). Median BNP measurement was 814 [666–1,306] pg/ml, whereas median troponin level was 500 [341–1,833] ng/L. Maximum troponin level was 8,752 ng/L, while maximum BNP dosage was 4,145 pg/ml. In 10 patients, BNP was elevated but normal troponin levels were observed, while only one patient had increased troponin but normal BNP.
Echocardiographic findings on admission
Echocardiographic findings on admission, at discharge, and at 3-month follow-up are presented in Table 2.
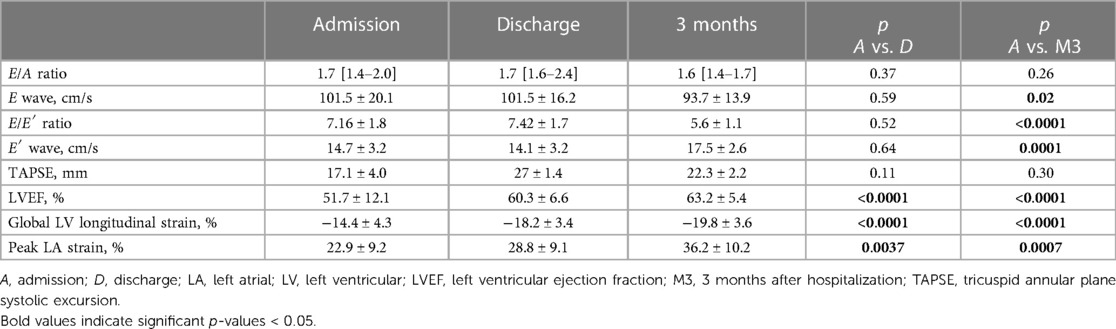
Table 2. Echocardiographic characteristics of MIS-C patients on admission, at discharge, and at 3-month follow-up.
Mean LV ejection fraction on admission was 49 ± 11.8%: seven patients (18%) presented with severe LV dysfunction (LVEF ≤ 35%), and 14 (36%) with moderately impaired LV function (LVEF between 36% and 54%). Ten patients presented with evidence of segmental LV wall motion abnormality, especially within the anterior and septal wall. One patient had severe biventricular dysfunction. LV ejection fraction significantly improved at discharge (p < 0.001) and at 3-month follow-up (p < 0.001), while E/E' ratio decreased slightly at 3-month follow-up. Seventeen patients (44%) had evidence of significant but mild mitral regurgitation on admission, and nine patients (23%) had pericardial effusion. Seven patients (18%) showed evidence of coronary involvement (either dilatation or perivascular brightness of the coronary arteries).
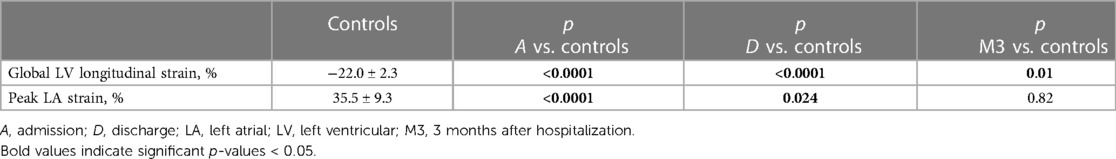
Mean global longitudinal LV strain improved significantly from admission (−14.4 ± 4.3%) to discharge (−18.2 ± 3.4%; p < 0.001) and 3-month follow-up (−19.8 ± 3.6; p < 0.001). Mean LA peak strain on admission was also improved at discharge and after 3 months (p < 0.001). As compared to controls (Table 3), global longitudinal LV strain was significantly impaired in MIS-C patients on admission and at discharge, and also at 3-month follow-up (p < 0.001, p < 0.001, and p = 0.01, respectively). Left atrial reservoir strain was impaired in MIS-C patients on admission (p < 0.001) and at discharge (p = 0.02), but returned to normal levels at 3-month follow-up (p = 0.82).
CMR findings
CMR was performed in 24 patients after a median delay of 6 days [4.3–10.3] after onset of symptoms. Myocardial edema was found in 14 patients (58%). LGE was identified in six patients (25%), among whom five patients presented with severe heart failure (71% of severe heart failure patients). LGE+ was more frequently observed in patients with heart failure.
Patients with LGE were older (LGE-: 8.9 [7.3–13.5] vs. LGE+: 14.1 [9.9–15.7]; p = 0.0003) and had higher body mass index (p = 0.03) as compared to patients without LGE. Patients with LGE also had lower LVEF (p = 0.004), decreased global LV longitudinal strain (p = 0.004), and left atrial reservoir strain (p = 0.05) (Figure 1).
There was no difference in peak CRP (p = 0.23) or leukocyte count (p = 0.94) according to the presence of LGE, but higher levels of ferritin (LGE-: 534.0 [315.2–1,000.0] vs. LGE+: 2,323.0 [1,394.5–3,345.5]; p = 0.007) were observed in patients with LGE. No difference in BNP or troponin was observed (for BNP, LGE-: 834.5 [427.9–1,472.5] vs. LGE+: 1,185.5 [442.9–2,027.1], p = 0.41; for troponin, LGE-: 109.0 [69.2–1,200.3] vs. LGE+: 1,495.0 [170.4-7,503.2], p = 0.10) (Figure 2). Patients with LGE were more likely to have a prolonged hospital stay (p = 0.02).
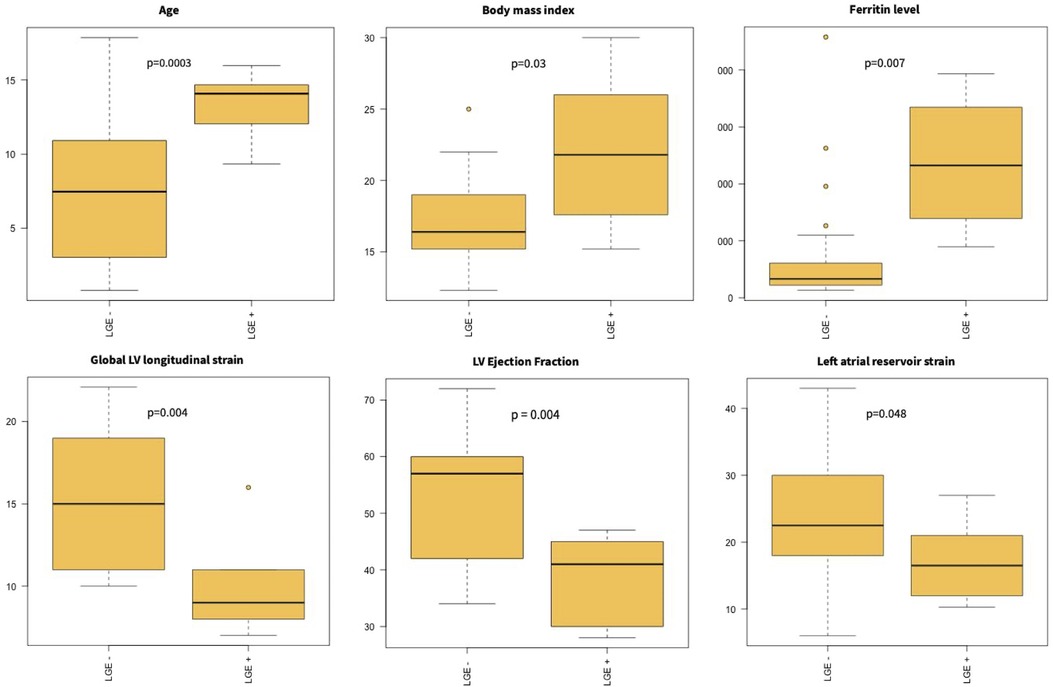
Figure 2. Myocardial injury in MIS-C. Patients with LGE were older and had higher BMI. They had higher ferritin levels and lower left ventricular ejection fraction and longitudinal strain on admission.
Clinical evolution
The most common treatments administered during the acute phase included aspirin in 38 patients (97.4%), low-molecular-weight heparin anticoagulation in 10 (25.6%), intravenous immunoglobulin in 38 (97.4%), intravenous corticosteroids in 36 (92.3%), and anakinra in 1 (2.6%). Eighteen patients (46%) required PICU admission, 14 of these (36%) requiring vasopressor or inotrope support: 5 patients (13%) required norepinephrine infusion, and dobutamine was required in 14 (36%).
All patients had recovered normal LVEF upon discharge and all survived after a median follow-up period of 7.5 months [4.5–9.7]. Three months after discharge from hospital, MIS-C patients did not differ significantly from controls in terms of LA strain, but global LV longitudinal strain was still impaired (p = 0.01).
Patients with LGE on initial CMR underwent an exercise test and a control CMR 6 months after MIS-C. All six of these patients had a normal control CMR with no residual LGE or myocardial edema, as well as a normal exercise test.
Discussion
Our study demonstrates the presence of tissue-characterized myocarditis with LGE in one-quarter of our MIS-C patient cohort who underwent CMR within the first month after hospitalization. LGE occurred more frequently in older children and was associated with higher BMI, higher levels of ferritin, lower LVEF, lower LV global longitudinal, and LA strain. Myocardial injury on CMR was associated with a prolonged hospital stay. Three months after discharge, LV global longitudinal strain was still impaired as compared to a control population, while other echocardiographic parameters had normalized.
Our findings are supported by recent studies (11, 12) showing evidence of CMR myocarditis in approximately 16%–35% of MIS-C patients. Studies investigating the presence of LGE during the acute phase of MIS-C are scarce, and preliminary studies have not identified LGE in MIS-C patients (13, 14). LGE has probably been underestimated in those studies as a result of the delay between hospitalization and MRI (2–3 months), as compared to the CARDOVID cohort (11), where the median delay for CMR was 28 days, and our study (6 days). LGE findings on CMR reflected myocardial injury. As LGE was not related to LV ejection fraction, myocardial injury could be found even in the presence of preserved or mildly reduced LV systolic function. LGE can suggest active inflammation but is less effective in detecting “borderline myocarditis” (15). This underlines the importance of CMR within a reasonable time delay in order to detect LGE, as the extent of LGE will decrease over time, explaining the number of false negatives obtained when performing an MRI late after the onset of MIS-C. Indeed, children with LGE should probably undergo strict cardiovascular follow-up given the potential risk of myocardial scarring and fibrosis, which might lead to arrythmia. Residual myocardial damage 6-9 months after MIS-C, as assessed by CMR, appears to be minimal according to a recent study (16), supporting the role of early MRI in detecting high-risk patients. In contrast, the American College of Rheumatology Clinical Guidance for MIS-C recommends cardiac MRI 2 to 6 months after MIS-C diagnosis in patients with LV ejection fraction <50% during the acute phase of illness (17). Our data and previous studies suggest that LGE occurs earlier than 2 months and is not related to LV systolic dysfunction. CMR is helpful to determine the presence and extent of myocardial inflammation, suggested typically by LGE. In the absence of LGE, after complete recovery from MIS-C, patients should not be advised against exercise, especially given that the prevalence of overweight in MIS-C is 25% (7).
Given the overall excellent cardiovascular outcome of MIS-C, the presence of LGE on CMR could help us detect children with a higher risk of cardiovascular complications. This should also be borne in mind when considering the resumption of sporting activity in school-aged patients. Indeed, myocarditis is a risk factor for sudden cardiac death in athletes (18–20). Both the European Society of Cardiology (21) and the American Heart Association (22) recommend abstinence from moderate- to high-intensity exercise for a period of 3–6 months after myocarditis. The timing for return to sport could be guided by CMR LGE, underlining its importance in detecting active inflammation.
The cardiovascular presentation of MIS-C varies widely from sub-clinical cardiac injury to myocarditis with severe heart failure. We report here that MIS-C with coronary artery dilatation or perivascular brightness is related to younger age at presentation. This supports low-dose aspirin treatment for at least 6 weeks (23) in young patients with Kawasaki-like presentation, as well as appropriate follow-up using coronary Z-scores. Kawasaki syndrome and MIS-C might belong to the common spectrum of pathogen-triggered hyperinflammatory state and share a common presentation in young children, whereas older children mostly have a clinical presentation involving heart failure, myocarditis, and gastrointestinal symptoms. On another hand, overweight has been found to be associated with the occurrence of MIS-C (7, 24), and in our study, BMI was related to the presence of LGE on MRI in MIS-C. This might explain the potentially poorer prognosis of MIS-C in obese children (25, 26).
Our study confirms the overall excellent prognosis of MIS-C. All patients were discharged alive, and LV ejection fraction had recovered after 3 months in all cases, even following severe cardiogenic shock. However, although we observed improvement in LV and LA strain over time, global LV longitudinal strain was still impaired 3 months post-discharge as compared to controls, suggesting subclinical myocardial dysfunction. We thus confirm that global LV longitudinal strain is last to normalize in MIS-C (12). The decrease in LA strain, although this improves over time, might suggest the presence of subtle diastolic dysfunction and may prompt prolonged follow-up in those children, especially in those with risk factors such as obesity.
Limitations
This was a single-center study, thus limiting the scope for extrapolation from our data. However, the early CMR scanning conducted in our patients increases the robustness of our data. Although LGE provides information on myocardial inflammation and necrosis status, this does not offer any clues regarding the pathogenesis of MIS-C. Our data were collected mostly during the outbreaks of the alpha, delta, and omicron variants of COVID. Whether MIS-C related to future variants will lead to the same conclusions remains unknown.
Conclusion
MIS-C physiopathology remains unclear. The possible presence of myocarditis with myocardial injury prompts us to make the generalization that early CMR scanning should be performed in MIS-C patients whenever possible. Standard and speckle-tracking echocardiography are especially useful for diagnosis but also for follow-up. Stratifying cardiac risk in MIS-C patients using CMR and echocardiography will help us to individualize follow-up and probably avoid potential cardiac complications in this young population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by IRB CHU de NICE—local ethics committee and CCPPRB for the studies involving humans because this was an observational study without any intervention, we were reporting our standard of care in MIS-C. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LS: Data curation, Investigation, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. JD: Formal Analysis, Investigation, Project administration, Software, Writing – review & editing. DD: Investigation, Writing – review & editing. JB: Conceptualization, Investigation, Supervision, Writing – review & editing. MA: Investigation, Supervision, Writing – review & editing. AD: Investigation, Project administration, Writing – review & editing. SB: Investigation, Project administration, Writing – review & editing. EG: Investigation, Writing – review & editing. JL: Visualization, Writing – review & editing. LG: Data curation, Formal Analysis, Investigation, Supervision, Writing – review & editing. PM: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. (2021) 4:e2116420. doi: 10.1001/jamanetworkopen.2021.16420
2. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
3. Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. (2020) 142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360
4. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. Br Med J. (2020) 370:m3249. doi: 10.1136/bmj.m3249
5. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. (2021) 325:1074–87. doi: 10.1001/jama.2021.2091
6. Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. (2020) 20:453–4. doi: 10.1038/s41577-020-0367-5
7. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. (2021) 180:2019–34. doi: 10.1007/s00431-021-03993-5
8. Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2021) 39:3037–49. doi: 10.1016/j.vaccine.2021.01.054
9. US Centers for Disease Control and Prevention Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) (2020). Available at: https://emergency.cdc.gov/han/2020/han00432.asp
10. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. (2018) 72:3158–76. doi: 10.1016/j.jacc.2018.09.072
11. Aeschlimann FA, Misra N, Hussein T, Panaioli E, Soslow JH, Crum K, et al. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J Cardiovasc Magn Reson. (2021) 23:140. doi: 10.1186/s12968-021-00841-1
12. Sirico D, Basso A, Sabatino J, Reffo E, Cavaliere A, Biffanti R, et al. Evolution of echocardiographic and cardiac magnetic resonance imaging abnormalities during follow-up in patients with multisystem inflammatory syndrome in children. Eur Heart J Cardiovasc Imaging. (2022) 23:1066–74. doi: 10.1093/ehjci/jeac096
13. Bartoszek M, Małek ŁA, Barczuk-Falęcka M, Brzewski M. Cardiac magnetic resonance follow-up of children after pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 with initial cardiac involvement. J Magn Reson Imaging. (2022) 55:883–91. doi: 10.1002/jmri.27870
14. Webster G, Patel AB, Carr MR, Rigsby CK, Rychlik K, Rowley AH, et al. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. (2021) 23:86. doi: 10.1186/s12968-021-00786-5
15. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. (2009) 53:1475–87. doi: 10.1016/j.jacc.2009.02.007
16. DiLorenzo MP, Farooqi KM, Shah AM, Channing A, Harrington JK, Connors TJ, et al. Ventricular function and tissue characterization by cardiac magnetic resonance imaging following hospitalization for multisystem inflammatory syndrome in children: a prospective study. Pediatr Radiol. (2023) 53:394–403. doi: 10.1007/s00247-022-05521-5
17. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. (2021) 73:e13–29. doi: 10.1002/art.41616
18. Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. (2015) 132:10–9. doi: 10.1161/CIRCULATIONAHA.115.015431
19. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. (2009) 119:1085–92. doi: 10.1161/CIRCULATIONAHA.108.804617
20. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:2636–48, (2648a–2648d). doi: 10.1093/eurheartj/eht210
21. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42:17–96. doi: 10.1093/eurheartj/ehaa605
22. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American heart association and American college of cardiology. J Am Coll Cardiol. (2015) 66:2356–61. doi: 10.1016/j.jacc.2015.09.034
23. Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national delphi process. Lancet Child Adolesc Health. (2021) 5:133–41. doi: 10.1016/S2352-4642(20)30304-7
24. Rhedin S, Lundholm C, Horne A, Smew AI, Osvald EC, Haddadi A, et al. Risk factors for multisystem inflammatory syndrome in children—a population-based cohort study of over 2 million children. Lancet Reg Health Eur. (2022) 19:100443. doi: 10.1016/j.lanepe.2022.100443
25. Gawlik AM, Berdej-Szczot E, Chmiel I, Lorek M, Antosz A, Firek-Pedras M, et al. A tendency to worse course of multisystem inflammatory syndrome in children with obesity: multiOrgan inflammatory syndromes COVID-19 related study. Front Endocrinol (Lausanne). (2022) 13:934373. doi: 10.3389/fendo.2022.934373
Keywords: COVID-19, multisystem inflammatory syndrome in children (MIS-C), myocarditis, cardiac magnetic resonance, speckle-tracking imaging, cardiac injury
Citation: Scarduelli L, De Guillebon De Resnes J-M, Ducreux D, Bernardor J, Afanetti M, Dupont A, Barthelemy S, Gondon E, Leporati J, Giovannini-Chami L and Moceri P (2023) Cardiac manifestations of MIS-C: cardiac magnetic resonance and speckle-tracking data. Front. Cardiovasc. Med. 10:1288176. doi: 10.3389/fcvm.2023.1288176
Received: 3 September 2023; Accepted: 9 October 2023;
Published: 6 November 2023.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Lilia Oreto, Mediterranean Pediatric Cardiology Center, ItalyAhmet Sert, Selçuk Üniversitesi Tıp Fakültesi Hastanesi, Türkiye
© 2023 Scarduelli, De Guillebon De Resnes, Ducreux, Bernardor, Afanetti, Dupont, Barthelemy, Gondon, Leporati, Giovannini-Chami and Moceri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pamela Moceri moceri.p@chu-nice.fr
Abbreviations 2D, two-dimensional; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; CRP, C-reactive protein; EF, ejection fraction; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; MIS-C, multisystem inflammatory syndrome in children; PICU, pediatric intensive care unit; RT-PCR, reverse transcriptase polymerase chain reaction.
†ORCID Pamela Moceri orcid.org/0000-0002-0741-1609
 Lorenzo Scarduelli1,2
Lorenzo Scarduelli1,2  Julie Bernardor
Julie Bernardor Sébastien Barthelemy
Sébastien Barthelemy Pamela Moceri
Pamela Moceri