Abstract
Two orthologues of the gene encoding the Na+-Cl− cotransporter (NCC), termed ncca and nccb, were found in the sea lamprey genome. No gene encoding the Na+-K+-2Cl− cotransporter 2 (nkcc2) was identified. In a phylogenetic comparison among other vertebrate NCC and NKCC sequences, the sea lamprey NCCs occupied basal positions within the NCC clades. In freshwater, ncca mRNA was found only in the gill and nccb only in the intestine, whereas both were found in the kidney. Intestinal nccb mRNA levels increased during late metamorphosis coincident with salinity tolerance. Acclimation to seawater increased nccb mRNA levels in the intestine and kidney. Electrophysiological analysis of intestinal tissue ex vivo showed this tissue was anion absorptive. After seawater acclimation, the proximal intestine became less anion absorptive, whereas the distal intestine remained unchanged. Luminal application of indapamide (an NCC inhibitor) resulted in 73% and 30% inhibition of short-circuit current (Isc) in the proximal and distal intestine, respectively. Luminal application of bumetanide (an NKCC inhibitor) did not affect intestinal Isc. Indapamide also inhibited intestinal water absorption. Our results indicate that NCCb is likely the key ion cotransport protein for ion uptake by the lamprey intestine that facilitates water absorption in seawater. As such, the preparatory increases in intestinal nccb mRNA levels during metamorphosis of sea lamprey are likely critical to development of whole animal salinity tolerance.
Similar content being viewed by others
Introduction
Lampreys are one of two surviving lineages of jawless fishes of the group Agnatha. Agnathans appear to have diverged during the late Ordovician period ~ 450 million years ago (mya)1,2 and persisted through several confirmed mass extinctions on Earth3,4. Adult lampreys have remained morphologically conserved based on a limited fossil record and have inhabited marine and estuarine environments from at least the late Devonian period ~ 360 mya5. Two episodes of whole-genome duplication likely occurred prior to the divergence of Agnatha6. Additional whole-genome duplications have been reported in the ancestors of modern salmonids7, castomids (suckers)8, and common carp9, and additional partial genome duplications have been reported elsewhere in fishes10. Given the basal phylogenetic position of Agnatha and the many genomic and phenotypic evolutionary transitions that have occurred since the divergence of this group, molecular studies in this group are important for understanding and contextualizing physiological shifts throughout vertebrate evolution.
Modern lampreys are iono- and osmoregulators, in that they maintain their plasma osmolality at approximately one-third that of seawater (SW) regardless of external salinity. This osmoregulatory strategy is found in nearly all other extant vertebrate lineages, with the notable exception of elasmobranchs, coelacanths, and lungfishes11,12. The persistence of an osmoregulatory system over evolutionary time underscores its adaptive importance, particularly considering the extensive increases in phenotypic complexity that has occurred in derived fishes13.
Like marine teleosts, lampreys ingest large amounts of SW, which are progressively processed by distinct intestinal regions to compensate for passive water loss14. The gut desalinates ingested SW until it is nearly iso-osmotic with respect to the blood14,15, thus facilitating net water absorption16. Intestinal water absorption occurs primarily via two possible paths: (i) transcellular, in which aquaporins17, Na+-glucose transporters18,19, and/or Na+-K+-2Cl− (NKCC2)/Na+-Cl− (NCC) cotransporter proteins are involved20; (ii) paracellular diffusion across the tight apical junction complexes21,22.
The osmotic force for water absorption from the lumen is driven by salt transport and facilitated along the gastrointestinal tract by luminal CaCO3 precipitation21. This process begins with ingested SW filling lateral intercellular spaces between ion-transporting enterocytes23. The intestinal water absorption from the lumen into the serosa likely occurs by osmosis following Cl− molecular transport21. Note that most studies reporting “macro” osmotic gradients in the intestinal lumen and blood of marine fishes show no differences in osmotic pressure24. The mechanism for luminal Cl− uptake is species- and tissue-dependent but always includes a member of the SLC family of solute-carrying proteins such as NKCC2, NCC, and/or Cl−/HCO3− exchanger25,26,27. The active transport of Cl− across the intestinal epithelium is associated with high levels of Na+/K+-ATPase (NKA) activity28. The basolateral NKA produces favorable electrochemical gradients for the absorption of Cl− via the SLC cotransporters and anion exchangers localized in the apical and basolateral membranes of enterocytes14,20,24. Excess monovalent ions that are incorporated into the blood plasma are secreted across the gills29.
The NCC is perhaps most well-known for its crucial role in renal ion reabsorption in the mammalian distal convoluted tubule, where 5–10% of the filtered Na+ and Cl− are reabsorbed30,31,32. Expression of an orthologous NCC (termed NCC1) in teleost fishes acclimated to freshwater (FW) environments has been shown to be highly expressed in the kidney, presumably fulfilling a similar role in NaCl absorption33,34. Fishes have another NCC gene (termed NCC2), first discovered and named by Hiroi et al.35, which is classically referred to as a ‘fish-specific NCC’, although it has not been found in sharks34. In teleost fishes, NCC2 is localized to the apical membrane of branchial ionocytes and is responsible for branchial NaCl absorption in FW36,37,38,39. Within the NKCC1/NKCC2/NCC gene superfamily, NCC1 and NCC2 form distinct lineages, indicating that this gene family has functionally diverged on an evolutionary timescale40.
In teleost fishes, NKCC2 is the predominant cation-chloride cotransporter that facilitates apical ion absorption in the SW intestine27,28,41. Ion and water uptake by the intestine has been shown to be inhibited by bumetanide, a selective inhibitor of NKCC20. The NCC2 appears to have a role in Na+ and Cl− removal from ingested SW in the esophagus and intestine of the Japanese42,43 and European eel44. However, information regarding the importance of NCC in intestinal ion and water uptake in other fish species is still scarce20.
Although these underlying mechanisms for intestinal SW osmoregulation have been elucidated in teleost fishes, whether such molecular mechanisms are present in the lamprey intestine was unknown prior to the present study28. Thus, we aimed to functionally characterize the putative molecular pathway for intestinal ion absorption during SW osmoregulation in the sea lamprey (Petromyzon marinus). Sea lamprey has a complex anadromous life cycle marked by a dramatic metamorphosis from a benthic filter-feeding larva (termed ‘ammocoete’) into a parasitic juvenile that migrates from FW to SW. After ~ 2 years at sea, the adults migrate upstream to spawn. Although the larval-juvenile metamorphosis has been morphologically conserved from at least the early Cretaceous, ~ 125 mya45, recent studies on fossil records suggest that ammocoetes are specializations of modern lampreys rather than a relic of ancient lampreys46.
We hypothesized that intestinal ion absorption in SW-acclimated sea lamprey occurred by an NKCC and/or NCC. We present the discovery of two functionally divergent NCC isoforms in lamprey, which appear to be basal in structure and function to the NCC isoforms of more derived fishes. Moreover, we show that NCC and not NKCC2 is the likely pathway for ion absorption in the intestine of marine lampreys.
Results
Tissue profile and molecular characterization
We comprehensively surveyed cDNA sequences encoding relevant ion-transporting proteins in the sea lamprey genome. Partial sequences of orthologous cDNAs encoding two distinct NCC-like proteins were discovered. The accession numbers of these newly identified sea lamprey NCC sequences are BK014291 and BK014292, which we have named “NCCa” and “NCCb”, respectively. We could not identify a gene encoding an NKCC2-like protein in any available genome for different lamprey species. Deduced amino acid sequences for sea lamprey NCCa and NCCb shared the highest sequence identity to known NCC families; molecular phylogenetic analysis grouped NCCa and NCCb with other NCC sequences (Fig. 1). NCCa occupied a basal position among the NCC1 and NCC2 clades. NCCb was basally positioned within the clade of the conventional, kidney-specific NCC1 sequences.
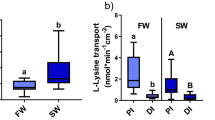
In tissue profiles, mRNA abundance of ncca and nccb showed drastically different expression patterns. The ncca was expressed predominately in the gills and kidney at ~ 400-fold higher levels than other tissues in FW-acclimated juvenile sea lamprey (Fig. 2A). Acclimation to SW resulted in ~ 90% reduction of ncca in the gills and kidney. The nccb was predominately expressed in the anterior intestine (AI), posterior intestine (PI), and kidney at ~ 1000-fold higher levels than other tissues in SW-acclimated sea lamprey (Fig. 2B). The nccb mRNA in the AI and kidney of SW-acclimated sea lamprey was 5- and twofold higher, respectively, than in FW-acclimated sea lamprey.
Gene expression of ncca (A) and nccb (B) isoforms in a tissue profile of juvenile sea lamprey acclimated to either FW or SW. Abbreviations: B, brain; P, pituitary; G, gill; H, heart; L, liver; AI, anterior intestine; PI, posterior intestine; K, kidney; M; muscle. Values are relative to the FW brain tissue. Each column represents mean ± SEM (n = 3). *Significant differences within a given tissue between environmental salinities (two-way ANOVA, Tukey’s post hoc).
In additional metamorphic and salinity experiments, nccb in the intestine increased during the late stages of metamorphosis and then increased further after SW acclimation. The nccb mRNA levels increased six and fivefold in the AI and PI, respectively, throughout the larvae-to-juvenile metamorphosis (Fig. 3A,B). In lab-acclimated sea lamprey, intestinal nccb mRNA was significantly higher in both the AI (ninefold) and PI (5.5-fold) in FW-acclimated juveniles compared to larvae (Fig. 3C,D). Acclimation to SW further increased sea lamprey nccb mRNA abundance in the AI (twofold) and PI (1.5-fold).
Gene expression of nccb in the anterior, AI (A) and posterior intestine, PI (B) during metamorphosis of the sea lamprey. Gene transcription of nccb in the AI (B) and PI (C) for larvae, and FW- and SW-acclimated juvenile sea lamprey. Each column represents mean ± SEM (n = 4–19). Different superscript letters indicate significant differences within intestinal regions among life stages (A and B; P < 0.05, one-way ANOVA, Tukey’s post hoc). *Significant differences within the same environmental salinities between groups (B and C; unpaired Student’s t-test).
Electrophysiological properties of sea lamprey intestine
Briefly, we measured ‘transepithelial’ electrophysiology possibly due to coordinated fluxes across the apical and basolateral membranes. The resulting paracellular Na-diffusion flux (i.e., sodium and chloride inwards and Na diffusing paracellularly outwards), a mechanism common to other NaCl absorbing epithelia, sets up a transepithelial electric potential (TEP)47. Therefore, no particular mechanism dictates the global TEP across the epithelium, such as electroneutral NKCC/NCC. The combined activity generates this potential, and a large TEP ex vivo is characteristic of epithelia with robust transepithelial transport activity. Chloride is the primary physiologic anion and the ion suspected of carrying the majority of the current (Isc). The TEP and Isc values observed for FW- and SW-acclimated sea lamprey intestine are consistent with a preferential anion-absorbing tissue. In the AI, Isc was significantly less negative after SW acclimation (Fig. 4A). Isc of the AI of SW-acclimated sea lamprey was significantly less negative than that of the PI.
(A) Short-circuit current (Isc, μA cm−2), and (B) transepithelial electric resistance (TER, Ω·cm2) in the anterior (AI) and posterior (PI) intestine of FW- and SW-acclimated juvenile sea lamprey. Each column represents mean ± SEM (n = 8–15). Different letters indicate significant differences within intestinal regions between environmental salinities. *Significant differences between intestinal regions within salinities (two-way ANOVA, Tukey’s post hoc).
The transepithelial electric resistance (TER) did not change across intestinal regions and/or salinities (Fig. 4B).
To investigate whether NKCC2 and/or NCCb are involved in intestinal ion absorption mechanisms in the intestine of the juvenile sea lamprey, increasing concentrations of either bumetanide in preparations from FW- and SW-acclimated juveniles, or indapamide in preparations from SW-acclimated juveniles, were added to the luminal side of the AI (Fig. 5A) and PI (Fig. 5B). Note that indapamide was employed just in SW-acclimated juveniles since their sensitivity was more remarkable than FW-acclimated ones (data not shown). There was no significant effect of bumetanide at any of the concentrations tested (0.1–2.0 mM). In contrast, increasing concentrations of indapamide resulted in significant inhibition of the baseline Isc, and the effect appeared to be dose-dependent. The inset figures are original traces of the impact on the Isc of a single application of indapamide (0.4 mM) following the last application of 0.4 mM bumetanide. These data are consistent with the presence of an NCC-mediated anion absorptive pathway in both the AI and PI regions of the SW-acclimated sea lamprey intestinal tract.
Impact of increasing concentrations of bumetanide or indapamide on basal short circuit current (Isc, μA cm−2) in the anterior (AI; A) or posterior (PI; B) intestine of juvenile sea lamprey acclimated to FW and/or SW. After mounting the tissues, the Isc was allowed to stabilize (i.e., baseline level) before adding the respective inhibitors. Note, the grey circles represent the mean response of intestinal tissues of FW-acclimated (open triangles) and SW-acclimated (open squares) sea lamprey at each concentration of bumetanide tested. In contrast, the effect of increasing concentrations of indapamide were only examined in intestinal tissues of SW-acclimated sea lamprey. The inset figures are examples of the impact on the Isc of a single application of indapamide (0.4 mM) following the last 0.4 mM bumetanide application. Data are the mean ± SEM (n = 2–6). *Significant differences between the basal Isc and the Isc after it reached its maximal change following drug application at each concentration of drug tested (one-sample t-test).
The magnitude of the indapamide effects varied between intestinal regions and with environmental salinity (Fig. 6). In FW, approximately 40% and 25% of the baseline anion absorption was indapamide-sensitive in the AI and PI, respectively. In SW, indapamide-sensitive anion absorption was significantly higher in the AI than in the PI. Indapamide-sensitive anion absorption in the AI was 73% of baseline anion absorption in SW-acclimated juvenile sea lamprey, compared to only 30% of that PI.
Percent inhibition of baseline short circuit current (%) in response to luminal indapamide (0.4 mM) in FW- and SW-acclimated juvenile sea lamprey. All data are the mean ± SEM (n = 5–8). Different letters indicate significant differences within intestinal regions between environmental salinities. *Significant differences between intestinal regions within environmental salinities (two-way ANOVA, Tukey’s post hoc).
Intestinal water absorption
Whole intestine water absorption under iso-osmotic conditions decreased significantly by 64% and 41% after the serosal addition of ouabain (500 µM) or luminal addition of indapamide (400 µM), respectively (Fig. 7).
Intestinal water absorption (% of control) in whole intestines of juvenile sea lamprey acclimated to SW. Ouabain (500 µM) was added to the serosal side, and indapamide (400 µM) was added to the luminal side. All data are presented as mean ± SEM (n = 3–4). Different letters indicate significant differences among groups (one-way ANOVA, Tukey’s post hoc).
Discussion
It has been hypothesized that a first-round of whole-genome duplication (1R) in vertebrates led to an initial divergence of NKCC from NCC and that a second round of whole-genome duplication (2R) in the vertebrate lineage resulted in multiple NKCC and NCC isoforms, NKCC1/NKCC2 and NCC1/NCC248. Although Agnathans radiated after 1R or 2R is still unresolved6,49, recent studies in noggin genes in lampreys suggest two rounds (2R) of ancient genome duplication50.
Our molecular phylogenetic analysis showed that the NCC1 and NCC2 of bony fishes were grouped into distinct NCC1 (conventional, kidney NCC) and NCC2 (ray-finned fish-specific gill NCC) clades. Sea lamprey NCCb was clearly positioned at a basal position within the NCC1 clade. Sea lamprey NCCa was not explicitly located within either the NCC1 or NCC2 clades and was instead positioned basal to both NCC clades. All retrieved NKCC1-, NCCa-, and NCCb-like proteins from other lamprey species were grouped with the respective identified sequences of the sea lamprey (Petromyzon marinus).
The dramatic changes in nccb transcription during the latest stages of development and in response to environmental changes are good indicators of functional relevance. We found that nccb mRNA levels were significantly upregulated in the later stages of metamorphosis, particularly in post-metamorphic juveniles which were captured while actively migrating toward the sea. Barany et al.14,51 observed increases in intestinal NKA transcription, mRNA, and activity in the late stages of metamorphosis. Both NKA and NCCb might be important to the development of SW tolerance that occurs late in metamorphosis. Based on our transcriptional and pharmacological experiments, NCCb in the intestine was further upregulated in juveniles after exposure to SW. Furthermore, the expression and activity of NCCb in SW were higher in AI than in PI. These changes in intestinal NCCb expression and activity during metamorphosis and SW-acclimation reflect similar intestinal NKA activity and expression14, indicating that both of these transporters play a critical role in developing salinity tolerance in lamprey. This salinity-responsive pattern for NCCb is similar to reports on the apical NKCC2 in the intestine of euryhaline teleosts transferred to higher salinities27,52,53, and on the basolateral NKCC1 in the gills of sea lamprey54. The SW adaptive changes in gill NKCC1 expression have been shown to be driven by corticosteroid signaling55. It has also been shown that the intestine of post-metamorphic juvenile sea lamprey highly expresses a corticosteroid receptor51,56. Thus, whether SW-adaptive anion absorption via NCCb in the AI is also under corticosteroid control should be investigated.
Although the present study was explicitly focused on functional characterization of the intestinal NCCb isoform, we posit a potential mechanism for branchial ion uptake and renal NaCl reabsorption in FW-acclimated sea lamprey, the NCCa isoform. In our mRNA tissue profile, ncca was highly expressed in the gills of FW-acclimated juvenile lamprey and significantly down-regulated after SW acclimation. This apparent FW-specific role for ncca in the gills may explain the apparent FW-specific regulation of an ncc-like orthologue reported in the gills of up-stream migrating adult sea lamprey57. This salinity-specific regulation of gill NCC in order to adapt to different salinities is similar to what has been described of gill NCC2 in teleosts35,39,58.
The expression of both NCCs, NCCa and NCCb, in the sea lamprey kidney, is unique among vertebrates and warrants further investigation. The sea lamprey ncca was abundant in the kidney of FW-acclimated juveniles and strongly down-regulated after SW acclimation. By contrast, nccb mRNA levels in the kidney were upregulated after SW acclimation. The typical salinity-responsive pattern of the conventional kidney NCC1 in fishes is that it is abundant in FW and down-regulated in SW33. This indicates that NCCa in sea lamprey kidney functions similar to the conventional, kidney-specific NCC involved salt retention of teleosts in FW. The expression of the sea lamprey ncca in both the gills and kidney of FW-acclimated sea lamprey is particularly interesting because, in teleost fishes, the NCC in the gills (NCC2) and kidney (NCC1) are two distinct NCC isoforms33,35,37. It is important to note that the elasmobranch NCC also exhibits FW-specific function in the gills and kidney34,59, similar to the expression pattern of the sea lamprey NCCa.
In our effort to functionally characterize the intestinal NCCb isoform, we used an electrophysiological approach to elucidate the underlying mechanisms contributing to intestinal ion transport in response to environmental salinity. The anion-absorptive properties of the sea lamprey intestine reported here agree with what has previously been shown in the sea lamprey intestine14. Surprisingly, absorptive Isc in the AI was higher in FW-acclimated juvenile sea lamprey. It is known that FW-acclimated sea lamprey exhibits some minimal drinking14,60. Thus, it could be that the ion uptake capacity of the FW-acclimated AI offers extra osmoregulatory support by facilitating ion uptake in ion-poor environments, suggesting that intestinal ion uptake from imbibed FW might be more important than previously thought. In this case, the AI would supplement branchial uptake in FW. Although intestinal ion uptake from feed could supplement ion uptake by the gill in freshwater larvae, feeding by migrating anadromous juveniles does not usually occur until fish reaches seawater. In SW, higher anion-absorptive Isc was detected in the PI than in the AI. This demonstrates that these intestinal regions have different functional responses to environmental salinity, similar to what has also been previously reported in teleosts27,41,61. We anecdotally observed intestinal precipitates only in the intestine of SW-acclimated juveniles (data not shown). Thus, the reduction in anion absorption in the AI of SW-acclimated juveniles may also indicate a functional switch in intestinal physiology to secrete HCO3− and/or H+62 into the AI lumen to lower luminal osmolality and facilitate net water absorption63,64. Although no direct reports of measurements of intestinal fluid HCO3− concentrations or secretion exists for lamprey, reports by Rankin65 show a substantial cation–anion change difference among measured ions in the intestinal lumen of SW-acclimated lamprey. Moreover, this observation and what it may imply for evolutionary aspects of intestinal HCO3− secretion by marine fishes was discussed by Taylor and Grosell66. Still, further research is needed to determine if a similar gut intestinal fluid alkalinization mechanism occurs in sea lamprey.
In the juvenile sea lamprey, the apparent lack of sensitivity to bumetanide indicates that anion absorption by NKCC2 is unlikely to be present in the sea lamprey intestine. This is further supported by our inability to identify a gene encoding NKCC2 in the published genomes of sea lamprey, western brook lamprey, Pacific lamprey, or Arctic lamprey. We cannot rule out the possibility that a sea lamprey NKCC2 could be insensitive to bumetanide67,68 and that nkcc2 is present but has yet to be detected in the available lamprey genomes. Note that no significant effects in Isc were obtained with either chlorothiazide or hydrochlorothiazide, which were initially employed to target a putative NCC. It also should be noted that NKCC1 has been identified and characterized in sea lamprey gills54 but is present only at very low levels in the intestine. Moreover, we were able to identify NKCC1-, NCCa-, and NKCCb-like proteins in the available different lamprey species genomes. Therefore, we can surmise two possible explanations for the lack of a sea lamprey nkcc2: (i) Agnathans diverged after 2R, but without a functional role, an nkcc2 gene was eventually lost; and (ii) Agnathans diverged before 2R, and an nkcc2 gene never existed in sea lamprey.
Our results demonstrate that an indapamide-sensitive mechanism (i.e., NCCb) on the apical membrane appears to be a major contributor to anion absorption in the lamprey intestine. Overall lumen-to-serosa ion influx along the intestinal tract was observed, with marked differences depending on intestinal regions. In SW-acclimated sea lamprey juveniles, approximately 70 and 30% of the net absorptive currents in the AI and PI, respectively, are mediated by NCCb. With NKCC2 ruled out by the lack of bumetanide sensitivity, any further speculation about the mechanisms facilitating the residual Isc that is NCCb-independent (i.e., insensitive to indapamide) is beyond the scope of this investigation. Further studies are required to elucidate whether the NKCC2/NCC-independent mechanisms of Cl− absorption, which have been shown in the teleost intestine28, are also present in lampreys. It is also possible that indapamide simply did not fully inhibit all NCCb activity, even at the highest tested doses. Further pharmacological characterization of the sea lamprey NCCs is warranted.
The contribution of NCCb to anion-absorption in the AI is about twice as high in SW compared to FW. Thus, increased environmental salinity may cause a shift of Cl− absorption mechanisms41, which agrees with previously reported increases in drinking rate, intestinal NKA activity, and intestinal water absorption14, as well as increased gill Cl− secretory mechanisms54. The higher sensitivity of Isc to indapamide in the AI than the PI is consistent with higher nccb transcription in this region.
To better understand how intestinal water absorption was related to NCCb activity, we characterized indapamide-sensitive intestinal water transport in SW-acclimated sea lamprey juveniles. As expected, the serosal addition of ouabain inhibited active water absorption, demonstrating NKA is critical involvement in this process, as has previously been shown in teleosts and sea lamprey14,69. The luminal addition of indapamide also inhibited water absorption to a similar degree as the addition of ouabain. These results indicate that the activity of NCCb in the sea lamprey intestine is a critical ion absorptive mechanism that is functionally coupled to water absorption.
In summary, our finding that an NCCb, and not NKCC2, functions in the intestine offers new insights into the diversification of the vertebrate NKCC/NCC protein superfamily as it might be related to partial and/or whole-genome duplication events. Although the results reported here are an important discovery and describe specific differences in the osmoregulatory roles of NKCCs and NCCs between lamprey and later-derived fishes, further studies are still needed to complete our understanding of ion-absorptive pathways in the gill, kidney, and intestine of the sea lamprey.
Materials and methods
Animals and experimental designs
All experiments were carried out in accordance with USGS guidelines and approved by the USGS Institutional Animal Care and Use Committee (Protocol No. LB00A3O-117). In addition, the study reported here also is in accordance with ARRIVE guidelines. Sea lamprey juveniles were caught in the Sawmill River, a tributary of the Connecticut River (Montague, MA, USA), by electrofishing (ammocoetes and stages 1–7) or Fyke net capture (considered ‘migrating juveniles’) from July to November 2018. For the metamorphic series, lamprey were sampled in the field immediately upon capture and staged according to the descriptions presented by Youson et al.70. Salinity acclimation experiments were carried out under laboratory conditions at the USGS Conte Anadromous Fish Research Center (Turners Falls, MA, USA) and acclimated to experimental salinity conditions for least 3 weeks prior to sampling. Experimental SW was prepared by mixing artificial sea salt (Crystal Sea Salt, Baltimore, MD, USA) and dechlorinated municipal FW. Lamprey in FW and SW were kept under natural photoperiod conditions and at a constant temperature of 15 ºC in 60 L recirculating glass aquaria equipped with mechanical, chemical, and biological filtration and monitored daily. The animals were not offered food because they naturally stop feeding during metamorphosis and do not resume until they begin parasitic feeding once they reach the ocean.
Sampling protocol
For all tissue collection, lamprey were euthanized using MS-222 (400 mg L−1 buffered with NaHCO3, pH 7.0) (Argent Chemical Laboratories, Redmond, WA, USA). Tissue was collected from the gills and two sections of the intestine: (i) the AI, corresponding to 1.5–2 cm after the end of the esophagus; and (ii) the PI, which corresponds to a section of distal intestine, 2–3 cm in length, delimited by the rectal sphincter. All samples were immediately frozen and stored at − 80 °C until analysis, except in cases of measurements of intestinal water permeability and electrophysiology, where the tissues were used immediately after dissection.
Gene identification and molecular phylogenetic analysis
Gene-specific primers were designed based on sea lamprey genomic information available from the SIMRbase (https://genomes.stowers.org/). The identity of annotated genes was confirmed using sequence identity comparison and NCBI blast analyses.
The deduced amino acid sequences of the obtained annotated genes were predicted using the Translate tool provided by the Expasy bioinformatics resource portal (https://www.expasy.org). Phylogenetic analysis was carried out on a balanced selection of vertebrate NKCC1, NKCC2 and NCC peptides available from NCBI GenBank, Ensemble Genomes and SIMRbase, using a ClustalW alignment (https://www.ebi.ac.uk/clustalw) and the neighbor-joining method with bootstrap analysis for 1000 cycles implemented by MEGA X software. Accession numbers for the peptides used in the phylogenetic analysis were: lancelet (NKCC/NCC-like, XP_035666237.1), human (NKCC1, U30246; NKCC2, NM_000338; NCC1, X91220), mouse (NKCC1, NP_033220.2; NKCC2, NP_899197.2; NCC1, NP_062288.1), rat (NCC1, NP_062218.3; KCC1, NM_019229.2), tilapia (NKCC1, AY513737; NKCC2, AY513739; NCC2, EU518934), eel (NKCC1, AJ486858; NKCC2, AJ564602; NCC1, AJ564604; NCC2, AJ564606), zebrafish (NCC1, NP_001038545; NCC2a, NP_001154850; NCC2b, EF591989; NCC2c, NP_001128603.1), medaka (NCC2a, KJ489428; NCC2b, KJ489429), mefugu (NCC1, AB479211), flounder (NCC1, L11615), gar (NCC1, ENSLOCG00000007841; NCC2, ENSLOCG00000000965), sturgeon (NCC1, XP_033895975.2; NCC2, XP_034770356.1), coelacanth (NCC1, XM_014494272), elephant fish (NKCC1, AB769492; NKCC2, AB769493; NCC1, AB769494), dogfish (NKCC1, U05958; NKCC2a, AF521915), bullshark (NCC1, AB769491), houndshark (NKCC1, AB669487; NKCC2, AB769486; NCC1, AB769487), arctic lamprey (NCCa-like, KE993714.1; NCCb-like, APJL01019054.1); western brook lamprey (NKCC1-like, LPT_00008897-P; NCCa-like, LPT_00002495-RA; NCCb-like, LPT_00012662-RA); pacific lamprey (NKCC1-like, ETRf_mk00026850-P; NCCa-like, ETRf_mk00025483-RA; NCCb-like, ETRf_mk00025494-RA); sea lamprey (NKCC1, MK779970.1; NCCa, BK014291; NCCb, BK014292).
RNA isolation and quantitative real-time PCR
Total RNA was extracted from frozen tissues using TRIzol reagent (Molecular Research Center Inc., Cincinnati, OH, USA) following the manufacturer’s protocol. The total RNA concentration and purity of each sample were determined spectrophotometrically using a Take3 microvolume plate reader (BioTek Instruments, Inc., Winooski, VT, USA), and RNA integrity was assessed using gel electrophoresis. Only high-purity RNA (1.9 < A260/A280 > 2.2) were used for cDNA synthesis. First-strand cDNA synthesis was accomplished using a High-Capacity Reverse Transcription Kit following the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA, USA). Quantitative real-time PCR (RT-qPCR) was carried out in 10 µL reactions containing 5 ng cDNA template (estimated from the input of total RNA), 150 nM forward and reverse primers, and SYBR Select master mix (ThermoFisher, Waltham, MA, USA). All RT-qPCR reactions were performed in optical 96-well reaction plates in a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Inc., Foster City, CA, USA) covered with adhesive seals. The thermocycling procedure was as follows: an initial step of 50 °C (2 min) followed by 95 °C (2 min), and then 40 consecutive cycles of 95 °C (15 s), 60 °C (1 min), and 72 °C (30 s). Melting curves were used to ensure that only a single PCR product was amplified and to verify the absence of primer-dimer artifacts. A pool of concentrated cDNA from all samples for each tissue was used for a standard curve, which was a five-point, serial tenfold dilution from 50 ng to 5 pg. The linearity and efficiency of amplification for each primer combination was assessed. Control reactions with RNase-free water (no template control) and RNA (no reverse transcriptase during cDNA synthesis) were included in the analysis to ensure the absence of primer-dimerization and genomic DNA contamination. For all pairs of primers, linearities (R2) and amplification efficiencies were in the ranges 0.998–1 and 0.923–0.939, respectively. The relative gene expressions were quantified using the ΔΔCT method71, corrected for efficiencies72, and referenced to the relative mRNA expression of the difference between the target gene and an endogenous reference gene (eef1α; gene encoding eukaryotic elongation factor 1-alpha) for each sample. Primer sequence details are shown in Table 1.
Chemicals
Bumetanide (a selective NKCC2 inhibitor), ouabain (a selective NKA inhibitor), and indapamide (a selective NCC inhibitor) were purchased from ThermoFisher (Waltham, MA, USA) and prepared as concentrated stocks in dimethyl sulfoxide (DMSO) except ouabain, which was dissolved in lamprey serosal saline. The final volume of DMSO never exceeded 0.2%, and no effect of DMSO at this concentration was observed on multiple tissues tested (data not shown).
Electrophysiology in ussing chambers
Anterior and posterior intestinal tissues were dissected, washed, and mounted in Ussing chambers. The tissues were opened longitudinally, flattened, and placed between the chambers containing 1.2 mL of serosal saline (in mM: 128.0 NaCl, 1.2 NaH2PO4, 5.0 NaHCO3, 4.0 KCl, 2.4 CaCl2, 0.9 MgSO4, 0.9 MgCl2, 5.5 glucose; 270 mOsm/kgH2O; pH 7.8). During the experiments, the tissue was bilaterally gassed with humidified 0.5% CO2 in oxygen, and the temperature was maintained at 15 °C. The TEP (in mV) was referenced to the luminal side, where a positive TEP indicates that the luminal side is positively charged with respect to the serosal side. The Isc (in μA cm−2) was monitored by clamping epithelia to 0 mV expressed as negative for the absorption of anions. The TER (in Ω·cm2) was manually calculated (Ohm's Law) using the current deflections induced by a 10 mV pulse at the beginning and the end of the experiment. After the tissue achieved a steady state, which usually occurred between 30 and 40 min after mounting, all data were digitally recorded using the Lab-Trax-4 acquisition system (World Precision Instruments, Sarasota, FL, USA) and LabScribe3 software (iWorx Systems Inc., Dover, NH, USA). Ag–AgCl electrodes were used as short‐circuiting electrodes to measure transepithelial electrical properties. The electrodes were connected to the serosal and luminal sides using PE90 tubing containing solidified 3 mM KCl and 2% agar. A high impedance automatic dual voltage clamp (EVC 4000‐4, World Precision Instruments, Sarasota, FL) was used for voltage clamping and monitoring TEP. Under these conditions, the measured Isc is a function of transcellular ion transport that requires an energy input (active transport).
Determination of iso-osmotic in vitro intestinal water absorption
Water absorption was determined following previous methods14. Briefly, the intestinal sections were isolated, flushed and then incubated for 30 min in lamprey serosal saline bubbled with a physiological gas mixture (humidified 0.5% CO2 in oxygen). Saline was maintained at 15 °C and pre-gassed for at least 30 min before experimentation. The ends of the intestinal samples were sealed at each end using dental line and placed in a beaker containing serosal saline supplied with the gas mixture. The intestinal preparations were weighed every 15 min for 1 h. At the end of the experiment, each intestinal section was opened, placed on graph paper, and then photographed. The surface area of the intestinal segment within the tied region was determined using ImageJ software (NIH, Bethesda, MD).
Statistical analysis
Statistical comparisons across experimental procedures were performed using unpaired Student’s t-test, one-sample t-test, one-way ANOVA, and two-way ANOVA, as indicated in the figure legends. All data are represented as the mean ± SEM. Prior to these statistical analyses, both normality and equal variance were confirmed. When ANOVA yielded significant differences, Tukey's post hoc test was used to identify these. Statistical significance was accepted at P < 0.05.
Data availability
All data generated or analyzed during this study are available in Science Data Bank with the following https://doi.org/10.11922/sciencedb.01279.
References
Kuraku, S. & Kuratani, S. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 23, 1053–1064 (2006).
Miyashita, T. et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Proc. Natl. Acad. Sci. USA. 116, 2146–2151 (2019).
MacLeod, N. The Great Extinctions: What Causes Them and how They Shape Life (Firefly Books, 2013).
Corso, J. D. et al. Extinction and dawn of the modern world in the Carnian (Late Triassic). Sci. Adv. 6, 1–13 (2020).
Gess, R. W., Coates, M. I. & Rubidge, B. S. A lamprey from the Devonian period of South Africa. Nature 443, 981–984 (2006).
Holland, L. Z. & Ocampo Daza, D. A new look at an old question: When did the second whole genome duplication occur in vertebrate evolution?. Genome Biol. 19, 2–5 (2018).
Wang, S., Hard, J. J. & Utter, F. Genetic variation and fitness in salmonids. Conserv. Genet. 3, 321–333 (2002).
Uyeno, T. & Smith, G. R. Tetraploid origin of the karyotype of catostomid fishes. Science 175, 644–646 (1972).
David, L., Blum, S., Feldman, M. W., Lavi, U. & Hillel, J. Recent duplication of the common carp (Cyprinus carpio L.) Genome as revealed by analyses of microsatellite loci. Mol. Biol. Evol. 20, 1425–1434 (2003).
Vrijenhoek, R. C. The evolution of clonal diversity in poeciliopsis. In Evol. Genet. Fishes 399–429 https://doi.org/10.1007/978-1-4684-4652-4_8 (1984).
Griffith, R. W. Chemistry of the body fluids of the coelacanth, Latimeria chalumnae. Proc. R. Soc. Lond.Ser. B Biol. Sci. 208, 329–347 (1980).
Pang, P. K. T., Griffith, R. W. & Atz, J. W. Osmoregulation in elasmobranchs. Integr. Comp. Biol. 17, 365–377 (1977).
Donoghue, P. C. J. & Purnell, M. A. Genome duplication, extinction and vertebrate evolution. Trends Ecol. Evol. 20, 312–319 (2005).
Barany, A., Shaughnessy, C. A., Fuentes, J., Mancera, J. M. & McCormick, S. D. Osmoregulatory role of the intestine in the sea lamprey (Petromyzon marinus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R410–R417 (2020).
Skadhauge, E. The mechanism of salt and water absorption in the intestine of the eel (Anguilla anguilla) adapted to waters of various salinities. J. Physiol. 204, 135–158 (1969).
Grosell, M. & Taylor, J. R. Intestinal anion exchange in teleost water balance. Compar. Biochem. Physiol. Part A Mol. Integr. Physiol. 148, 14–22 (2007).
Cerdà, J. & Finn, R. N. Piscine aquaporins: An overview of recent advances. J. Exp. Zool. A. Ecol. Genet. Physiol. 313, 623–650 (2010).
Loo, D. D. F., Wright, E. M. & Zeuthen, T. Water pumps. J. Physiol. 542, 53–60 (2002).
Zeuthen, T. Water-transporting proteins. J. Membr. Biol. 234, 57–73 (2010).
Whittamore, J. M. Osmoregulation and epithelial water transport: Lessons from the intestine of marine teleost fish. J. Comp. Physiol. B. 182, 1–39 (2012).
Grosell, M. The role of the gastrointestinal tract in salt and water balance. In Fish Physiology Vol. 30 (Elsevier Inc., 2010).
Sundell, K. & Sundh, H. Intestinal fluid absorption in anadromous salmonids: Importance of tight junctions and aquaporins. Front. Physiol. 3, 388 (2012).
Larsen, E. H., Willumsen, N. J., Møbjerg, N. & Sørensen, J. N. The lateral intercellular space as osmotic coupling compartment in isotonic transport. Acta Physiol. 195, 171–186 (2009).
Larsen, E. H. et al. Osmoregulation and Excretion. In Comprehensive Physiology 405–573 (American Cancer Society, 2014). https://doi.org/10.1002/cphy.c130004.
Grosell, M. Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiol. 202, 421–434 (2011).
Watanabe, T. & Takei, Y. Molecular physiology and functional morphology of SO42− excretion by the kidney of seawater-adapted eels. J. Exp. Biol. 214, 1783–1790 (2011).
Gregorio, S. F. et al. Adaptation to different salinities exposes functional specialization in the intestine of the sea bream (Sparus aurata L.). J. Exp. Biol. 216, 470–479 (2013).
Shaughnessy, C. A. & Breves, J. P. Molecular mechanisms of Cl− transport in fishes: New insights and their evolutionary context. J. Exp. Zool. Paet A Ecol. Integr. Physiol. 335, 207–216 (2021).
McCormick, S. D. et al. Physiological and endocrine changes in Atlantic salmon smolts during hatchery rearing, downstream migration, and ocean entry. Can. J. Fish. Aquat. Sci. 70, 105–118 (2013).
Plotkin, M. D. et al. Localization of the thiazide sensitive Na-Cl cotransporter, rTSC1, in the rat kidney. Kidney Int. 50, 174–183 (1996).
Reilly, R. F. & Ellison, D. H. Mammalian distal tubule: Physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 80, 277–313 (2000).
Yang, S. S. et al. Generation and analysis of the thiazide-sensitive Na+-Cl-cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome. Hum. Mutat. 31, 1304–1315 (2010).
Kato, A. & Romero, M. F. Regulation of electroneutral NaCl absorption by the small intestine. Annu. Rev. Physiol. 73, 261–281 (2011).
Takabe, S., Inokuchi, M., Yamaguchi, Y. & Hyodo, S. Distribution and dynamics of branchial ionocytes in houndshark reared in full-strength and diluted seawater environments. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 198, 22–32 (2016).
Hiroi, J., Yasumasu, S., McCormick, S. D., Hwang, P. P. & Kaneko, T. Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J. Exp. Biol. 211, 2584–2599 (2008).
Inokuchi, M., Hiroi, J., Watanabe, S., Lee, K. M. & Kaneko, T. Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 151–158 (2008).
Hwang, P. P. Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 212, 1745–1752 (2009).
Hsu, H. H., Lin, L. Y., Tseng, Y. C., Horng, J. L. & Hwang, P. P. A new model for fish ion regulation: Identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes). Cell Tissue Res. 357, 225–243 (2014).
Breves, J. P. et al. Clc-2c is regulated by salinity, prolactin and extracellular osmolality in tilapia gill. J. Mol. Endocrinol. 59, 391–402 (2017).
Takei, Y., Hiroi, J., Takahashi, H. & Sakamoto, T. Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physiol. Integr. Comp. Physiol. 307, R778–R792 (2014).
Ruiz-Jarabo, I., Barany, A., Jerez-Cepa, I., Mancera, J. M. & Fuentes, J. Intestinal response to salinity challenge in the Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 204, 57–64 (2017).
Watanabe, S., Mekuchi, M., Ideuchi, H., Kim, Y. K. & Kaneko, T. Electroneutral cation-Cl- cotransporters NKCC2Β and NCCΒ expressed in the intestinal tract of Japanese eel Anguilla japonica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 159, 427–435 (2011).
Takei, Y. et al. Molecular mechanisms underlying active desalination and low water permeability in the esophagus of eels acclimated to seawater. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R231–R244 (2017).
Cutler, C. P. & Cramb, G. Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 149, 63–73 (2008).
Chang, M., Wu, F., Miao, D. & Zhang, J. Discovery of fossil lamprey larva from the Lower Cretaceous reveals its three-phased life cycle. Proc. Natl. Acad. Sci. https://doi.org/10.1073/pnas.1415716111 (2014).
Miyashita, T., Gess, R. W., Tietjen, K. & Coates, M. I. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 591, 408–412 (2021).
Allen, G. G. & Barratt, L. J. Origin of positive transepithelial potential difference in early distal segments of rat kidney. Kidney Int. 27, 622–629 (1985).
Hartmann, A. M., Tesch, D., Nothwang, H. G. & Bininda-Emonds, O. R. P. Evolution of the cation chloride cotransporter family: Ancient origins, gene losses, and subfunctionalization through duplication. Mol. Biol. Evol. 31, 434–447 (2014).
Smith, J. J. et al. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 50, 270–277 (2018).
Ermakova, G. V., Kucheryavyy, A. V., Zaraisky, A. G. & Bayramov, A. V. Discovery of four Noggin genes in lampreys suggests two rounds of ancient genome duplication. Commun. Biol. 3, 501 (2020).
Barany, A., Shaughnessy, C. A. & McCormick, S. D. Corticosteroid control of Na+/K+-ATPase in the intestine of the sea lamprey (Petromyzon marinus). Gen. Comp. Endocrinol. 307, 113756 (2021).
Sundh, H. et al. Development of intestinal ion-transporting mechanisms during smoltification and seawater acclimation in Atlantic salmon Salmo salar. J. Fish Biol. 85, 1227–1252 (2014).
Esbaugh, A. J. & Cutler, B. Intestinal Na+, K+, 2Cl− cotransporter 2 plays a crucial role in hyperosmotic transitions of a euryhaline teleost. Physiol. Rep. 4, e13028 (2016).
Shaughnessy, C. A. & McCormick, S. D. Functional characterization and osmoregulatory role of the Na+-K+-2Cl− cotransporter in the gill of sea lamprey (Petromyzon marinus), a basal vertebrate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R17–R29 (2020).
Shaughnessy, C. A., Barany, A. & McCormick, S. D. 11-Deoxycortisol controls hydromineral balance in the most basal osmoregulating vertebrate, sea lamprey (Petromyzon marinus). Sci. Rep. 10, 12148 (2020).
Shaughnessy, C. A. & McCormick, S. D. 11-Deoxycortisol is a stress responsive and gluconeogenic hormone in a jawless vertebrate, the sea lamprey (Petromyzon marinus). J. Exp. Biol. https://doi.org/10.1242/jeb.241943 (2021).
Ferreira-Martins, D., Coimbra, J., Antunes, C. & Wilson, J. M. Effects of salinity on upstream-migrating, spawning sea lamprey, Petromyzon marinus. Conserv. Physiol. 4(1), cov064 (2016).
Dymowska, A. K., Hwang, P. P. & Goss, G. G. Structure and function of ionocytes in the freshwater fish gill. Respir. Physiol. Neurobiol. 184, 282–292 (2012).
Imaseki, I. et al. Comprehensive analysis of genes contributing to euryhalinity in the bull shark, Carcharhinus leucas; Na+-Cl− cotransporter is one of the key renal factors upregulated in acclimation to low-salinity environment. J. Exp. Biol. 222, 1–13 (2019).
Rankin, J. C. Drinking in hagfishes and lampreys. In Symp. Soc. Exp. Biol. 1–17 (2002).
Alves, A., Gregório, S. F., Egger, R. C. & Fuentes, J. Molecular and functional regionalization of bicarbonate secretion cascade in the intestine of the European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 233, 53–64 (2019).
Ferreira-Martins, D. et al. A cytosolic carbonic anhydrase molecular switch occurs in the gills of metamorphic sea lamprey. Sci. Rep. 6, 1–11 (2016).
Carvalho, E. S. M., Gregório, S. F., Power, D. M., Canário, A. V. M. & Fuentes, J. Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 182, 1069–1080 (2012).
Wilson, R. W. & Grosell, M. Intestinal bicarbonate secretion in marine teleost fish—source of bicarbonate, pH sensitivity, and consequences for whole animal acid–base and calcium homeostasis. Biochim. Biophys. Acta Biomembr. 1618, 163–174 (2003).
Rankin, J. C. Chapter 4: osmotic and ionic osmoregulation in cyclostomes. In Ionic Regulation in Animals: A Tribute to Professor W. T. W. Potts (eds Potts, W. T. W. et al.) (Springer, 1997).
Taylor, J. R. & Grosell, M. Evolutionary aspects of intestinal bicarbonate secretion in fish. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 143, 523–529 (2006).
Isenring, P., Jacoby, S. C., Payne, J. A. & Forbush, B. Comparison of Na-K-Cl cotransporters: NKCC1, NKCC2, and the hek cell Na-K-Cl cotransporter*. J. Biol. Chem. 273, 11295–11301 (1998).
Moreno, E. et al. The European Eel NCCβ gene encodes a thiazide-resistant Na-Cl cotransporter. J. Biol. Chem. 291, 22472–22481 (2016).
Madsen, S. S. et al. Functional characterization of water transport and cellular localization of three aquaporin paralogs in the salmonid intestine. Front. Physiol. 2 SEP, 1–14 (2011).
Youson, J., Lee, J. & Potter, I. The distribution of fat in larval, metamorphosing, and young adult anadromous sea lampreys, Petromyzon marinus L. Can. J. Zool. 57, 237–246 (1979).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
Acknowledgements
We thank Amy Regish, Jessica Norstog, Diogo Ferreira Martins, Daniel Hall, Hadley Kerr, and Alec Daigle for help in collecting and sampling sea lamprey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Funding
This research was funded by two International Mobility Program Fellowships from the University of Cadiz, UCA-INTERNACIONAL- POSGRADO and PU/PP-EST/MV/2018-004 (to A. Barany) and the National Science Foundation Grant IOS-1558025 (to S.D. McCormick). CCMAR is supported by Portuguese national funds from the Foundation for Science and Technology (FCT) through project UIDB/04326/2020.
Author information
Authors and Affiliations
Contributions
A.B., C.A.S., and S.D.M., conception and design of research; C.A.S., and S.D.M collected sea lamprey juveniles; A.B., conducted all live animal experimentation; A.B., and C.A.S., performed phylogeny; A.B., performed ex-vivo, molecular and electrophysiological analyses, data curation, and statistical analyses; A.B., wrote original manuscript draft; A.B., C.A.S., R.P., J.F., J.M.M., and S.D.M., edited, revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barany, A., Shaughnessy, C.A., Pelis, R.M. et al. Tissue and salinity specific Na+/Cl− cotransporter (NCC) orthologues involved in the adaptive osmoregulation of sea lamprey (Petromyzon marinus). Sci Rep 11, 22698 (2021). https://doi.org/10.1038/s41598-021-02125-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02125-1
This article is cited by
Effects of the environment on the evolution of the vertebrate urinary tract
Nature Reviews Urology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.