Recommendations for the Management of Cardiomyopathy Mutation Carriers: Evidence, Doubts, and Intentions
Abstract
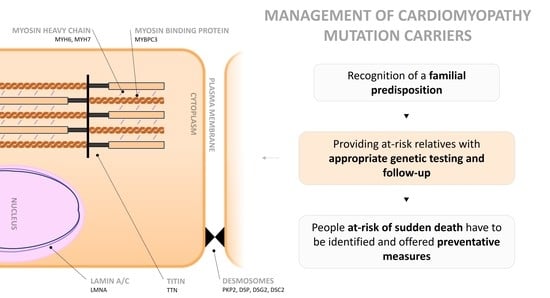
:1. Introduction
2. Methods
3. Hypertrophic Cardiomyopathy
3.1. Prevalence
3.2. Associated Genes
3.3. Diagnosis and Disease Mechanism
4. Dilated Cardiomyopathy
4.1. Prevalence
4.2. Associated Genes
4.3. Diagnosis and Disease Mechanism
5. Arrhythmogenic Cardiomyopathy
5.1. Prevalence
5.2. Associated Genes
5.3. Diagnosis and Disease Mechanism
6. Left Ventricular Noncompaction
6.1. Prevalence
6.2. Associated Genes
6.3. Diagnosis and Disease Mechanism
7. Genetic Testing
8. Baseline Evaluation, Follow-Up, and Management
9. Pregnancy
10. Physical Activity and Athletes’ Recommendations
11. Sudden Death
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Pereira, N.L. Genetics of Cardiomyopathy: Clinical and Mechanistic Implications for Heart Failure. Korean Circ. J. 2021, 51, 797–836. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Weiss, J.; Debus, J.D.; Stanasiuk, C.; Klauke, B.; Deutsch, M.A.; Fox, H.; Bax, J.; Ebbinghaus, H.; Gärtner, A.; et al. A homozygous DSC2 deletion associated with arrhythmogenic cardiomyopathy is caused by uniparental isodisomy. J. Mol. Cell. Cardiol. 2020, 141, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Rehm, H.L.; Menesses, A.; McDonough, B.; Roberts, A.E.; Kucherlapati, R.; Towbin, J.A.; Seidman, J.G.; Seidman, C.E. Shared genetic causes of cardiac hypertrophy in children and adults. N. Engl. J. Med. 2008, 358, 1899–1908. [Google Scholar] [CrossRef]
- Kaski, J.P.; Syrris, P.; Burch, M.; Tomé-Esteban, M.T.; Fenton, M.; Christiansen, M.; Andersen, P.S.; Sebire, N.; Ashworth, M.; Deanfield, J.E.; et al. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart 2008, 94, 1478–1484. [Google Scholar] [CrossRef]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 2003, 107, 2227–2232. [Google Scholar] [CrossRef]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef]
- Maron, B.J.; Carney, K.P.; Lever, H.M.; Lewis, J.F.; Barac, I.; Casey, S.A.; Sherrid, M.V. Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003, 41, 974–980. [Google Scholar] [CrossRef]
- Olivotto, I.; Maron, M.S.; Adabag, A.S.; Casey, S.A.; Vargiu, D.; Link, M.S.; Udelson, J.E.; Cecchi, F.; Maron, B.J. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005, 46, 480–487. [Google Scholar] [CrossRef]
- Judge, D.P.; Johnson, N.M. Genetic evaluation of familial cardiomyopathy. J. Cardiovasc. Transl. Res. 2008, 1, 144–154. [Google Scholar] [CrossRef]
- Ingles, J.; Bagnall, R.D.; Semsarian, C. Genetic Testing for Cardiomyopathies in Clinical Practice. Heart Fail. Clin. 2018, 14, 129–137. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Varma, P.K.; Neema, P.K. Hypertrophic cardiomyopathy: Part 1—Introduction, pathology and pathophysiology. Ann. Card. Anaesth. 2014, 17, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients with Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e558–e631. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, Epidemiology, and Global Burden of Cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- McCrohon, J.A.; Moon, J.C.; Prasad, S.K.; McKenna, W.J.; Lorenz, C.H.; Coats, A.J.; Pennell, D.J. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 2003, 108, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Shaw, L.K.; O’Connor, C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J. Am. Coll. Cardiol. 2002, 39, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.; Charron, P.; Gimeno-Blanes, J.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: Bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: The complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef]
- Taylor, M.R.; Slavov, D.; Ku, L.; Di Lenarda, A.; Sinagra, G.; Carniel, E.; Haubold, K.; Boucek, M.M.; Ferguson, D.; Graw, S.L.; et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation 2007, 115, 1244–1251. [Google Scholar] [CrossRef]
- Koelemen, J.; Gotthardt, M.; Steinmetz, L.M.; Meder, B. RBM20-Related Cardiomyopathy: Current Understanding and Future Options. J. Clin. Med. 2021, 10, 4101. [Google Scholar] [CrossRef]
- Gaertner, A.; Bloebaum, J.; Brodehl, A.; Klauke, B.; Sielemann, K.; Kassner, A.; Fox, H.; Morshuis, M.; Tiesmeier, J.; Schulz, U.; et al. The Combined Human Genotype of Truncating TTN and RBM20 Mutations Is Associated with Severe and Early Onset of Dilated Cardiomyopathy. Genes 2021, 12, 883. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Basso, C.; Corrado, D.; Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009, 373, 1289–1300. [Google Scholar] [CrossRef]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [CrossRef]
- Peters, S.; Trümmel, M.; Meyners, W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int. J. Cardiol. 2004, 97, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Boogerd, C.J.; Lacraz, G.P.A.; Vértesy, Á.; van Kampen, S.J.; Perini, I.; de Ruiter, H.; Versteeg, D.; Brodehl, A.; van der Kraak, P.; Giacca, M.; et al. Spatial transcriptomics unveils ZBTB11 as a regulator of cardiomyocyte degeneration in arrhythmogenic cardiomyopathy. Cardiovasc. Res. 2023, 119, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Zorio, E.; Jimenez-Jaimez, J.; Salguero-Bodes, R.; Zwart, R.; Gonzalez-Lopez, E.; Molina, P.; Bermúdez-Jiménez, F.; Delgado, J.F.; Braza-Boïls, A.; et al. Clinical characteristics and determinants of the phenotype in TMEM43 arrhythmogenic right ventricular cardiomyopathy type 5. Heart Rhythm. 2020, 17, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.; Seewald, A.; Rohrbach, M.; Brodehl, A.; Liedtke, D.; Williams, T.; Childs, S.J.; Gerull, B. Altered Expression of TMEM43 Causes Abnormal Cardiac Structure and Function in Zebrafish. Int. J. Mol. Sci. 2022, 23, 9530. [Google Scholar] [CrossRef]
- Brodehl, A.; Rezazadeh, S.; Williams, T.; Munsie, N.M.; Liedtke, D.; Oh, T.; Ferrier, R.; Shen, Y.; Jones, S.J.M.; Stiegler, A.L.; et al. Mutations in ILK, encoding integrin-linked kinase, are associated with arrhythmogenic cardiomyopathy. Transl. Res. J. Lab. Clin. Med. 2019, 208, 15–29. [Google Scholar] [CrossRef]
- Klauke, B.; Kossmann, S.; Gaertner, A.; Brand, K.; Stork, I.; Brodehl, A.; Dieding, M.; Walhorn, V.; Anselmetti, D.; Gerdes, D.; et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum. Mol. Genet. 2010, 19, 4595–4607. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef]
- Bosman, L.P.; Cadrin-Tourigny, J.; Bourfiss, M.; Aliyari Ghasabeh, M.; Sharma, A.; Tichnell, C.; Roudijk, R.W.; Murray, B.; Tandri, H.; Khairy, P.; et al. Diagnosing arrhythmogenic right ventricular cardiomyopathy by 2010 Task Force Criteria: Clinical performance and simplified practical implementation. EP Eur. 2020, 22, 787–796. [Google Scholar] [CrossRef]
- Bennett, C.E.; Freudenberger, R. The Current Approach to Diagnosis and Management of Left Ventricular Noncompaction Cardiomyopathy: Review of the Literature. Cardiol. Res. Pract. 2016, 2016, 5172308. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Heart J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Carrilho-Ferreira, P.; Almeida, A.G.; Pinto, F.J. Non-compaction cardiomyopathy: Prevalence, prognosis, pathoetiology, genetics, and risk of cardioembolism. Curr. Heart Fail. Rep. 2014, 11, 393–403. [Google Scholar] [CrossRef]
- Bhatia, N.L.; Tajik, A.J.; Wilansky, S.; Steidley, D.E.; Mookadam, F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J. Card. Fail. 2011, 17, 771–778. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011, 8, 1308–1339. [Google Scholar] [CrossRef]
- Stöllberger, C.; Blazek, G.; Wegner, C.; Winkler-Dworak, M.; Finsterer, J. Neuromuscular and cardiac comorbidity determines survival in 140 patients with left ventricular hypertrabeculation/noncompaction. Int. J. Cardiol. 2011, 150, 71–74. [Google Scholar] [CrossRef]
- Kulikova, O.; Brodehl, A.; Kiseleva, A.; Myasnikov, R.; Meshkov, A.; Stanasiuk, C.; Gärtner, A.; Divashuk, M.; Sotnikova, E.; Koretskiy, S.; et al. The Desmin (DES) Mutation p.A337P Is Associated with Left-Ventricular Non-Compaction Cardiomyopathy. Genes 2021, 12, 121. [Google Scholar] [CrossRef]
- Toste, A.; Branco, L.M.; Galrinho, A.; Lousinha, A.; Fiarresga, A.; Oliveira, M.M.; Abreu, J.; Mendes, J.J.; Ferreira, L.; Leal, A.; et al. Noncompaction cardiomyopathy—A review of eight cases. Port. J. Cardiol. 2010, 29, 1847–1864. [Google Scholar]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.C.S.; do Rêgo Aquino, P.L.; de Souza Silva, G.; Fabro, C.B. Left Ventricular Noncompaction: New Insights into a Poorly Understood Disease. Curr. Cardiol. Rev. 2021, 17, 209–216. [Google Scholar] [CrossRef]
- Vogiatzi, G.; Lazaros, G.; Oikonomou, E.; Lazarou, E.; Vavuranakis, E.; Tousoulis, D. Role of genetic testing in cardiomyopathies: A primer for cardiologists. World J. Cardiol. 2022, 14, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.G.; Vatta, M.; Ware, S.M. Genetic Evaluation of Cardiomyopathy-A Heart Failure Society of America Practice Guideline. J. Card. Fail. 2018, 24, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J.; Doolan, A.; Chiu, C.; Seidman, J.; Seidman, C.; Semsarian, C. Compound and double mutations in patients with hypertrophic cardiomyopathy: Implications for genetic testing and counselling. J. Med. Genet. 2005, 42, e59. [Google Scholar] [CrossRef]
- Pugh, T.J.; Kelly, M.A.; Gowrisankar, S.; Hynes, E.; Seidman, M.A.; Baxter, S.M.; Bowser, M.; Harrison, B.; Aaron, D.; Mahanta, L.M.; et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet. Med. 2014, 16, 601–608. [Google Scholar] [CrossRef]
- Lakdawala, N.K.; Funke, B.H.; Baxter, S.; Cirino, A.L.; Roberts, A.E.; Judge, D.P.; Johnson, N.; Mendelsohn, N.J.; Morel, C.; Care, M.; et al. Genetic testing for dilated cardiomyopathy in clinical practice. J. Card. Fail. 2012, 18, 296–303. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Kelly, M.A.; Rehm, H.L.; Lakdawala, N.K.; Funke, B.H. Inherited cardiomyopathies: Molecular genetics and clinical genetic testing in the postgenomic era. J. Mol. Diagn. 2013, 15, 158–170. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Semsarian, C.; Márquez, M.F.; Shamloo, A.S.; Ackerman, M.J.; Ashley, E.A.; Sternick, E.B.; Barajas-Martinez, H.; Behr, E.R.; Bezzina, C.R.; et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. EP Eur. 2022, 24, 1307–1367. [Google Scholar] [CrossRef]
- Colucci, W.S.; Kolias, T.J.; Adams, K.F.; Armstrong, W.F.; Ghali, J.K.; Gottlieb, S.S.; Greenberg, B.; Klibaner, M.I.; Kukin, M.L.; Sugg, J.E. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: The REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation 2007, 116, 49–56. [Google Scholar] [CrossRef]
- Yusuf, S.; Pitt, B.; Davis, C.E.; Hood, W.B., Jr.; Cohn, J.N. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 1992, 327, 685–691. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Cowan, J.; Morales, A.; Siegfried, J.D. Progress with genetic cardiomyopathies: Screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. Heart Fail. 2009, 2, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.M.; Gibbs, J.S.; Gohlke-Baerwolf, C.; Gorenek, B.; Iung, B.; et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 3147–3197. [Google Scholar] [CrossRef] [PubMed]
- Van Tintelen, J.P.; Pieper, P.G.; Van Spaendonck-Zwarts, K.Y.; Van Den Berg, M.P. Pregnancy, cardiomyopathies, and genetics. Cardiovasc. Res. 2014, 101, 571–578. [Google Scholar] [CrossRef]
- Jastrow, N.; Meyer, P.; Khairy, P.; Mercier, L.A.; Dore, A.; Marcotte, F.; Leduc, L. Prediction of complications in pregnant women with cardiac diseases referred to a tertiary center. Int. J. Cardiol. 2011, 151, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Sermer, M.; Colman, J.M.; Alvarez, A.N.; Mercier, L.A.; Morton, B.C.; Kells, C.M.; Bergin, M.L.; Kiess, M.C.; Marcotte, F.; et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 2001, 104, 515–521. [Google Scholar] [CrossRef]
- Autore, C.; Conte, M.R.; Piccininno, M.; Bernabò, P.; Bonfiglio, G.; Bruzzi, P.; Spirito, P. Risk associated with pregnancy in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 1864–1869. [Google Scholar] [CrossRef]
- Blatt, A.; Svirski, R.; Morawsky, G.; Uriel, N.; Neeman, O.; Sherman, D.; Vered, Z.; Krakover, R. Short and long-term outcome of pregnant women with preexisting dilated cardiomypathy: An NTproBNP and echocardiography-guided study. Isr. Med. Assoc. J. 2010, 12, 613–616. [Google Scholar]
- Castrini, A.I.; Skjølsvik, E.; Estensen, M.E.; Almaas, V.M.; Skulstad, H.; Lyseggen, E.; Edvardsen, T.; Lie, H.; Picard, K.C.I.; Lakdawala, N.K.; et al. Pregnancy and Progression of Cardiomyopathy in Women with LMNA Genotype-Positive. J. Am. Heart Assoc. 2022, 11, e024960. [Google Scholar] [CrossRef]
- Krul, S.P.; van der Smagt, J.J.; van den Berg, M.P.; Sollie, K.M.; Pieper, P.G.; van Spaendonck-Zwarts, K.Y. Systematic review of pregnancy in women with inherited cardiomyopathies. Eur. J. Heart Fail. 2011, 13, 584–594. [Google Scholar] [CrossRef]
- Ghosh, N.; Haddad, H. Recent progress in the genetics of cardiomyopathy and its role in the clinical evaluation of patients with cardiomyopathy. Curr. Opin. Cardiol. 2011, 26, 155–164. [Google Scholar] [CrossRef]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef]
- Maron, B.J.; Chaitman, B.R.; Ackerman, M.J.; Bayés de Luna, A.; Corrado, D.; Crosson, J.E.; Deal, B.J.; Driscoll, D.J.; Estes, N.A., 3rd; Araújo, C.G.; et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation 2004, 109, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Olshansky, B.; Heidbuchel, H.; Lawless, C.; Saarel, E.; Ackerman, M.; Calkins, H.; Estes, N.A.M.; Link, M.S.; Maron, B.J.; et al. Safety of Sports for Athletes with Implantable Cardioverter-Defibrillators: Long-Term Results of a Prospective Multinational Registry. Circulation 2017, 135, 2310–2312. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Haland, T.F.; Lie, O.H.; Ribe, M.; Bjune, T.; Leren, I.S.; Berge, K.E.; Edvardsen, T.; Haugaa, K.H. Vigorous exercise in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2018, 250, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Udelson, J.E.; Bonow, R.O.; Nishimura, R.A.; Ackerman, M.J.; Estes, N.A., 3rd; Cooper, L.T., Jr.; Link, M.S.; Maron, M.S. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation 2015, 132, e273–e280. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Myerburg, R.J.; Junttila, M.J. Sudden cardiac death caused by coronary heart disease. Circulation 2012, 125, 1043–1052. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Lesser, J.R.; Garberich, R.F.; McGriff, D.M.; Maron, M.S. Hypertrophic Cardiomyopathy in Children, Adolescents, and Young Adults Associated with Low Cardiovascular Mortality with Contemporary Management Strategies. Circulation 2016, 133, 62–73. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Atteya, G.; Lampert, R. Sudden Cardiac Death in Genetic Cardiomyopathies. Card. Electrophysiol. Clin. 2017, 9, 581–603. [Google Scholar] [CrossRef]
- Dec, G.W.; Fuster, V. Idiopathic dilated cardiomyopathy. N. Engl. J. Med. 1994, 331, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Murbræch, K.; Andreassen, A.K.; Hopp, E.; Aakhus, S.; Gullestad, L. Contemporary Outcome in Patients With Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2015, 116, 952–959. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Agbaedeng, T.A.; Roberts, K.A.; Colley, L.; Noubiap, J.J.; Oxborough, D. Incidence and predictors of sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy: A pooled analysis. EP Eur. 2022, 24, 1665–1674. [Google Scholar] [CrossRef]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.; Marchlinski, F.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A.; et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Eur. Heart J. 2015, 36, 3227–3237. [Google Scholar] [CrossRef]
- Girolami, F.; Ho, C.Y.; Semsarian, C.; Baldi, M.; Will, M.L.; Baldini, K.; Torricelli, F.; Yeates, L.; Cecchi, F.; Ackerman, M.J.; et al. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J. Am. Coll. Cardiol. 2010, 55, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Fressart, V.; Duthoit, G.; Donal, E.; Probst, V.; Deharo, J.C.; Chevalier, P.; Klug, D.; Dubourg, O.; Delacretaz, E.; Cosnay, P.; et al. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Spectrum of mutations and clinical impact in practice. EP Eur. 2010, 12, 861–868. [Google Scholar] [CrossRef] [PubMed]


| HCM | All patients, and once a mutation is found, cascade testing of first-degree family members. |
| ACM | |
| DCM | Patients with positive family history. |
| Patients presenting at younger age, with LV ejection fraction <35%, and a history of ventricular tachycardia or arrhythmia. | |
| LVNC | Expert opinion recommends testing of patients and family members. |
| Children should be tested above 10–12 years old, or more prematurely if family history of early-onset disease. | |
| HCM | DCM | ACM | LVNC | ||
|---|---|---|---|---|---|
| Sarcomere | ACTC1—102540 | X | X | X | |
| TNNT2—191045 | X | X | X | ||
| TNNI3—191044 | X | X | |||
| MYH7—160760 | X | X | X | ||
| MYBPC3—600958 | X | X | X | ||
| TPM1—191010 | X | X | |||
| MYL2—160781 | X | ||||
| MYL3—160790 | X | ||||
| TNNC1—191040 | X | X | |||
| TTN—188840 | X | X | X | ||
| MYH6—160710 | X | X | |||
| Z-Disc | FLNC—102565 | X | X | X | |
| TCAP—604488 | X | X | |||
| MYOZ2—605377 | X | X | |||
| CSRP3—601225 | X | X | |||
| ACTN2—102573 | X | X | |||
| MYPN—608517 | X | ||||
| Cytoskeleton | DYS—300377 | X | |||
| DES—125660 | X | X | X | ||
| LDB3—605906 | X | X | |||
| SGCD—601411 | X | ||||
| PDLIM3—605906 | X | ||||
| VCL—193065 | X | X | |||
| CRYAB—123590 | X | ||||
| Nuclear envelope | LMNA—150330 | X | |||
| EMD—300384 | X | ||||
| ILK—602366 | X | X | |||
| LAP2—150320 | X | ||||
| LAMA4—600133 | X | ||||
| TMEM43—605676 | X | ||||
| Ion channel | SCN5A—600163 | X | |||
| ABCC9—601439 | X | ||||
| Mitochondria | FKRP—606596 | X | |||
| tRNA | X | ||||
| TAZ—300394 | X | X | |||
| ND1 | X | ||||
| FRDA—229300 | X | ||||
| ANT1—103220 | X | ||||
| Transcription factor | EYA4—603550 | X | |||
| Sarcoplasmatic reticulum | PLN—172405 | X | X | X | |
| JPH2—605779 | X | X | |||
| Transmembrane | PSEN1—104311 | X | |||
| PSEN2—600759 | X | ||||
| Storage disease | PRKAG2—602743 | X | |||
| LAMP2—309060 | X | ||||
| GLA—300644 | X | ||||
| GAA—606800 | X | ||||
| Desmosome | PKP2—602861 | X | |||
| DSP—125645 | X | ||||
| DSG2—125671 | X | ||||
| DSC2—125645 | X | ||||
| JUP—173325 | X | ||||
| Growth factor | TGFB3—190230 | X | |||
| Other | PTPN11—176876 | X | |||
| CAV3—601253 | X | ||||
| Infancy | Adolescence | Adulthood | Elderly | |
|---|---|---|---|---|
| HCM | 1–2 years | 1–3 years | 5 years | 5 years |
| DCM | 2–3 years | |||
| ACM | 5 years | 3 years | ||
| LVNC | No relevant data were collected | |||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, J.F.; Martins, E. Recommendations for the Management of Cardiomyopathy Mutation Carriers: Evidence, Doubts, and Intentions. J. Clin. Med. 2023, 12, 4706. https://doi.org/10.3390/jcm12144706
Couto JF, Martins E. Recommendations for the Management of Cardiomyopathy Mutation Carriers: Evidence, Doubts, and Intentions. Journal of Clinical Medicine. 2023; 12(14):4706. https://doi.org/10.3390/jcm12144706
Chicago/Turabian StyleCouto, José F., and Elisabete Martins. 2023. "Recommendations for the Management of Cardiomyopathy Mutation Carriers: Evidence, Doubts, and Intentions" Journal of Clinical Medicine 12, no. 14: 4706. https://doi.org/10.3390/jcm12144706