Published online Apr 15, 2024. doi: 10.4239/wjd.v15.i4.606
Peer-review started: December 31, 2023
First decision: January 16, 2024
Revised: January 22, 2024
Accepted: February 27, 2024
Article in press: February 27, 2024
Published online: April 15, 2024
Coronavirus disease 2019 (COVID-19) is a disease that caused a global pandemic and is caused by infection of severe acute respiratory syndrome coronavirus 2 virus. It has affected over 768 million people worldwide, resulting in approximately 6900000 deaths. High-risk groups, identified by the Centers for Disease Control and Prevention, include individuals with conditions like type 2 diabetes mellitus (T2DM), obesity, chronic lung disease, serious heart conditions, and chronic kidney disease. Research indicates that those with T2DM face a hei
Core Tip: This manuscript explored the complex connection between type 2 diabetes mellitus and coronavirus disease 2019 (COVID-19), emphasizing the heightened susceptibility of diabetic individuals. It revealed the crucial involvement of the renin-angiotensin system and angiotensin converting enzyme (ACE)/ACE2 enzymes, clarifying how decreased ACE2 expression in diabetes amplifies renin-angiotensis system activity, contributing to inflammation and fibrosis. While ACE inhibitors exhibit therapeutic potential, concerns arise regarding their potential to facilitate viral entry. The review suggested that innovative approaches, including recombinant human ACE2 therapy and the detection of epigenetic signatures, present promising avenues for addressing COVID-19 and its complications in type 2 diabetes mellitus.
- Citation: Shukla AK, Awasthi K, Usman K, Banerjee M. Role of renin-angiotensin system/angiotensin converting enzyme-2 mechanism and enhanced COVID-19 susceptibility in type 2 diabetes mellitus. World J Diabetes 2024; 15(4): 606-622
- URL: https://www.wjgnet.com/1948-9358/full/v15/i4/606.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i4.606
Coronavirus disease 2019 (COVID-19) is caused by a novel virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which globally expanded from Wuhan, China at the end of 2019. The World Health Organization declared COVID-19 a global pandemic on March 11, 2020 (World Health Organization Situation Reports 51). According to weekly epidemiological updates globally, over 4500 deaths and more than 836000 new COVID-19 cases had been recorded from June 19 to July 16, 2023. More than 6.9 million deaths and 768 million confirmed cases were recorded worldwide[1]. Most severe COVID-19 cases have been observed in elderly people or comorbid individuals, including type 2 diabetes mellitus (T2DM), hypertension, cardiovascular diseases (CVDs), chronic renal and lung disorders, and cancer[2-5]. According to a meta-analysis including 1527 patients in China, patients with diabetes or hypertension had a two-fold increase in risk of severe COVID-19 or requiring intensive care unit (ICU) admission, while it increased to three-fold in patients with cer
To effectively manage patients with comorbid disorders, it is crucial to comprehend the relationship between COVID-19 and chronic diseases. Additionally, it enables medical professionals to lessen the side effects linked to this disease. Hence, understanding these mechanisms will help develop a potential treatment for COVID-19. Herein, this review offered proof of the severe COVID-19 manifestation in T2DM and other chronic illnesses, including heart disease, hypertension, and myocardial infarction. More importantly, it analyzed the specific management techniques for patients with the aforementioned comorbid disorders to obtain the greatest therapeutic outcome, described in detail the molecular pathways through which COVID-19 causes these diseases, and highlighted the importance of these strategies.
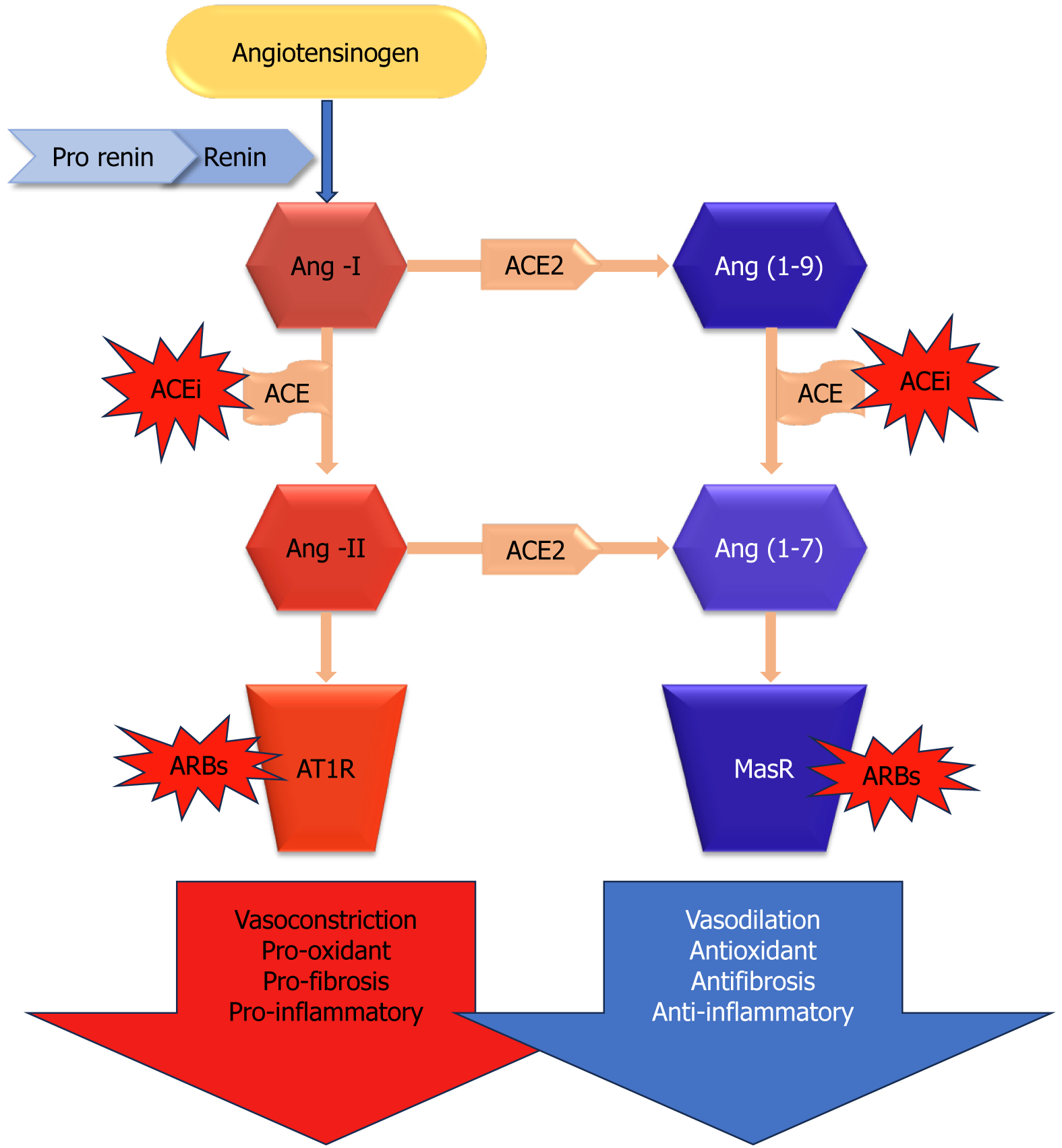
Angiotensin converting enzyme 2 (ACE2) is a key enzyme in the renin-angiotensin-aldosterone system (RAAS), which plays a critical role in the regulation of several physiological processes in the human body. ACE2, a component of RAAS, oversees vasoconstriction to manage blood pressure, electrolyte and fluid balance, and renal salt reabsorption[13]. In response to diminished renal blood flow, juxtaglomerular cells convert prorenin to renin, which is then released into the circulation spontaneously. Plasma renin converts hepatic angiotensinogen to angiotensin I (Ang-I), and Ang-I is further transformed into the vasoconstrictor Ang-II by ACE (Figure 1). By converting Ang-II into Ang-(1-7), a vasodilator, ACE2 is essential for maintaining system balance[13]. Thus, ACE2 controls several pathological states, including oxidative stress, fibrosis, vasoconstriction, and inflammation (Figure 1). To mitigate RAAS hyperactivity, specific medications disrupt this system through various approaches. For instance, to reduce the synthesis of robust Ang-II, ACE enzyme inhibitors (ACEi) are commonly employed. Another method involves the use of angiotensin-II receptor blockers (ARBs) to inhibit angiotensin-II type 1 receptor (AT1R), thereby controlling an overactive RAAS[14].
Additionally seen in the glomerulus, ACE2 colocalizes with ACE on the apical surface of the proximal tubules[15]. Similar to ACE in distribution, ACE2 was shown to be confined to pancreatic acini and islets[16]. It has been shown that in diabetic animal models, ACE2 pharmacological suppression[15,17] and genetic ablation[18] both exacerbate albuminuria and the associated glomerular lesions. Additionally, diabetic nephropathy patients’ kidney biopsies[19] and diabetic rodent models[15] have demonstrated decreased glomerular expression of ACE2. Thus, in order to address renal impairment linked to diabetes, increasing the activity of this enzyme by therapeutic targeting of ACE2 has been suggested[15,20].
Changes in glucose tolerance and decreased first-phase insulin production have been seen in ACE2 knockout mice, indicating that diabetes development may be influenced by ACE2[21]. According to reports, pancreatic β cells contain collectrin, another ACE homolog, which may play a role in insulin production and β cell proliferation despite its unknown function[22]. Concentrating on comprehending the involvement of ACE2 in diabetes, emphasizing ACE2 within the kidney could emerge as a crucial focal point for addressing and preventing diabetic kidney disease. This progressive condition is marked by thickening and expansion of the mesangial matrix around the glomerular basement membrane, the presence of elevated protein in urine, and associated features linked to kidney disease. In recent times, several research studies have also suggested that the loss of podocytes[23-27] is connected to the deterioration of glomerulopathy[28]. The kidney exhibits a completely operational local renin-angiotensin system (RAS), with the ability to produce Ang-II in hyperglycemic circumstances[29,30] as observed by its activation[31].
The presence of ACE is reported in the kidney, pancreas, adipose tissue, and liver. Its expression level is elevated in the endocrine system involving the pancreas in diabetes and in the early stages of diabetic nephropathy. The proposition suggests that ACE2 plays an equal role in compensating for both diabetic nephropathy and diabetes. Furthermore, it appears that mice lacking ACE2 also had higher blood glucose levels following fasting, as evidenced by their elevated fasting blood glucose levels. A novel method for examining the role of ACE2 in diabetes research may be found in mice[32]. On the apical surface of the proximal tubules and in the glomerulus, ACE2 colocalizes with ACE[15]. Similar to ACE, ACE2 was shown to be distributed in the pancreas and localized to acini and islets[16].
More than 20 years ago, Chappell et al[33] discovered that the pancreas, like the kidney, expresses significant components of a local angiotensin peptide-producing machinery. With decreased levels of Ang-(1-7) and undetectable quantities of Ang-I, the investigators found that Ang-II was the most prevalent angiotensin peptide in the pancreas. As it has been speculated to do in the kidneys and other organs, Ang-(1-7) may mitigate the effects of Ang-II in the pancreas[34]. In comparison to the plasma, both peptides were found in much larger concentrations in the pancreas[33]. Ang-II has the ability to inhibit blood flow in a dose-dependent manner and delay the generation of insulin in the Langerhans islets, as revealed by Carlsson et al[34]. The effects of AngII are consistent with increasing blood flow by RAS blockage with ACE inhibitors or AngII receptor antagonists[34]. Moreover, it has been demonstrated that these medications lessen pancreatic fibrosis and inflammation[35]. In a noteworthy study, Tikellis et al[16] found that in the Zucker diabetic fatty rat, RAS inhibition enhanced structural parameters in relation to increased first-phase insulin secretion and reduced islet fibrosis. Given clinical evidence that RAAS suppression may be associated with a decreased risk of T2D with a new start, these findings are particularly noteworthy[36]. Though more recent studies have cast doubt on these results[37], ongoing current investigations have demonstrated that RAAS blockage aids in the prevention of T2DM.
Based on animal research, it was hypothesized that ACE/ARB therapy increases ACE2 expression. After the reco
Over time, expression of ACE2 and functioning in several tissues decrease in complications such as T2DM and hypertension[19,41-43]. The mechanisms behind the reported decrease in ACE2 in these illnesses are unclear. One explanation for declining levels of ACE2 might be its depletion from tissues. Cell-membrane-bound ACE2 has been discovered to be cleaved by a sheddase, disintegrin, and metalloproteinase (ADAM17) or tumor necrosis factor-α (TNF-α) converting enzyme[44], releasing some of the tissue-resident protein into the bloodstream. In fact, ADAM17 may be the cause of ACE2 shedding in T2DM patients due to its elevation in human carotid atherosclerotic plaques[45]. Interestingly, the pancreas, brain, adipose tissues, and vascular smooth muscle cells all express more ADAM17 when exposed to Ang-II[46,47]. The phenomenon of ACE2 downregulation in response to RAS overactivity in the islets of mice can be attributed to ACE2 shedding mediated by ADAM17[42]. The surge in ADAM17 expression is lowered by ACE2 treatment, sug
SARS-CoV-2 belongs to the Beta-coronavirus genus, which encompasses other notably contagious viruses including the Middle East respiratory syndrome coronavirus and the SARS coronavirus (SARS-CoV)[48]. SARS-CoV and SARS-CoV2 have about 80% sequence similarity[49]. Both viruses enter cells by binding to the ACE2 with their spike proteins (S-protein)[49]. An extraordinarily high rate of human transmission makes SARS-CoV-2 different[50]. Indeed, SARS-CoV-2 exhibits a heightened attraction to ACE2, contributing to its increased transmissibility. Through its S-protein, SARS-CoV-2 binds to human cells. ACE2[51], which is widely expressed on the surfaces of pulmonary alveolar epithelial cells, venous and arterial endothelial cells, arterial smooth muscle cells, and small intestine enterocytes, is one of the main SARS-CoV-2 receptors[38,52-55].
The S-protein is split into S1 and S2 subunits after viral infection[54]. While the S2 subunit aids in membrane fusion, the S1 subunit directly interacts with the host cell’s ACE2 receptor through its receptor binding domain[54]. SARS-CoV-2 and SARS-CoV have very similar receptor binding regions. In contrast, the SARS-CoV-2 binding site exhibits enhanced stability and compactness, displaying a greater affinity for ACE2 and its ability to infect the host cell is increased by the presence of a Furin at the cleavage site[56,57]. After the binding is complete, ACE2 activity is downregulated[54]. ACE2 downregulation is due to the activation of ADAM-17/TNF-α converting enzyme by the SARS S-protein, which cleaves and releases ACE2[44,58]. Increased circulating levels of ACE2 were found interconnected with the severity of lung injury and the viral load[56]. The downregulation of ACE2 after SARS-CoV-2 infection increases Ang-II concentration in the lungs causing severe lung injury. Another study reported ACE2 downregulation in the heart and severe heart injury[59].
There are a number of potential causes for the increased severity of COVID-19 in individuals with concomitant illnesses. The increased expression of ACE2 in certain organs, including the kidneys, small intestine, lungs, and heart[52,56,60], might elucidate the severe manifestation of COVID-19 in specific patient groups. This is because ACE2 serves as the functional receptor through which SARS-CoV-2 enters cells[61]. In addition, the overactive immune response to the virus that contributes significantly to the disease severity is known as the “cytokine storm”[62,63]. It is intriguing to note that COVID-19 was shown to trigger the abrupt beginning of long-term organ damage in people who did not already have chronic illnesses. One of these consequences is cardiac injury[64], along with kidney injury[65], renal injury[65], liver damage[67], gastrointestinal disorders[66], and acute or chronic diabetes[68]. This makes COVID-19 more challenging and increases the likelihood that many infected people may develop chronic morbidities that are incapacitating. Interestingly, there is a scarcity of clinical data that supports these pathophysiological processes.
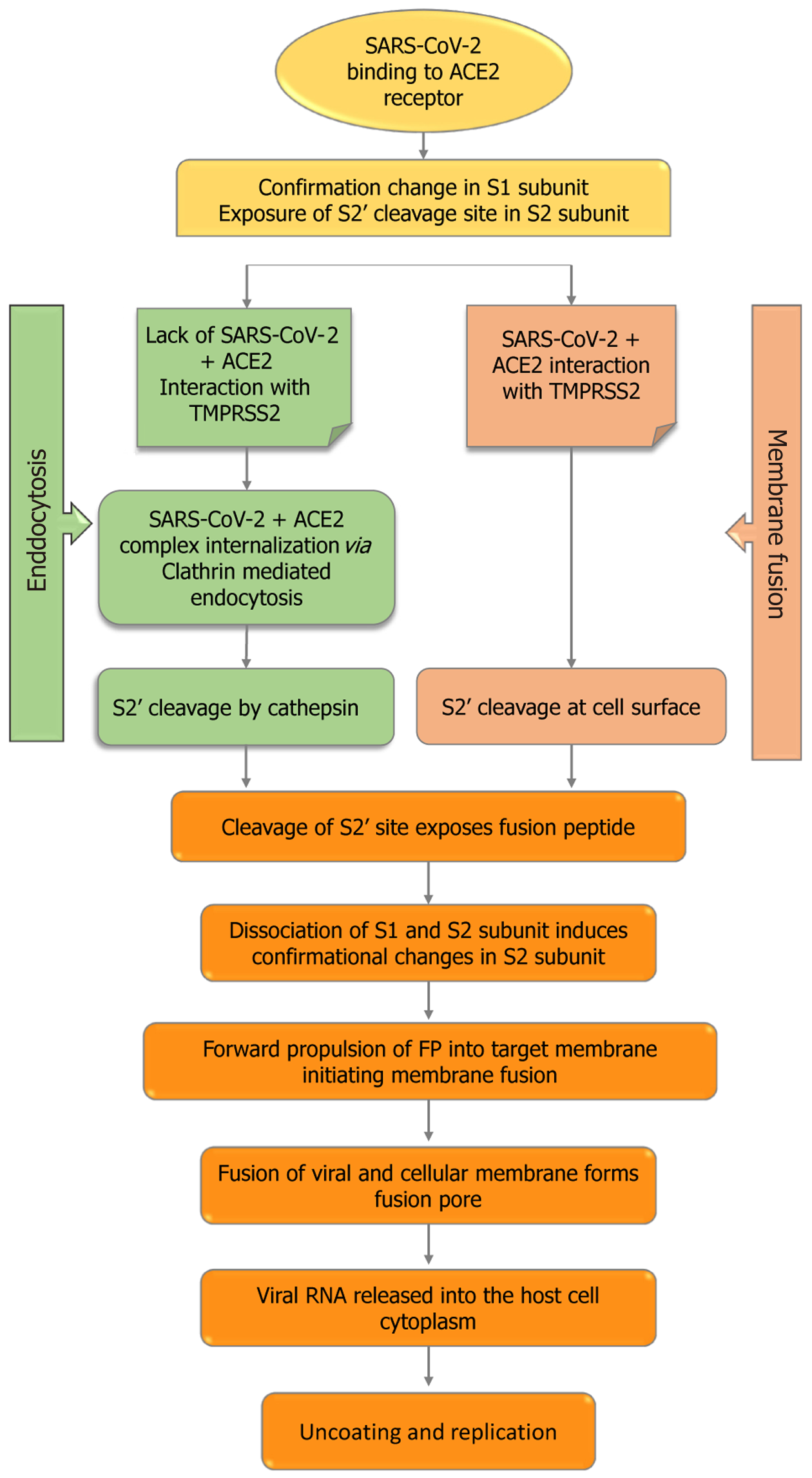
Typically, coronaviruses require two cleavage events of the S-protein for their entry process (Figure 2). Two cleaves occur: One at the S1-S2 subunit junction and the other at the S2’ location inside the S2 subunit[69]. Regarding SARS-CoV-2, the polybasic sequence cleaves at the S1-S2 border as the virus matures in an infected cell. Nevertheless, once ACE2 binds, the target cell cleaves at the S2’ location. The S2’ cleavage site in the S2 subunit is made visible by conformational changes in the S1 subunit caused by the interaction between the virus and ACE2. The particular proteases that cause the cleavage at the S2’ site differ according to the SARS-CoV-2 entry pathway that is selected.
When transmembrane protease, serine 2 (TMPRSS2) is insufficient in the target cell or when a virus-ACE2 complex is not in contact with TMPRSS2, the virus-ACE2 complex is internalized via clathrin-mediated endocytosis[70]. Cathepsins within endolysosomes cleave the S2’ site as a result of this internalization, and they require an acidic environment to function. Conversely, in the presence of TMPRSS2, S2’ cleaves at the cell surface. The fusion peptide is exposed in both routes by the cleavage of the S2’ site, and the S2 subunit undergoes major conformational changes as a result of S2 spli
Increased blood glucose and glycosylation product synthesis in patients with diabetes could enhance the working of RAAS, notably Ang-II. When exposed to excessive amounts of Ang-II, a variety of tissues can stimulate dihydro nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and increase oxidative stress, which can lead to increased insulin resistance, complications from diabetes, and a worse prognosis for COVID-19[71-73]. Moreover, Ang-II worsens diabetes by promoting fibrosis, cell death, and a reduction in the islet blood supply and early stages of insulin synthesis[73]. Ang-I is converted to Ang-(1-9) by ACE2, whereas Ang-II is converted to Ang-(1-7). These two physiologically active downstream peptides encourage vasodilation, which has been linked to anti-inflammatory, antifibrotic, and antiproliferative properties[74]. In addition to partially protecting islet cells and preventing diabetes, ACE2 suppresses conventional RAAS activation[75]. These receptors are downregulated as a result of the interaction of SARS-CoV-2 and ACE2, which is followed by membrane fusion and viral entry into the cell. This exacerbates cellular damage, hyperinflammation, and respiratory failure[76,77]. Increased ACE2 insufficiency may exacerbate the dysregulation between the “protective” ACE2 Ang-(1-7) Mas receptor axis and the “adverse” ACE Ang-II AT1R axis following viral invasion[77]. Insulin secretion may be hampered by ACE2 downregulation upon viral entry if Ang-II is not inhibited. These factors may play a role in the rapid degradation of pancreatic cell function and the development of diabetic ketoacidosis[78], suggesting that the prognosis for patients with diabetes after SARS-CoV-2 infection may be bad. Nevertheless, it is uncertain if ACE2 has a mechanistic role in the development of dysglycemia or elevated complications in diabetics[79]. Evidence suggests that RAS inhibitors, which include ACEi and ARBs, increase the expression of ACE2, which may have a protective effect against diabetes and hypertension but also make it easier for viruses to enter host cells, which may increase the likelihood or severity of infection[80]. However, recent studies have not demonstrated that using ACEi or ARBs raises the likelihood of SARS-CoV-2 infection or the risk of serious consequences in COVID-19 individuals[74,81-83].
Diabetes is considered to be one of the leading causes of death worldwide. Elderly people with T2DM are more severely prone to infections such as pneumonia and influenza[84,85]. The International Diabetes Federation estimated that 537 million adult individuals were diagnosed with diabetes in 2021[86], with this figure anticipated to climb to 643 million by 2030 and 783 million by 2045[87].
Several studies could not find a clear association between COVID-19 severity and diabetes[12,88]. However, studies in Italy[89] and China[2] showed the perilous threat of mortality and severe COVID-19 in older patients with some chronic diseases, including diabetes. Diabetes has been identified as an elevated risk for severe COVID-19 since the pandemic began. It is indeed one of the most frequently documented comorbidities among COVID-19 patients who were brought to the ICU and later passed away[3,11]. In a study that included 52 COVID-19 patients who were brought to the ICU, 32 (61%) of the patients had passed away 28 d after discharge; the most prevalent comorbidities in these patients were diabetes (22%) and cerebrovascular illnesses (22%)[4].
A different study involving 44672 individuals with SARS-COV-2 found that the COVID-19 case fatality rate was 7.3% in diabetic individuals and 2.3% in non-diabetic individuals[3]. Numerous variables may be responsible for the severe presentation, even if the cause of the poor prognosis in patients with diabetes is unknown[90]. First of all, poorly managed diabetes compromises the ability of the immune system to fight viral infections[91]. The capacity to eliminate viruses is particularly hampered by poor T cell function, which weakens the natural defense system[92]. Second, plasminogen levels are increased in diabetic individuals[93]. This particular protein cleaves the S-protein of SARS-CoV-2, which enhances the viral cellular entry; further, it leads to an increase in the viral contagion and infestation of the virus[93]. Alongside, inflammatory biomarkers like interleukin-6 (IL-6), C-reactive protein, and D-dimer were found to have increased levels in diabetic SARS-COV-2 individuals in comparison to those without diabetes, which indicates that the risk of worsening COVID-19 outcomes might be increased due to T2DM[64].
Due to their renal protective properties, ACEi is frequently prescribed to diabetic patients. This class of drugs raises the expression of ACE2, due to which viral entrance could be facilitated into the cells of the host[94]. FURIN and TMPRSS are two additional genes linked with cellular entry of SARS-CoV-2 that remain upregulated in T2DM, according to mic
Data are scarce regarding the advancement of diabetic complications and glucose metabolism in individuals having COVID-19. According to retrospective research, 10% of patients with COVID-19 and T2DM had at least one episode of hypoglycemia (< 3.9 mmol/L)[5]. However, SARS-CoV-2 infection in diabetic individuals may result in elevated stress levels and the release of more hyperglycemic hormones, such as glucocorticoids and catecholamines, which raises blood sugar levels[96].
Numerous vascular and metabolic problems associated with diabetes might impact our body’s ability to respond to infections[97]. Hyperglycemia as well as insulin resistance enhance the synthesis of adhesion molecules, which promote inflammation of tissues, and increase the production of proinflammatory cytokines, advanced glycation end products, and oxidative stress[97,98]. The undivulged phenomena that increases the risk of infestation in patients with diabetes may be this inflammatory response[97].
Some studies have reported an association of hyperglycemia with defects in immunity[99]. Compliment activation dysfunction[100] and abnormal delayed-type hypersensitivity reaction[99] have been reported in diabetic individuals. Studies conducted in vitro revealed that exposure to elevated concentrations of glucose enhanced influenza virus infection and replication in pulmonary epithelial cells, suggesting elevated viral replication in vivo during hyperglycemic circumstances[101].
COVID-19 epidemiology and its relationship to cardiometabolic diseases are poorly known. Current statistics reveal that people with COVID-19 and diabetes mellitus have a higher risk of death in comparison with individuals without diabetes mellitus. Resistance to insulin is caused by COVID-19 in individuals, resulting in persistent metabolic problems that did not exist before infection. Patients with diabetes are more vulnerable to SARS-CoV-2 infection than individuals who do not have diabetes. With infection, ACE2 expression declines, magnifying Ang-II activity and leading to insulin resistance, an increased immunological response, and severe SARS-COV-2 infection[102]. Exocrine and endocrine pancreatic synthesis of ACE2 is likely to be associated with an increased presentation of diabetes in subgroups of critically unwell SARS-CoV-2 infected persons[79]. Through binding to the ACE2 receptor as seen in SARS-Cov-1[103], SARS-CoV-2 promotes pancreatic islet destruction and the development of acute diabetes[4].
ACE2 is one of the main receptors of both SARS-CoV-2[51] and SARS-CoV[104]. When CoVs get introduced to host cells, the CoVs attach to specific ACE2 receptors with the help of their S-proteins. The S-protein is then cleaved by host cell protease, allowing the virus to enter and multiply within the host cells[51]. Patients with diabetes and hypertension frequently use medications called ACEi and ARBs[105]. These drugs are known to increase the ACE2 concentration, and thus their use is controversial as it might negatively affect the outcomes of COVID-19 patients[55]. On the other hand, some recommended ARBS and ACEi might be beneficial[106].
SARS-CoV-2 infection has been potentiated by primary complications that are illustrated as T2DM and its associated metabolic comorbidities. Mentioned below are the other related comorbidities and their possible mechanisms involved in the pathophysiology of SARS-CoV-2 infection and different strategies involved in the treatment of COVID-19.
The initial study in China indicated a high occurrence (30%) of hypertension in severe SARS-COV-2 cases[107], which is not very surprising as 23.2% of the adult population of China are estimated to have hypertension[108]. According to another report, out of 406 patients who died from COVID-19, 39.7% of patients had hypertension[109], which is higher than the general population. Hypertension may also result in other cardiovascular risk factors like hypertension-mediated organ damage, diabetes, or cardiovascular complications[110], and their prevalence increases with age.
Italy was the first European country that was most severely affected by COVID-19[111,112]. According to a report published on June 18, 2020 by Epidemiology of Public Health, the median age of patients in Italy who died with COVID-19 was 82 years, and 66.8% deceased COVID-19 patients had pre-existing hypertension (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths). These numbers were much higher than reported in China but were consistent with estimated high prevalence of hypertension of this age group in Italy[113]. Furthermore, 17% and 30% of deceased patients with COVID-19 were being treated with ARBs and ACEi, respectively. These data suggest that COVID-19 patients with hypertension exhibit an increased fatality rate as compared with overall infected patients, with elderly patients being at greatest risk.
A recent study conducted to understand the role of immune dysregulation in hypertension[114] provided the possible mechanism linking immune dysregulation and COVID-19 severity. An increased level of systemic IL-7, IL-6, IL-2, C-X-C motif chemokine 10, chemokine ligand 2, granulocyte colony-stimulating factor, and TNF-α was observed in COVID-19 patients[7]. Interestingly, these are the same cytokines that are associated with hypertension development in interventional[114], clinical observational[115], and experimental studies[116,117].
Another study reported dysregulation of CD8+ and CD4+ cells in hypertension[118], indicating elevated productivity of proinflammatory cytokines, which includes those related with COVID-19 (IL-6, IL-7, IL-17, TNF-α, and interferon-γ)[119]. Hypertension was associated with a distinct immune senescent profile of CD8+ cells[114,119,120], which were likely to overproduce cytokines but are weak in antiviral defense. These mechanisms significantly contribute to acc
ARBs are usually used for the treatment of hypertension and are considered the main therapy for hypertension and associated renal and cardiovascular comorbidities[110]. Several reports showed upregulation of ACE2 upon treatment with ACEi and/or ARBs[123]. Hence, some authors hypothesized that RAAS blockers may enhance the susceptibility and COVID-19 risk. They recommended avoiding ARBs and ACEi during the COVID-19 pandemic[40,124]. Several studies conducted to investigate the effect of RAS blockade on ACE2 showed variable results. These variations may depend on different pathological conditions, like hypertension vs normotension, animal strains used, organs investigated, and most importantly type and dosage of the drug. Moreover, very little human research has been conducted to look at how RAS blockage affects the expression of ACE2. A study showed that olmesartan, an ARB, can significantly increase urinary ACE2 in hypertensive patients when treated for > 1 year[125]. Similar results were seen in patients with diabetic nephropathy upon treatment with olmesartan for 24 wk[126]. On the other hand, urinary ACE2 levels were not affected by ACEi or ARBs in diabetic patients[127].
There is no evidence that shows the usage of ACEi and/or ARBs that directly enhances the susceptible SARS-CoV-2 and its severity and infectivity. Therefore, it is recommended to continue the use of RAAS blockers in stable COVID-19 patients with hypertension and other related diseases, like T2DM, heart failure, and chronic kidney disease[110,128].
Several studies of COVID-19 showed extreme variability ranging from asymptomatic to some mild symptoms like dry cough, fatigue, and fever. According to some early reports, a high prevalence and association of comorbidities was seen with severe COVID-19 and mortality[2,4,5,7,10,12,82]. Among different comorbidities, the involvement of heart-related ailments appeared more significant. In a report from the Chinese Control for Disease Control and Prevention of 72314 cases, 1023 deaths were reported among 44672 confirmed cases (i.e. 2.3% mortality rate). However, this percentage went up to 10.5% in instances of diabetes, 7.3% in cases of cardiovascular illness, 6.3% in situations of chronic respiratory conditions, and 6.0% in cases of hypertension[3].
Myocardial damage during COVID-19 might be asymptomatic, similar to other acute diseases. It can only be identified by detecting troponin levels beyond the 99th percentile upper reference range. Patients with CVDs were shown to be particularly affected by COVID-19[2], and many of them had myocardial stress[9], active myocardial damage[5,9,129], and cardiomyopathy[9] while they were unwell. There was an increase in the cardiac troponin levels in 8%-12% of unselected COVID-19 patients[5,7,64,129]. Other studies also reported higher plasma levels of N-terminal pro-brain natriuretic peptide in cases with myocardial injury, although these increased levels were not independently associated with the outcomes[64,129].
Some of the cardiac complications, like cardiomegaly, hypotension, and heart failure were previously seen in SARS-CoV infections[130]. Acute myocarditis or myocardial damage may lead to severe cardiac dysfunction or heart failure in COVID-19 cases. This is often difficult to diagnose and controversial. Although very few COVID-19-associated acute myocarditis cases were reported[131-134], they might be severe with low cardiac output and hypotension that needed end-stage heart failure inotropic therapy. The endomyocardial biopsy showed limited or no myocardial necrosis and different levels of myocardial inflammation[131-134].
Only 1 of the 2 patients who underwent endomyocardial biopsy met the criteria for acute myocarditis[133]. SARS-CoV-2 was also detected in macrophages but not in cardiomyocytes in a different instance. The biopsy results from these cells revealed only modest interstitial cardiac inflammation and some non-specific alterations in heart myocytes, such as lipid droplets and myofibrillar lysis[134]. These data indicate that the virus does not have any direct pathogenetic role, even though it can reside within the heart[5,135]. Hence, it can be assumed that apart from viral infection, other mechanisms responsible for myocardial injury may exist[5,135,136].
Cardiomyocytes, coronary endothelial cells, and cardiac fibroblasts all have ACE2 on their surface. It has been discovered that individuals with diabetes, cardiovascular conditions, and those using ARBs and/or ACEi had elevated levels of ACE2[136-140]. This has been reported in myocardium tissue samples of patients with end-stage heart failure[139,140], experimental models[137,138], and circulating plasma levels of ACE2[140]. ACE2 knockout mice develop heart failure with reduced ejection fraction and systolic dysfunction in left ventricle[59]. Several studies conducted on experimental models showed that ACE2 gene overexpression reduced fibrosis, oxidative stress, and myocardial hypertrophy, which improved the diastolic function of the left ventricle[141,142]. Notably, ACE2 also has immunomodulatory properties, both indirectly by decreasing Ang-II concentration, which favors inflammation and interacts with macrophages[56,143].
For the first time, Sama et al[140] assessed the levels of ACE2 in circulation in a group of patients with heart failure that included 1485 males and 537 females from Europe. The findings were confirmed in a separate survey. The ACE2 plasma levels were found to be increased in males in both the cohorts explaining high susceptibility and fatality rate in males[140], and this elevated ACE2 concentration was consistent with a higher prevalence and severity of COVID-19[2,3,140]. Consequently, elevated ACE2 expression may heighten the sensitivity to COVID-19 and may intensify the infection by augmenting the viral load within the cell. Because the administration of ARBs/ACEi upregulates the expression of ACE2, there are concerns regarding the usage of these drugs, which have not yet been verified[40,144-146].
Secondly, SAR-CoV-2 infection downregulates ACE2 and thus increases the Ang-II levels, which stimulates AT1R. Acute respiratory distress syndrome (ARDS), heart and/or lung damage, and other COVID-19 problems may be primarily brought on by elevated Ang-II activity[145,147,148]. This demonstrates the protective function of ACE2, suggesting that the use of ARBs may mitigate the organ damage caused by COVID-19. When the ACE2 concentration in two heart failure cohorts (validation cohort: 1123 males and 575 females; index cohort: 1485 males and 537 females) was assessed, there was no correlation seen between RAAS inhibitor medication and increased plasma ACE2 concentration[140]. This study suggested that ARB/ACEi treatment may not increase COVID-19 vulnerability through elevated plasma ACE2 levels[140].
At present several things remain uncertain regarding cardiac injury before COVID-19. The clinical implication of detecting myocardial injury remains uncertain as cardiac injury can only be diagnosed using biomarker measurements. At present there is no specific treatment. Regarding the use of ARBs and/or ACEi, no evidence is found to support a higher risk of COVID-19 or severity in individuals who are on these medications. Therefore, their use should be continued until any study proves its harmful effects.
There are several mechanisms through which COVID-19 may promote myocardial damage. COVID-19 has harmful effects caused by sympathetic activation, fever, and tachycardia with elevated energy expenditure and myocardial oxygen consumption[149]. Prolonged bed rest is another consequence of acute COVID-19, and this infection can cause thrombosis, a serious complication[150]. Another feature of COVID-19 is hypoxemia, which is associated with elevated oxidative stress brought on by the production of reactive oxygen species, damage to the mitochondria, intracellular acidosis, and even cell apoptosis[11,88,149,151].
Several more pathways are connected to the aberrant inflammatory reactions brought on by COVID-19. After 7-10 d from the start of COVID-19, a hyperinflammatory reaction with cytokine release (cytokine storm) may happen. These reactions may result in cardiac failure, thromboembolic events, shock, renal failure, and possibly multiorgan failure. They may increase the risk of pneumonia and ARDS[5,7,64,129]. The oxidative stress and activation of the inflammatory response may lead to severe clinical conditions in these patients, and this might elevate the possibility of mortality in these individuals with heart failure[152,153].
An association was seen among different inflammatory markers (like ferritin, C-reactive protein, IL-6, and d-dimer) and major complications and high mortality[5,7,129]. A constant increase in inflammatory markers was also associated with myocardial damage in line with hyperinflammation, which leads to cardiac dysfunction[64,129]. Currently, the beneficial roles of anti-inflammatory therapies in COVID-19 are being studied[154,155]. On the other hand, drugs working on endothelial functions, like ACEi or ARBs and statins may also be beneficial[156].
Treatment plans for patients with diabetes were determined based on their blood glucose and/or glycated hemoglobin levels, as well as their urine ketone levels, at the time of admission. At the time of admission, several factors were considered, including age, nutritional status, food intake, the existence of different organ dysfunctions, and conditions related to the heart and brain. This took into account both the potential risks associated with fluctuations in blood glucose levels and the severity of the patient’s condition[157].
As per the guidelines provided by the United Kingdom National Diabetes Inpatient COVID Response Group[158,159], it was recommended that all patients hospitalized with COVID-19 should diligently monitor their blood glucose levels. This would enable prompt identification of any changes in glucose levels. Individuals who had a history of diabetes or who showed up with blood glucose levels more than 12 mmol/L were regularly checked (2-4 times per hour) and treated for their diabetes. The aim for blood glucose management might be changed to 7-12 mmol/L if insulin therapy is implemented and blood glucose levels continue to surpass the 12 mmol/L threshold or if oral medication is not practical. When treatment for COVID-19-related symptoms did not provide the desired outcomes and real blood glucose readings did not match expectations, more frequent blood glucose monitoring (4-6 times per hour) was recommended. When blood glucose levels exceeded 15 mmol/L, subcutaneous administration of short-acting insulin was performed as required.
Dexamethasone emerged as a prominent treatment option for severe COVID-19 cases due to its demonstrated ability to mitigate fatalities among individuals requiring ventilator and oxygen therapies. However, given its classification as a glucocorticoid, special caution was urged for COVID-19 patients also managing diabetes when utilizing dexamethasone, to avert the onset of ketoacidosis. In scenarios involving dexamethasone treatment, patients and healthcare practitioners were advised to increase the frequency of blood glucose level assessments to ensure optimal glycemic control. Alternatively, consideration for alternate therapeutic approaches was recommended for patients grappling with uncontrolled hyperglycemia[160].
Adenoviral ACE2, also known as recombinant human ACE2 (rhACE2), has been used as a therapeutic intervention in animal models of sickness and a human trial including 44 patients with ARDS[161]. rhACE2 is a crucial negative regulator of Ang-II and inhibits harmful cardiac remodeling[142]. When rhACE2 significantly increases ACE2 activity in the circulation, Ang-II levels significantly decrease and Ang-(1-7) synthesis from Ang-II increases. A chimeric fusion of immunoglobulin fragment Fc region restored induced hypertension in mice and increased total Ang-II-conversion activities in blood by up to 100 times.
For COVID-19 patients, Verma et al[162] suggested a combination treatment that included rhACE2 and GapmeR technology. While rhACE2 stops the virus from infecting host cells, GapmeR is an antisense single-stranded DNA molecule that binds to the SARS-CoV-2 RNA. The resulting DNA-RNA hybrid is then destroyed by intracellular RNAase H[162]. For the treatment of this sickness, cyclodextrin soluble ACE2 (sACE2) insertion has been suggested as an alternative method[163]. Many aerosolized sACE2 compounds that may be inhaled directly into the lungs, intravenous sACE2 infusions, and ocular and nasal drops made from cyclodextrin sACE2 inclusion compounds have all been proposed as treatments for COVID-19[164].
Recent trials have shown that RAAS inhibitors and increased ACE2 levels are beneficial for COVID-19 individuals. As mentioned earlier, ACE2 is downregulated when it binds to the SARS-CoV-2 S-protein, which raises Ang-II levels. Breathing problems and lung congestion result from this[148,165]. Therefore, some drugs that increase ACE2 concentration may help lessen the severity of COVID-19[106]. Patients treated with RAS inhibitor had a lower mortality rate than patients taking other hypertensive drugs, according to many retrospective studies on people with COVID-19 and hypertension[166,167].
Lam et al[168] showed that hypertensive COVID-19 patients who continued to use ACEi or ARBs had better clinical results and were advised to stick with their current course of treatment. Meng et al[169] separated their study subjects into two separate groups: Those taking RAS inhibitors (including 17 participants); and those not taking RAS inhibitors (including 25 participants). As opposed to those receiving on-RAS inhibitors, they found that those receiving RAS inhibitors had lower viral loads, lowered serum IL-6, increased blood CD8+ and CD3+ T cells, and decreased sickness severity. Thus, medications such as RAS inhibitors have been associated with better clinical results in COVID-19 hypertension patients[14].
Several clinical trials have been performed for the efficacy and safety of potential alternatives, including arbidol, chloroquine phosphate, tocilizumab, ribavirin, and remdesivir, among others. Chloroquine and its hydroxy analog hydroxychloroquine are a promising pharmacological option for patients with diabetes as it is a broad-spectrum antiviral drug and is commonly used to treat malaria and autoimmune diseases. Chloroquine increases endosomal pH and inhibits ACE2 glycosylation, thereby blocking the SARS-CoV entry in the host cell[170]. It has been shown that chloroquine increases the C-peptide response, which indicates enhanced function of the pancreatic β cell[171]. Several studies showed improved glycemic control by hydroxychloroquine in decompensated diabetic patients, which were refractory to other antidiabetic drugs[171,172].
The severity of the condition, age, the existence of comorbidities, and problems associated with diabetes, among other things, should all be taken into consideration while developing therapeutic methods and ideal glucose levels. A greater number of COVID-19 tests in outpatient diabetes clinics may exhibit a positive impact on their outcome.
Identifying the epigenetic signatures linked to COVID-19 and their changes during viral entry and infection (such as going from asymptomatic to mildly symptomatic, severe infection, and long-lasting symptoms) could be helpful in facilitating prompt diagnosis and the advancement of treatments that could lessen the severity of the virus and its associated mortality. Major metabolic issues such as T2DM, hypertension, and CVD are factors that lead to COVID-19 patient death. Finding epigenetic markers linked to these comorbidities and their impact on COVID-19 severity may also be helpful in guiding treatment to prevent the development of sequelae that increase the catastrophic death rate associated with COVID-19[173].
Early diagnosis, early isolation, and early management might prove to be important in controlling COVID-19. ACE2 is the major receptor for the SARS virus and the interaction between the S-protein and ACE2 is proposed to be the potential factor for infectivity[174,175]. There are concerns regarding the use of RAAS blockers as they may alter ACE2 and whether the altered ACE2 expression is responsible for COVID-19 virulence[39,40,124,146]. There are uncertainties regarding the association of elevated ACE2 expression and infectivity of SARS-CoV-2. The available evidence suggests that RAAS inhibitors should be continued until it is demonstrated that there is an association of ARB/ACEi use with increased severity of COVID-19.
AKS acknowledges the Research and Development Scheme, Government of Uttar Pradesh, Lucknow, India for providing a Junior Research Fellowship. The authors also acknowledge ICMR, Department of Biotechnology (DBT), Department of Science and Technology (DST), New Delhi, India, and Centre of Excellence, Higher Education, Government of Uttar Pradesh, Lucknow, India.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nassar G, France S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhang YL
| 1. | World Health Organization. COVID-19 Weekly Epidemiological Update (Edition 152). 2023. [cited 20 July 2023]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2023. [Cited in This Article: ] |
| 2. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Zhou J, Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical Characteristics of Refractory Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73:e4208-e4213. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049-6057. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019;11. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Shukla AK, Banerjee M. Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update. High Blood Press Cardiovasc Prev. 2021;28:129-139. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067-3075. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin- angiotensin system in the ZDF rat. Diabetes. 2004;53:989-997. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614-623. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438-451. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610-1616. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529-538. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Niu MJ, Yang JK, Lin SS, Ji XJ, Guo LM. Loss of angiotensin-converting enzyme 2 leads to impaired glucose homeostasis in mice. Endocrine. 2008;34:56-61. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Akpinar P, Kuwajima S, Krützfeldt J, Stoffel M. Tmem27: a cleaved and shed plasma membrane protein that stimulates pancreatic beta cell proliferation. Cell Metab. 2005;2:385-397. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Schmid H, Henger A, Cohen CD, Frach K, Gröne HJ, Schlöndorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol. 2003;14:2958-2966. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225-233. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031-1035. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341-1344. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342-348. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16:1733-1741. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Bader M, Peters J, Baltatu O, Müller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med (Berl). 2001;79:76-102. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Konoshita T, Wakahara S, Mizuno S, Motomura M, Aoyama C, Makino Y, Kawai Y, Kato N, Koni I, Miyamori I, Mabuchi H. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care. 2006;29:848-852. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193-202. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Chappell MC, Millsted A, Diz DI, Brosnihan KB, Ferrario CM. Evidence for an intrinsic angiotensin system in the canine pancreas. J Hypertens. 1991;9:751-759. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127-133. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Kuno A, Yamada T, Masuda K, Ogawa K, Sogawa M, Nakamura S, Nakazawa T, Ohara H, Nomura T, Joh T, Shirai T, Itoh M. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010-1019. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Cooper ME, Tikellis C, Thomas MC. Preventing diabetes in patients with hypertension: one more reason to block the renin-angiotensin system. J Hypertens Suppl. 2006;24:S57-S63. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225-1237. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;27. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I-converting enzyme type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes. 2010;59:2540-2548. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab. 2013;304:E874-E884. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392-397. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem. 2005;280:30113-30119. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396-2401. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, Nakashima H, Eguchi K, Eguchi S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:e133-e137. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781-R788. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729-734. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 2020;56:106054. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Gilbert GL. Commentary: SARS, MERS and COVID-19-new threats; old lessons. Int J Epidemiol. 2020;49:726-728. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020;382:1653-1659. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364-374. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727-11734. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, Sasazuki T, Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809-7814. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618-625. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097-1100. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, Hu B, Yip CC, Tsang JO, Huang X, Chai Y, Yang D, Hou Y, Chik KK, Zhang X, Fung AY, Tsoi HW, Cai JP, Chan WM, Ip JD, Chu AW, Zhou J, Lung DC, Kok KH, To KK, Tsang OT, Chan KH, Yuen KY. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14-e23. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Abdin SM, Elgendy SM, Alyammahi SK, Alhamad DW, Omar HA. Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci. 2020;257:118054. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339-1348. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020. [cited 10 January 2024]. Available from: https://europepmc.org/article/PPR/PPR111788. [Cited in This Article: ] |
| 68. | Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-Onset Diabetes in Covid-19. N Engl J Med. 2020;383:789-790. [PubMed] [DOI] [Cited in This Article: ] |
| 69. | Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3-20. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722-8729. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186-194. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132-2139. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Sankrityayan H, Kale A, Sharma N, Anders HJ, Gaikwad AB. Evidence for Use or Disuse of Renin-Angiotensin System Modulators in Patients Having COVID-19 With an Underlying Cardiorenal Disorder. J Cardiovasc Pharmacol Ther. 2020;25:299-306. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726-730. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16:297-298. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14-20. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020;41. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: Outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr. 2020;14:283-287. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305-307. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med. 2020;382:2441-2448. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, Folarin A, Roguski L, Noor K, Shek A, Zakeri R, Shah AM, Teo JTH, Dobson RJB. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. 2020;22:967-974. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | McDonald HI, Nitsch D, Millett ER, Sinclair A, Thomas SL. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabet Med. 2014;31:606-614. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Li S, Wang J, Zhang B, Li X, Liu Y. Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study. Diabetes Metab J. 2019;43:319-341. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | International Diabetes Federation. IDF Diabetes Atlas. 10th ed. 2021. [cited 10 January 2024]. Available from: https://diabetesatlas.org/atlas/tenth-edition/. [Cited in This Article: ] |
| 87. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131-1134. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Gupta R, Hussain A, Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74:864-870. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of Infection in Type 1 and Type 2 Diabetes Compared With the General Population: A Matched Cohort Study. Diabetes Care. 2018;41:513-521. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. T-cell activation and cardiovascular risk in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin Immunol. 2020;210:108313. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Ji HL, Zhao R, Matalon S, Matthay MA. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol Rev. 2020;100:1065-1075. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Fang HJ, Yang JK. Tissue-specific pattern of angiotensin-converting enzyme 2 expression in rat pancreas. J Int Med Res. 2010;38:558-569. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Singh MK, Mobeen A, Chandra A, Joshi S, Ramachandran S. A meta-analysis of comorbidities in COVID-19: Which diseases increase the susceptibility of SARS-CoV-2 infection? Comput Biol Med. 2021;130:104219. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59:99-104. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. 2018;34:575-584. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26:259-265. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Ilyas R, Wallis R, Soilleux EJ, Townsend P, Zehnder D, Tan BK, Sim RB, Lehnert H, Randeva HS, Mitchell DA. High glucose disrupts oligosaccharide recognition function via competitive inhibition: a potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology. 2011;216:126-131. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Kohio HP, Adamson AL. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444:301-309. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Govender N, Khaliq OP, Moodley J, Naicker T. Insulin resistance in COVID-19 and diabetes. Prim Care Diabetes. 2021;15:629-634. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193-199. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164:542-552. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537-540. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-534. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Wang Z, Chen Z, Zhang L, Wang X, Hao G, Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, Wang J, Zhu M, Weintraub WS, Gao R; China Hypertension Survey Investigators. Status of Hypertension in China: Results From the China Hypertension Survey, 2012-2015. Circulation. 2018;137:2344-2356. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) - China, 2020. China CDC Wkly. 2020;2:113-122. [PubMed] [Cited in This Article: ] |
| 110. | Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021-3104. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Grasselli G, Pesenti A, Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA. 2020;323:1545-1546. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225-1228. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Tocci G, Nati G, Cricelli C, Parretti D, Lapi F, Ferrucci A, Borghi C, Volpe M. Prevalence and control of hypertension in the general practice in Italy: updated analysis of a large database. J Hum Hypertens. 2017;31:258-262. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G, Mikolajczyk TP, Schramm-Luc A, Furtak A, Matusik P, Koziol J, Drozdz M, Munoz-Aguilera E, Tomaszewski M, Evangelou E, Caulfield M, Grodzicki T, D'Aiuto F, Guzik TJ. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459-3470. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol. 2019;19:517-532. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Carnevale D, Wenzel P. Mechanical stretch on endothelial cells interconnects innate and adaptive immune response in hypertension. Cardiovasc Res. 2018;114:1432-1434. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114:1547-1563. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Perrotta M, Lori A, Carnevale L, Fardella S, Cifelli G, Iacobucci R, Mastroiacovo F, Iodice D, Pallante F, Storto M, Lembo G, Carnevale D. Deoxycorticosterone acetate-salt hypertension activates placental growth factor in the spleen to couple sympathetic drive and immune system activation. Cardiovasc Res. 2018;114:456-467. [PubMed] [DOI] [Cited in This Article: ] |
| 119. | Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68:123-132. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126-133. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Ketelhuth DFJ. The immunometabolic role of indoleamine 2,3-dioxygenase in atherosclerotic cardiovascular disease: immune homeostatic mechanisms in the artery wall. Cardiovasc Res. 2019;115:1408-1415. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Ketelhuth DFJ, Lutgens E, Bäck M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O'Neill L, Weber C, Evans PC. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res. 2019;115:1385-1392. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10:410-414. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781-782. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Furuhashi M, Moniwa N, Mita T, Fuseya T, Ishimura S, Ohno K, Shibata S, Tanaka M, Watanabe Y, Akasaka H, Ohnishi H, Yoshida H, Takizawa H, Saitoh S, Ura N, Shimamoto K, Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15-21. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Abe M, Oikawa O, Okada K, Soma M. Urinary angiotensin-converting enzyme 2 increases in diabetic nephropathy by angiotensin II type 1 receptor blocker olmesartan. J Renin Angiotensin Aldosterone Syst. 2015;16:159-164. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Mariana CP, Ramona PA, Ioana BC, Diana M, Claudia RC, Stefan VD, Maria KI. Urinary angiotensin converting enzyme 2 is strongly related to urinary nephrin in type 2 diabetes patients. Int Urol Nephrol. 2016;48:1491-1497. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13-e115. [PubMed] [DOI] [Cited in This Article: ] |
| 129. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [PubMed] [DOI] [Cited in This Article: ] |
| 130. | Yu CM, Wong RS, Wu EB, Kong SL, Wong J, Yip GW, Soo YO, Chiu ML, Chan YS, Hui D, Lee N, Wu A, Leung CB, Sung JJ. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140-144. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819-824. [PubMed] [DOI] [Cited in This Article: ] |
| 132. | Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. [PubMed] [DOI] [Cited in This Article: ] |
| 133. | Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861-1862. [PubMed] [DOI] [Cited in This Article: ] |
| 134. | Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911-915. [PubMed] [DOI] [Cited in This Article: ] |
| 135. | Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648-1655. [PubMed] [DOI] [Cited in This Article: ] |
| 136. | Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707-1712. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970-976. [PubMed] [DOI] [Cited in This Article: ] |
| 138. | Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281-H2290. [PubMed] [DOI] [Cited in This Article: ] |
| 139. | Ohtsuki M, Morimoto SI, Izawa H, Ismail TF, Ishibashi-Ueda H, Kato Y, Horii T, Isomura T, Suma H, Nomura M, Hishida H, Kurahashi H, Ozaki Y. Angiotensin converting enzyme 2 gene expression increased compensatory for left ventricular remodeling in patients with end-stage heart failure. Int J Cardiol. 2010;145:333-334. [PubMed] [DOI] [Cited in This Article: ] |
| 140. | Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020;41:1810-1817. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783-790. [PubMed] [DOI] [Cited in This Article: ] |
| 142. | Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717-728, 18 p following 728. [PubMed] [DOI] [Cited in This Article: ] |
| 143. | Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107:888-897. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | Aronson JK, Ferner RE. Drugs and the renin-angiotensin system in covid-19. BMJ. 2020;369:m1313. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc. 2020;9:e016219. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. [PubMed] [DOI] [Cited in This Article: ] |
| 147. | Raiden S, Nahmod K, Nahmod V, Semeniuk G, Pereira Y, Alvarez C, Giordano M, Geffner JR. Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J Pharmacol Exp Ther. 2002;303:45-51. [PubMed] [DOI] [Cited in This Article: ] |
| 148. | Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112-116. [PubMed] [DOI] [Cited in This Article: ] |
| 149. | Violi F, Cangemi R, Falcone M, Taliani G, Pieralli F, Vannucchi V, Nozzoli C, Venditti M, Chirinos JA, Corrales-Medina VF; SIXTUS (Thrombosis-Related Extrapulmonary Outcomes in Pneumonia) Study Group. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin Infect Dis. 2017;64:1486-1493. [PubMed] [DOI] [Cited in This Article: ] |
| 150. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [PubMed] [DOI] [Cited in This Article: ] |
| 151. | Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509-517. [PubMed] [DOI] [Cited in This Article: ] |
| 152. | Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, Zannad F, Zwinderman AH, Hillege HL, van Veldhuisen DJ, Kakkar R, Voors AA, van der Meer P. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21:965-973. [PubMed] [DOI] [Cited in This Article: ] |
| 153. | van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425-435. [PubMed] [DOI] [Cited in This Article: ] |
| 154. | Agarwal S, June CH. Harnessing CAR T-cell Insights to Develop Treatments for Hyperinflammatory Responses in Patients with COVID-19. Cancer Discov. 2020;10:775-778. [PubMed] [DOI] [Cited in This Article: ] |
| 155. | Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, Richards D, Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407-1409. [PubMed] [DOI] [Cited in This Article: ] |
| 156. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] |
| 157. | Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, Amadou C, Arnault G, Baudoux F, Bauduceau B, Borot S, Bourgeon-Ghittori M, Bourron O, Boutoille D, Cazenave-Roblot F, Chaumeil C, Cosson E, Coudol S, Darmon P, Disse E, Ducet-Boiffard A, Gaborit B, Joubert M, Kerlan V, Laviolle B, Marchand L, Meyer L, Potier L, Prevost G, Riveline JP, Robert R, Saulnier PJ, Sultan A, Thébaut JF, Thivolet C, Tramunt B, Vatier C, Roussel R, Gautier JF, Gourdy P; CORONADO investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500-1515. [PubMed] [DOI] [Cited in This Article: ] |
| 158. | Sinclair A, Dhatariya K, Burr O, Nagi D, Higgins K, Hopkins D, Patel M, Kar P, Gooday C, Howarth D, Abdelhafiz A, Newland-Jones P, O'Neill S. Guidelines for the management of diabetes in care homes during the Covid-19 pandemic. Diabet Med. 2020;37:1090-1093. [PubMed] [DOI] [Cited in This Article: ] |
| 159. | Rayman G, Lumb A, Kennon B, Cottrell C, Nagi D, Page E, Voigt D, Courtney H, Atkins H, Platts J, Higgins K, Dhatariya K, Patel M, Narendran P, Kar P, Newland-Jones P, Stewart R, Burr O, Thomas S; London Inpatient Diabetes Network-COVID-19. Guidelines for the management of diabetes services and patients during the COVID-19 pandemic. Diabet Med. 2020;37:1087-1089. [PubMed] [DOI] [Cited in This Article: ] |
| 160. | Rayman G, Lumb AN, Kennon B, Cottrell C, Nagi D, Page E, Voigt D, Courtney HC, Atkins H, Higgins K, Platts J, Dhatariya K, Patel M, Newland-Jones P, Narendran P, Kar P, Burr O, Thomas S, Stewart R. Dexamethasone therapy in COVID-19 patients: implications and guidance for the management of blood glucose in people with and without diabetes. Diabet Med. 2021;38:e14378. [PubMed] [DOI] [Cited in This Article: ] |
| 161. | Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, Hardes K, Powley WM, Wright TJ, Siederer SK, Fairman DA, Lipson DA, Bayliffe AI, Lazaar AL. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. [PubMed] [DOI] [Cited in This Article: ] |
| 162. | Verma NK, Fazil MHUT, Duggan SP, Kelleher D. Combination Therapy Using Inhalable GapmeR and Recombinant ACE2 for COVID-19. Front Mol Biosci. 2020;7:197. [PubMed] [DOI] [Cited in This Article: ] |
| 163. | Latil M, Camelo S, Veillet S, Lafont R, Dilda PJ. Developing new drugs that activate the protective arm of the renin-angiotensin system as a potential treatment for respiratory failure in COVID-19 patients. Drug Discov Today. 2021;26:1311-1318. [PubMed] [DOI] [Cited in This Article: ] |
| 164. | Sun P, Lu X, Xu C, Wang Y, Sun W, Xi J. CD-sACE2 inclusion compounds: An effective treatment for coronavirus disease 2019 (COVID-19). J Med Virol. 2020;92:1721-1723. [PubMed] [DOI] [Cited in This Article: ] |
| 165. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [PubMed] [DOI] [Cited in This Article: ] |
| 166. | Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Yuan Y, Loomba R, Liu PP, Li H. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res. 2020;126:1671-1681. [PubMed] [DOI] [Cited in This Article: ] |
| 167. | Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li Q, Li W, Yang S, Zhao X, Zhao Y, Wang H, Liu Y, Yin Z, Zhang R, Wang R, Yang M, Hui C, Wijns W, McEvoy JW, Soliman O, Onuma Y, Serruys PW, Tao L, Li F. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058-2066. [PubMed] [DOI] [Cited in This Article: ] |
| 168. | Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu SK, Skopicki HA, Singer AJ, Duong TQ. Continued In-Hospital Angiotensin-Converting Enzyme Inhibitor and Angiotensin II Receptor Blocker Use in Hypertensive COVID-19 Patients Is Associated With Positive Clinical Outcome. J Infect Dis. 2020;222:1256-1264. [PubMed] [DOI] [Cited in This Article: ] |
| 169. | Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757-760. [PubMed] [DOI] [Cited in This Article: ] |
| 170. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [PubMed] [DOI] [Cited in This Article: ] |
| 171. | Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas--a randomized trial. Diabetes Res Clin Pract. 2002;55:209-219. [PubMed] [DOI] [Cited in This Article: ] |
| 172. | Rekedal LR, Massarotti E, Garg R, Bhatia R, Gleeson T, Lu B, Solomon DH. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum. 2010;62:3569-3573. [PubMed] [DOI] [Cited in This Article: ] |
| 173. | Kgatle MM, Lawal IO, Mashabela G, Boshomane TMG, Koatale PC, Mahasha PW, Ndlovu H, Vorster M, Rodrigues HG, Zeevaart JR, Gordon S, Moura-Alves P, Sathekge MM. COVID-19 Is a Multi-Organ Aggressor: Epigenetic and Clinical Marks. Front Immunol. 2021;12:752380. [PubMed] [DOI] [Cited in This Article: ] |