Exploratory Full-Field Mechanical Analysis across the Osteochondral Tissue—Biomaterial Interface in an Ovine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Production
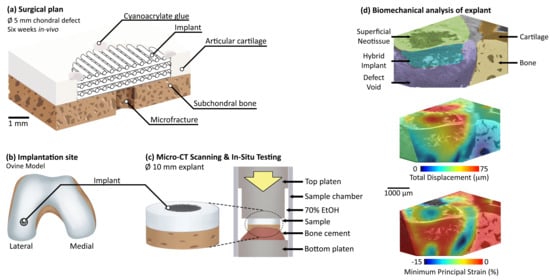
2.2. Surgical Procedure
2.3. Micro-CT Imaging and In Situ Mechanics
3. Results
3.1. In Situ Mechanical Loading and Deformation Visualisation
3.2. Error Quantification
3.3. Micro-CT Scanning Protocol
4. Discussion
4.1. Key Findings
4.2. Comparison to Literature
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, B.J.; Brown, W.E.; Keown, T.; Hu, J.C.; Athanasiou, K.A. Overcoming Challenges in Engineering Large, Scaffold-Free Neocartilage with Functional Properties. Tissue Eng. Part A 2018, 24, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Guettler, J.H.; Demetropoulos, C.K.; Yang, K.H.; Jurist, K.A. Osteochondral deflects in the human knee—Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am. J. Sports Med. 2004, 32, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.L.; Schenck, R.C., Jr.; Wascher, D.C.; Treme, G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 2016, 8, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckwalter, J.A.; Saltzman, C.; Brown, T. The impact of osteoarthritis: Implications for research. Clin. Orthop. Relat. Res. 2004, 427, S6–S15. [Google Scholar] [CrossRef]
- Samuels, J.; Krasnokutsky, S.; Abramson, S.B. Osteoarthritis: A tale of three tissues. Bull. NYU Hosp. Jt. Dis. 2008, 66, 244–250. [Google Scholar] [PubMed]
- Fodor, P.; Solyon, A.; Fodor, R.; Catoi, C.; Tabaran, F.A.; Lăcătuş, R.; Trambitas, C.; Bataga, T. Role of the Biomimetic Scaffolds in the Regeneration of Articular Tissue in Deep Osteochondral Defects in a Rabbit Model. Rev. Chim. 2018, 69, 201–207. [Google Scholar] [CrossRef]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly Osteochondral Regeneration in a Sheep Model Using a Novel Nano-Composite Multilayered Biomaterial. J. Orthop. Res. 2010, 28, 116–124. [Google Scholar] [CrossRef]
- Urbanek, O.; Kołbuk, D.; Wróbel, M. Articular cartilage: New directions and barriers of scaffolds development—Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 396–410. [Google Scholar] [CrossRef]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef]
- Schulz, R.M.; Bader, A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur. Biophys. J. 2007, 36, 539–568. [Google Scholar] [CrossRef]
- Ghouse, S.; Reznikov, N.; Boughton, O.R.; Babu, S.; Ng, K.G.; Blunn, G.; Cobb, J.P.; Stevens, M.M.; Jeffers, J.R. The design and in vivo testing of a locally stiffness-matched porous scaffold. Appl. Mater. Today 2019, 15, 377–388. [Google Scholar] [CrossRef]
- Darnell, M.; Mooney, D.J. Leveraging advances in biology to design biomaterials. Nat. Mater. 2017, 16, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Wazen, R.M.; Currey, J.A.; Guo, H.; Brunski, J.B.; Helms, J.A.; Nanci, A. Micromotion-induced strain fields influence early stages of repair at bone-implant interfaces. Acta Biomater. 2013, 9, 6663–6674. [Google Scholar] [CrossRef] [Green Version]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using X-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Madi, K.; Staines, K.A.; Bay, B.K.; Javaheri, B.; Geng, H.; Bodey, A.J.; Cartmell, S.; Pitsillides, A.A.; Lee, P.D. In situ characterization of nanoscale strains in loaded whole joints via synchrotron X-ray tomography. Nat. Biomed. Eng. 2019, 4, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, G.; Peña Fernández, M.; Davis, S.; Karali, A.; Kao, A.P.; Blunn, G. Full-Field Strain Uncertainties and Residuals at the Cartilage-Bone Interface in Unstained Tissues Using Propagation-Based Phase-Contrast XCT and Digital Volume Correlation. Materials 2020, 13, 2579. [Google Scholar] [CrossRef]
- Sukjamsri, C.; Geraldes, D.M.; Gregory, T.; Ahmed, F.; Hollis, D.; Schenk, S.; Amis, A.; Emery, R.; Hansen, U. Digital volume correlation and micro-CT: An in-vitro technique for measuring full-field interface micromotion around polyethylene implants. J. Biomech. 2015, 48, 3447–3454. [Google Scholar] [CrossRef]
- Tozzi, G.; Dall’Ara, E.; Palanca, M.; Curto, M.; Innocente, F.; Cristofolini, L. Strain uncertainties from two digital volume correlation approaches in prophylactically augmented vertebrae: Local analysis on bone and cement-bone microstructures. J. Mech. Behav. Biomed. Mater. 2017, 67, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Rapagna, S.; Berahmani, S.; Wyers, C.E.; van den Bergh, J.P.; Reynolds, K.J.; Tozzi, G.; Janssen, D.; Perilli, E. Quantification of human bone microarchitecture damage in press-fit femoral knee implantation using HR-pQCT and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2019, 97, 278–287. [Google Scholar] [CrossRef]
- Peña Fernaández, M.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Full-Field Strain Analysis of Bone-Biomaterial Systems Produced by the Implantation of Osteoregenerative Biomaterials in an Ovine Model. ACS Biomater. Sci. Eng. 2019, 5, 2543–2554. [Google Scholar] [CrossRef] [Green Version]
- Le Cann, S.; Tudisco, E.; Perdikouri, C.; Belfrage, O.; Kaestner, A.; Hall, S.; Tägil, M.; Isaksson, H. Characterization of the bone-metal implant interface by Digital Volume Correlation of in-situ loading using neutron tomography. J. Mech. Behav. Biomed. Mater. 2017, 75, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Peña Fernández, M.; Black, C.; Dawson, J.; Gibbs, D.; Kanczler, J.; Oreffo, R.O.; Tozzi, G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayne, J.S.; McDowell, C.L.; Shields, K.J.; Tuan, R.S. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005, 11, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Tallia, F.; Russo, L.; Li, S.; Orrin, A.L.; Shi, X.; Chen, S.; Steele, J.A.; Meille, S.; Chevalier, J.; Lee, P.D.; et al. Bouncing and 3D printable hybrids with self-healing properties. Mater. Horiz. 2018, 5, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tallia, F.; Mohammed, A.A.; Stevens, M.M.; Jones, J.R. Scaffold channel size influences stem cell differentiation pathway in 3-D printed silica hybrid scaffolds for cartilage regeneration. Biomater. Sci. 2020. [Google Scholar] [CrossRef]
- Clark, J.N.; Garbout, A.; Ferreira, S.A.; Javaheri, B.; Pitsillides, A.A.; Rankin, S.M.; Jeffers, J.R.T.; Hansen, U. Propagation phase-contrast micro-computed tomography allows laboratory-based three-dimensional imaging of articular cartilage down to the cellular level. Osteoarthr. Cartil. 2020, 28, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Morgan, E.F. Accuracy and precision of digital volume correlation in quantifying displacements and strains in trabecular bone. J. Biomech. 2007, 40, 3516–3520. [Google Scholar] [CrossRef] [Green Version]
- Peña Fernández, M.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Optimization of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Buljac, A.; Taillandier-Thomas, T.; Helfen, L.; Morgeneyer, T.F.; Hild, F. Evaluation of measurement uncertainties of digital volume correlation applied to laminography data. J. Strain Anal. Eng. Des. 2018, 53, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Roux, S.; Hild, F.; Viot, P.; Bernard, D. Three-dimensional image correlation from X-ray computed tomography of solid foam. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1253–1265. [Google Scholar] [CrossRef] [Green Version]
- Hickey, D.S.; Hukins, D.W. Effect of methods of preservation on the arrangement of collagen fibrils in connective tissue matrices: An x-ray diffraction study of annulus fibrosus. Connect Tissue Res. 1979, 6, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Lowe, T.; Shearer, T. Optimal Contrast Agent Staining of Ligaments and Tendons for X-Ray Computed Tomography. PLoS ONE 2016, 11, e0153552. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Scan Protocol | Voltage (kV) | Current (µA) | SOD (mm) | ODD (mm) | Voxel Size (µm) | Number of Projections | Exposure Time Per Projection (s) | Liquid Medium |
|---|---|---|---|---|---|---|---|---|
| Coarse | 80 | 87.5 | 40 | 95 | 10.2 | 2401 | 5 | PBS |
| DVC study | 60 | 83 | 26 | 172 | 4.5 | 1201 | 16 | 70% EtOH |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, J.N.; Heyraud, A.; Tavana, S.; Al-Jabri, T.; Tallia, F.; Clark, B.; Blunn, G.W.; Cobb, J.P.; Hansen, U.; Jones, J.R.; et al. Exploratory Full-Field Mechanical Analysis across the Osteochondral Tissue—Biomaterial Interface in an Ovine Model. Materials 2020, 13, 3911. https://doi.org/10.3390/ma13183911
Clark JN, Heyraud A, Tavana S, Al-Jabri T, Tallia F, Clark B, Blunn GW, Cobb JP, Hansen U, Jones JR, et al. Exploratory Full-Field Mechanical Analysis across the Osteochondral Tissue—Biomaterial Interface in an Ovine Model. Materials. 2020; 13(18):3911. https://doi.org/10.3390/ma13183911
Chicago/Turabian StyleClark, Jeffrey N., Agathe Heyraud, Saman Tavana, Talal Al-Jabri, Francesca Tallia, Brett Clark, Gordon W. Blunn, Justin P. Cobb, Ulrich Hansen, Julian R. Jones, and et al. 2020. "Exploratory Full-Field Mechanical Analysis across the Osteochondral Tissue—Biomaterial Interface in an Ovine Model" Materials 13, no. 18: 3911. https://doi.org/10.3390/ma13183911