Prior to the Covid vaccine rollout, We Are Change Chicago published “Immune Enhancement: Significant Cause for Concern over Covid-19 Vaccine Safety,” presenting the findings of dozens of scientific studies on coronavirus vaccines that resulted in antibody dependent enhancement (ADE), i.e., worse outcomes from exposure to the virus. In a paper reviewing many of the same studies, the risk was sufficiently obvious for the authors to conclude that
“The specific and significant COVID-19 risk of ADE should have been and should be prominently… disclosed to research subjects… and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.”
Even a summary report on a meeting of experts including members of CEPI which has been pledged $300 million by the Bill and Melinda Gates Foundation (1, 2) and Wellcome (1, 2) and including Ralph S. Baric who has been involved in coronavirus gain of function research and “…has collaborations with… Moderna… and Pfizer,” acknowledges that “Data are needed on whether antibody waning could increase the risk of enhanced disease on exposure to virus in the long term” (1, 2). It should therefore have been equally obvious to vaccine developers and policy makers that extreme caution is warranted. Apparently it was not. In its briefing document to the FDA. Pfizer wrote:
“Available data do not indicate a risk of vaccine-enhanced disease, and conversely suggest effectiveness against severe disease within the available follow-up period. However, risk of vaccine-enhanced disease over time, potentially associated with waning immunity, remains unknown and needs to be evaluated further in ongoing clinical trials and in observational studies that could be conducted following authorization and/or licensure.”
A Pfizer document on vaccine associated enhanced disease (VAED) and Vaccine Associated Enhanced Respiratory Disease (VAERD) reports 317 potentially relevant cases from 12/01/2022 to 02/28/2022. Of these, 138 were considered serious and 101 were confirmed COVID-19 cases. Of the 101, 75 were considered “severe, resulting in hospitalisation, disability, life-threatening consequences or death. None of the 75 cases could be definitively considered as VAED/VAERD…. [B]ased on the current evidence, VAED/VAERD remains a theoretical risk for the vaccine. Surveillance will continue.” Pfizer’s informed consent documents for potential participants in studies on boosters state: “Although not seen to date, it cannot yet be ruled out that the study vaccine could make a later COVID-19 illness more severe.” A CDC document describing VAERS monitoring standard operating procedure for COVID-19 vaccines identifies 8 adverse events of special interest as potentially related to VAED, while an FDA document identifies VAED and VAERD as “important potential risks.”
In October of 2021, Brian Hooker, a study author (1, 2, 3) and Children’s Health Defense‘s Chief Scientific Officer most well known for exposing a correlation between the MMR vaccine and autism as presented in the film “Vaxxed: From Coverup to Catastrophe” warned that “we’re one step away” from ADE.
ADE is more likely as new variants arise and vaccine effectiveness wanes. Effectiveness is waning, even with booster doses as reported in the UK Health Security Agency’s Technical Briefing 33:
“Repeated VE analysis continues to show lower VE for symptomatic Omicron disease compared to Delta. There is evidence of waning of protection against symptomatic disease with increasing time after dose 2, and by 10 weeks after the booster dose, with a 15 to 25% reduction in vaccine effectiveness after 10 weeks. This waning is faster for Omicron than for Delta infections.”
So, are we experiencing ADE with Omicron? Government compiled datasets from multiple countries seem to suggest the possibility, showing more COVID-19 cases and deaths in the vaccinated compared to the unvaccinated. See for example: UK, New South Wales. The argument has been made that this is because more people are vaccinated. So more cases and deaths among the vaccinated simply means that vaccines do not work, not that they enhance disease. However, looking at numbers of cases per 100,000 people reveals higher rates among the vaccinated. See for example: Ontario, Scotland).
Likewise, analyses of data by The Exposé from the UK, Canada, New Zealand, Germany, and Scotland show that rates are higher in the vaccinated in these countries which is attributed variously to ADE or Vaccine induced Acquired Immune Deficiency Syndrome (VAIDS) (more on this below).
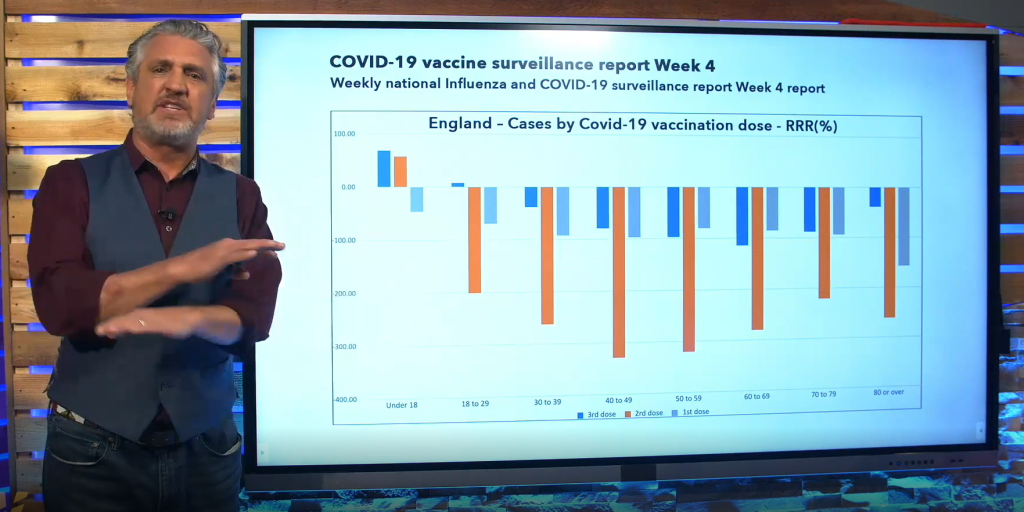
Relative risk reduction calculations based on UK data presented on The Highwire show an increased risk of contracting Covid-19 in the vaccinated compared to the unvaccinated in 7/8 age groups. Note that the risk is over 300% greater in the age 40-59 group.
VAERS is an additional source of data which shows that COVID-19 adverse event reports are now up to 1,216,787, including 26,693 deaths. Using Bradford-Hill Criteria, a very strong argument for a causal relationship can be made (1, 2, 3).
An analysis using UK COVID-19 mortality data and US COVID-19 vaccine mortality data (based on VAERS data and a separate analysis that determined the VAERS death underreporting factor to be 20), concludes: “COVID vaccine inoculations increase risk of death and produce a net negative benefit… for all age groups younger than 60 years old…. For those over 60 years old, the benefit of COVID inoculations is… a 0.0016%… to a 0.125% reduction in likelihood of death.”
An additional consideration is reports of excess mortality. The most prominent example is that of OneAmerica, an Indiana life insurance company, having reported a 40% increase in deaths. The company’s CEO states, “Just to give you an idea of how bad that is… a one-in-200-year catastrophe would be 10% increase over pre-pandemic…. So 40% is just unheard of.” He further states, “What the data is showing to us is that the deaths that are being reported as COVID deaths greatly understate the actual death losses among working-age people from the pandemic.” Also see similar reports from India: Life Insurance Death Claims Shoot 41%, Up 3.5x in 2021 and Germany: Board Member of Large German Insurance Company Blows the Whistle on COVID Vaccines.
UK data reveals that “The non-Covid excess has been running about as high at any point in the last ten years [for the time of year] since about mid-July.” (1, 2)
An analysis (1, 2) of excess mortality in Germany reports “The correlation is + .31, is amazingly high and especially in an unexpected direction. Actually, it should be negative, so that one could say: The higher the vaccination rate, the lower the excess mortality. However, the opposite is the case and this urgently needs to be clarified…. The higher the vaccination rate, the higher the excess mortality.”
Edward Dowd has analyzed CDC data and found that the millennial age group suffered a record rate of excess deaths in 2021. Thomas Renz has released data from the Defense Medical Epidemiology Database provided to him by whistleblowers indicating a nearly 1000% increase in disease and injury in 2021 compared to the previous 5 years.
Also, several studies have presented findings of negative vaccine effectiveness. One study found -19% effectiveness of the AstraZeneca Vaccine. Another reports, “…receipt of 2 doses of COVID-19 vaccines was not protective against Omicron infection at any point in time, and VE was –38%… 120-179 days and –42% 180-239 days after the second dose.”
It is important to note that none of these findings are certain indicators of ADE. Another possible explanation for these findings is that the vaccines are causing VAIDS, i.e., negatively impacting people’s immune systems via other means. One potential mechanism is that “…the SARS–CoV–2 spike protein significantly inhibits DNA damage repair, which is required for effective V(D)J recombination in adaptive immunity,” and therefore, “…the spike protein might impede adaptive immunity and underscore the potential side effects of full-length spike-based vaccines.”
A second study titled “The BNT162b2 mRNA Vaccine Against SARS-CoV-2 Reprograms Both Adaptive and Innate Immune Responses” found that “The response of innate immune cells to TLR4 and TLR7/8 ligands was lower after BNT162b2 vaccination….” “…we observed a significant reduction in the production if IFN-α… after the administration of the second dose of the vaccine. This may hamper the initial innate immune response against the virus….”
A third paper describes “…the extensively documented subversion of innate immunity, primarily via suppression of IFN-α and its associated signaling cascade. This supression will have a wide range of consequences, not the least of which will include the reactivation of latent viral infections and the reduced ability to effectively combat future infections.”
As to whether or not ADE is currently occurring then, Stephanie Seneff answers:
“There’s a crossover point at which the enhancing antibodies can be stronger than the protective antibodies, and that’s when you can get this antibody dependent enhancement that people have seen in the past with [other] coronavirus vaccines. We’re still trying to see if that’s the case with these vaccines. There is some evidence here and there, but it’s not sure yet.”
That people are being negatively impacted by vaccination is becoming more and more difficult to deny as more and more datasets and studies are released. The fact that to what extent this may be attributed to ADE remains unclear is very concerning, especially in the context of mandates and the absolute assurances of safety provided by politicians, regulatory officials, and mainstream media outlets.
For those who want to dive into the available scientific literature on the topic, conclusions from most of the relevant papers are categorized below. Asterisks indicate greater relevance in the context of this article. Briefly, an autopsy study released just last week found an increased rate of viral dissemination in the vaccinated compared to the unvaccinated. ADE definitively can occur in vitro, while there is intriguing but minimal evidence of it occurring in vivo. Some in vitro studies concluded that ADE does not occur. A majority of in vivo studies, largely of poor quality (brief follow up periods, small sample sizes, and conflicts of interest), report the absence of ADE. Various antibody attributes imply the possibility of ADE. Finally, authors provide warning after warning that ADE should be taken seriously.
Does occur:
In People Who Died After Breakthrough Infections Despite Partial or Full Vaccination:
*”Real-time RT-PCR (RT-qPCR) identified a significantly increased rate of generalized viral dissemination within organ systems in vaccinated cases versus nonvaccinated cases (45% vs. 16%, respectively; P = 0.008) mainly with Ct-values of higher than 25 in non-respiratory samples. However, vaccinated cases also showed high viral loads, reaching Ct-values below 10, especially in
the upper airways and lungs…. the potential role of antibody-dependent enhancement must also be ruled out in future studies.” “…a high rate of viral dissemination detected by RT-qPCR within the organ system was an unanticipated result in this study, which was especially accentuated in the partially vaccinated compared to fully vaccinated cases (11 of 16 [69%] vs. five of 13 [38%], respectively; P = 0.144). In several cases, RT-qPCR identified the RNA of SARS-CoV-2 in all investigated samples, including cerebrospinal fluid, CNS, and soft tissues. This is in strong contrast to… nonvaccinated lethal SARS-CoV-2 infections, in which the frequency of viral dissemination was rare, with a rate of only 16% (three of 19) instead of 69%.” Note that, as the authors point out, these findings are confounded by the fact that the vaccinated group also had more past medical histories of immunocompromising factors compared to the unvaccinated group.
https://www.nature.com/articles/s41379-022-01069-9.pdf
In Vitro:
*”Here, we show that ADE antibodies are produced by SARS-CoV-2 infection and the ADE process can be mediated by at least two different host factors, Fcγ receptor (FcγR) and complement component C1q…. FcγR- and/or C1q-mediated ADE were detected in 50% of the IgG-positive sera, whereas most of them showed neutralizing activity in the absence of FcγR and C1q. Importantly, ADE antibodies were found in 41.4% of the acute COVID-19 patients. Neutralizing activity was also detected in most of the IgG-positive sera, but it was counteracted by ADE in subneutralizing conditions in the presence of FcγR or C1q…. C1q-mediated ADE may particularly have a clinical impact since C1q is present at high concentrations in plasma and its receptors are ubiquitously expressed on the surfaces of many types of cells, including respiratory epithelial cells, which SARS-CoV-2 primarily infects.” “Our data highlight the importance of careful monitoring of the antibody properties in COVID-19 convalescent and vaccinated individuals.”
https://journals.asm.org/doi/epub/10.1128/spectrum.01553-21
*”Plasma from recovered patients of COVID-19 showed enhancement of SARS-CoV-2 infection of immune cells.” “…the antibody at suboptimal neutralizing concentration promotes virus entry into cells….” “These results also suggest that ADE may be more likely to occur at later time points after recovery from COVID-19 when the concentration of neutralizing antibodies elicited by the primary SARS-CoV-2 infection have waned to suboptimal neutralizing level.”
https://www.medrxiv.org/content/10.1101/2020.10.08.20209114v1.full.pdf
*”…our study revealed that SARS-CoV-2 infection induces antibodies that elicit ADE of infection….” “These results indicate that neutralization may occur with plasma containing sufficient neutralizing antibodies but that ADE-inducing antibodies may function at lower concentrations than neutralizing antibodies.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8546849/pdf/mbio.01987-21.pdf
*”…a specific subset of antibodies targeting the NTD domain… were found to enhance the binding of ACE2 to the spike protein….” “The replication of the authentic SARS-CoV-2 virus… increased more than four times in the presence of enhancing antibodies…. These data indicate that enhancing antibodies robustly augment infectivity of the SARS-CoV-2 virus….” “Enhancing and neutralizing antibodies… were detected in severe COVID-19 patients. The balance of enhancing and neutralizing antibody titers differed among patients. Because the effect of enhancing antibodies is affected by the level of neutralizing antibodies…, we quantified the overall effect by the difference in titers (enhancing/neutralizing). The observed titer difference was higher in severe patients than in non-severe patients…. our data suggest a correlation between enhancing antibodies and severe COVID-19.” “There is a possibility that the production of enhancing antibodies might be boosted by SARS-CoV-2 infection or vaccination.”
https://www.sciencedirect.com/science/article/pii/S0092867421006620
*”By clamping the NTD and the lipid raft, the antibody reinforces the attachment of the
spike protein to the cell surface and thus facilitates the conformational change of the RBD which is the next step of the virus infection process.” “Inasmuch as neutralizing antibodies overwhelm facilitating antibodies, ADE is not a concern. However, the emergence of SARS-CoV-2 variants may tip the scales in favor of infection enhancement. Our structural and modeling data suggest that it might be indeed the case for Delta variants.” “Since our data indicate that Delta variants are especially well recognized by infection enhancing antibodies targeting the NTD, the possibility of ADE should be further investigated as it may represent a potential risk for mass vaccination during the current Delta variant pandemic.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8351274/pdf/main.pdf
*”…anti-Spike IgG immune complexes generated from serum of severely ill COVID-19 patients induce a strong pro-inflammatory response by (otherwise immunosuppressive) human M2 macrophages, which is characterized by high production of classical cytokine storm mediators….” “These data indicate that anti-Spike IgG from severely ill COVID-19 patients does not only induce hyperinflammation by macrophages, but also may contribute to permeabilization of pulmonary endothelium and microvascular thrombosis.” “IgG immune complexes can be recognized by Fc gamma receptors…. all FcγRs contributed to anti-Spike-induced cytokine induction….” “…our data show that increased pathology by… IgG in COVID-19 patients most likely results from excessive immune activation.” “In addition to human alveolar macrophages, these FcγRs are expressed by various other myeloid immune cells, but also by airway epithelial cells, which are one of the main target cells of infection by SARS-CoV-2 and closely interact with activated macrophages.”
https://www.biorxiv.org/content/10.1101/2020.07.13.190140v1.full.pdf
*”Our results showed a minimum viral positivity in these immune cells without serum, whereas the addition of COVID-19 patient serum greatly enhanced viral infection by as much as a 7-fold increase in B cells and monocytes, or 5-fold increase in macrophages. Moreover, the second patient serum with a higher titer of viral antibody than the first serum demonstrated better efficacy in the context of enhancing viral infection. Taken together, SARS-CoV-2 infection of immune cells was enhanced in the presence of convalescent sera from COVID-19 patients, suggesting ADE.” “Similarly, more sgRNA was observed in the serum treated group, suggesting more replicating viruses in the ADE group.” ” Collectively, our data indicated that convalescent serum samples from COVID-19 patients caused excessive activation of the immune cascade.” “Our data indicated that ADE could occur to FcR-expression cells such as B cells, monocytes, or macrophages…. the ADE effect includes not only an enhancement of viral replication, but also an excessive immune response in these immune cells.” “Finally, the ADE effect may further dampen
our immune defense mechanism by causing the dysfunction of B cells or macrophages,
which eventually leads to impaired adaptive immunity.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8704563/pdf/viruses-13-02483.pdf
*”…the neutralizing antibody CB6, a mouse anti-S1 serum and convalescent plasma, induced ADE on cells expressing FcgRIIA/CD32A and low levels of endogenous ACE2. ADE occurred at sub-neutralizing antibody concentrations, indicating that unneutralized S protein was required for ADE. The enhanced infectivity of 614G variant was higher than that of 614D wildtype in the presence of antibodies, further suggesting that ADE may be influenced by virus strains with different ACE2-binding affinity.” “Among 93 plasma samples tested, 90 plasma exhibited canonical bell-shaped ADE curves, suggesting that most had the potential to cause ADE.” “The maximum infectivities in the presence of ADE differed significantly between scale 3 [severity] and scale 5 + 6 [severity] (p = 0.004), with convalescent plasma from scale 5 + 6 showing a stronger ADE effect compared with that of scale 3.” “…different antibodies may elicit ADE by different mechanisms, and FcgR may not be the only receptor required for ADE.” “We emphasize that although we found that most convalescent plasma exhibited ADE potential on Huh7-CD32A cells, they did not enhance virus infectivity of immune cell lines or PBMCs. Our results indicate that ADE might not be a common event in infected individuals. However, considering the diversity of antibodies and the variable status of immune cells in the human bodies, we cannot exclude the possibility that ADE may occur in special cases upon SARS-CoV-2 infection.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8719361/pdf/main.pdf
*”Here, we found that the Delta variant completely escaped from anti-N-terminal domain (NTD) neutralizing antibodies, while increasing responsiveness to anti-NTD infectivity-enhancing antibodies. Although Pfizer-BioNTech BNT162b2-immune sera neutralized the Delta variant, when four common mutations were introduced into the receptor binding domain (RBD) of the Delta variant (Delta 4+), some BNT162b2-immune sera lost neutralizing activity and enhanced the infectivity. Unique mutations in the Delta NTD were involved in the enhanced infectivity by the BNT162b2-immune sera.” “…epitopes of the enhancing antibodies, not neutralizing antibodies, are well conserved in most SARS-CoV-2 variants, including the Delta variant. Therefore, additional immunization of the spike protein derived from SARS-CoV-2 variants may boost enhancing antibodies more than the neutralizing antibodies in individuals who were previously infected with wild-type SARS-CoV-2 or immunized with vaccines composed of wild-type spike protein.”
https://www.biorxiv.org/content/10.1101/2021.08.22.457114v1.full.pdf
*”Antibodies to one group of these RBD epitopes mediate ADE of entry in Raji cells via an Fcg receptor-dependent mechanism. In contrast, antibodies targeting two other distinct epitope groups neutralize SARS-CoV-2 without ADE….” “Eleven of 48 antibodies (23%) significantly enhanced viral infection….” “Therefore, some SARS-CoV-2 anti-RBD and anti-S1 antibodies
induce ADE of viral entry in Raji cells through the Fcg receptor-dependent mechanism.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7802522/pdf/main.pdf
*”Serum from immunized mice indeed augmented SARS-CoV-2 replication by 5 and 8 times when diluted 10–4- and 10–5-fold, respectively….” “…nearly half… of serum samples from COVID-19 patients examined had the ability to cause ADE to a greater or lesser degree at different Ab concentrations. Experiments using representative ADE-causing sera demonstrated that ADE depends on FcR…. We observed an increasing tendency of anti-SARS-CoV-2 Ab quantity… in apparent and slight ADE groups compared with the no ADE group, although these differences did not reach statistical significance. …the apparent ADE group was further classified into two subgroups based on the Ab titer that caused ADE…: the frst subgroup (A-1) showed ADE peaks at high dilutions, i.e., at a lower concentration of serum…; and the second subgroup (A-2) showed ADE activity at the highest serum concentration examined….” “The addition of COVID-19 patient serum or serum from SARS-CoV-2-immunized mice to a culture of clone 35–40 cells together with live SARS-CoV-2 induced the production of IL-6…. The blockade of FcR resulted in the reduced production of IL-6, demonstrating FcR dependency in the enhancement of IL-6 production by COVID-19 patient serum…. ADE causing sera exhibited higher induced IL-6 production (…88.9% in Apparent, 72.7% in Slight, and 58.8% in None groups, and notably 100% in the A-2 subgroup)….” “Furthermore, in the future when SARS-CoV-2 diverges into several different serotypes like dengue virus has, ADE in SARS-CoV-2 will become a more important concern.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8660863/pdf/41598_2021_Article_3273.pdf
“We show here that SARS CoV-2 efficiently infects monocytes and macrophages without any cytopathic effect. Infection was associated with the secretion of immunoregulatory cytokines (IL-6, IL-10, 42 TGF-β).”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7928817/pdf/jiab044.pdf
“Here, we report that two neutralizing mAbs, MW01 and MW05, could enhance the infection of SARS-CoV-2 pseudovirus on FcγRIIB-expressing B cells…. Moreover, both macropinocytosis
and endocytosis are confirmed involving in ADE of SARS-CoV-2 pseudoviral infection.”
https://www.nature.com/articles/s42003-022-03207-0.pdf
In Vitro with Mouse Monoclonal Antibody 1Ba–3H When Binding to Gamma Variant:
*”Here, we reported two mouse monoclonal antibodies 7 Eb-4G and 1Ba–3H that specifically recognized the receptor-binding domain (RBD) of SARS-CoV-2 spike (S) protein…. Only 1Ba–3H exhibited the neutralizing activity for preventing the pseudotyped lentivirus from binding to the angiotensinconverting enzyme 2 (ACE2)-transfected HEK293T cells…. 1Ba–3H exhibited the neutralizing activity against the wild-type, Alpha, Delta, and Epsilon variants of SARS-CoV-2, but lost the neutralizing activity against Gamma variant in the plaque reduction assay. On the contrary, 1Ba–3H enhanced the cellular infection of Gamma variant in a dose-dependent manner. Our findings suggest that the antibody-dependent enhancement of infection mediated by the RBD-specific antibody for different SARS-CoV-2 variants must be considered while developing the NAb.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8918075/pdf/main.pdf
In Vitro with Non-engineered MW05 Monoclonal Antibody:
“Enhanced SARS-CoV-2 pseudovirus infection of Raji cells, but not of THP-1 or K562 cells, was observed for MW05…. These results indicate that FcγRIIB is the major FcγR contributing to the enhancement of SARS-CoV-2 infection mediated by MW05.” ” Introducing of LALA mutation to the Fc region (MW05/LALA) completely abolished the ADE activity of MW05. Further, potent prophylactic and therapeutic effects against SARS-CoV-2 were observed in rhesus monkeys. These results support the development of MW05/LALA for combating COVID-19.”
https://www.nature.com/articles/s41467-020-19568-1.pdf
In Vitro and in SARS-CoV-2 antibody infused Macaques and Mice:
*”Select RBD NAbs… demonstrated Fc receptor-g (FcgR)-mediated enhancement of virus infection in vitro, while five non-neutralizing NTD antibodies mediated FcgR-independent in vitro infection enhancement. However, both types of infection-enhancing antibodies protected from SARS-CoV-2 replication in monkeys and mice. Three of 46 monkeys infused with enhancing antibodies had higher lung inflammation scores compared to controls. One monkey had alveolar edema and elevated bronchoalveolar lavage inflammatory cytokines. Thus, while in vitro antibody-enhanced infection does not necessarily herald enhanced infection in vivo, increased lung inflammation can rarely occur in SARS-CoV-2 antibody-infused macaques.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8232969/pdf/main.pdf
In People Previously Infected with Covid-19 and Subsequently Vaccinated with Covishield or Covaxin:
“Individuals who encountered the viral antigen for the second time experienced either through vaccination or infection demonstrated exaggerated inflammatory response which is explained by the antibody-dependent enhancement phenomenon without lifethreatening complications.” “In our study, association of clinical parameters and disease infectivity among vaccinated individuals were assessed. The IL-6 count (pg/ml) during the active infective phase including home-based care, ferritin level (ng/ml), and LDH level (U/L) were clinically raised among vaccinated individuals significantly (p < 0.001) compared to non-vaccinated individuals.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8824716/pdf/main.pdf
In People Previously Infected with Covid-19 and Subsequently Vaccinated with Pfizer, AstraZeneca, or Moderna:
“Those who previously had COVID-19 had stronger adverse effects after the first dose of the vaccine against COVID-19, which could be partly dependent on weakened antibody-dependent enhancement (ADE)….”
https://www.mdpi.com/2076-393X/9/5/502/htm
In People Generally:
“…rapid and increased induction of antigen-specific IgG upon SARS-CoV-2 infection in adults may result in ADE and contribute to COVID-19 pathogenesis.” “Another potential mechanism of antibody-mediated pathogenesis in COVID-19 patients may be the production of autoantibodies…. SARS-CoV-2 infection in some patients induces autoimmune activation and production/amplification of autoantibodies. The autoantibodies contribute to COVID-19 pathogenesis through antibody-mediated organ damage.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7756132/pdf/11427_2020_Article_1859.pdf
“…the absence of fucose increases the capability of IgG to trigger antibody-dependent cell cytotoxicity (ADCC) via binding to IgG-specific Fc gamma receptor IIIa (FcgRIIIa) on natural killer (NK) cells, resulting in the enhancement of inflammatory cytokines produced by monocytes…. This might be the pathway that IgG afucosylation modulates cytokine storm during the active phase of the SARS-CoV-2 infection.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8495247/pdf/fimmu-12-748566.pdf
“We show here that IgG against nucleocapsid protein of alphacoronavirus NL63 and 229E correlate with… clinical severity…. These laboratory findings suggest possible ADE of SARS CoV-2 infection by previous alphacoronavirus immunity.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8067214/pdf/life-11-00298.pdf
“…adults may experience ADE where the S protein enhances entry into cells via Fc receptors, resulting in cytokine storms, which cause severe lung injury. Elderly may be even more susceptible to ADE because they have more afucosylated IgG, which has a higher affinity with Fc receptors.” “In the case of COVID-19, the critically ill show aggravated afucosylated-IgG responses against the viral spike protein. In contrast, those clearing the infection unaided show higher fucosylation levels of the anti spike protein IgG.” https://www.biorxiv.org/content/10.1101/2020.05.18.099507v1.full.pdf
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7450283/pdf/main.pdf
“Overall, the data shown here raise the possibility that immune complex-mediated activation of inflammatory FcγR pathways and the associated cytokine production… can promote progression to severe COVID-19.” “Here we show that severe COVID-19 patients produced a unique serologic signature, including increased IgG1 with afucosylated Fc glycans. This Fc modification on SARS-CoV-2 IgGs enhanced interactions with the activating FcγR, FcγRIIIa; when incorporated into immune complexes, Fc afucosylation enhanced production of inflammatory cytokines by monocytes, including IL-6 and TNF. These results show that disease severity in COVID-19 correlates with the presence of afucosylated IgG1, a pro-inflammatory IgG Fc modification.”
https://www.medrxiv.org/content/10.1101/2020.05.15.20103341v2.full.pdf
https://www.medrxiv.org/content/10.1101/2020.05.15.20103341v1.full.pdf
“IgM and IgG appear earlier, and their titers are significantly higher in severe patients than non-severe patients (p<0.05). The weak responders for IgG had a significantly higher viral clearance rate than that of strong responders (p= 0.011).”
https://www.medrxiv.org/content/10.1101/2020.03.24.20042382v1.full.pdf
“The C-reactive protein and D-dimer concentrations were significantly higher in the early IgG reponders….” “We observed that higher IgG responses during the acute phase were associated with several severity markers…. these data suggest the need for further studies to analyze and differentiate the early and late IgG reponders. The following should be investigated: i) the possible role of ADE….”
https://www.jstage.jst.go.jp/article/yoken/74/6/74_JJID.2020.799/_pdf/-char/en
“…ADE can be mediated via increased immune activation by Fc-mediated effector functions or immune complex formation. In the case of respiratory virus infections, the resulting immune cascade can contribute to lung disease. While the hallmarks of severe COVID-19 have features that overlap with this type of ADE, there is currently no definitive evidence to show ADE occurs with SARS-CoV-2 infection.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8054133/pdf/41577_2021_Article_542.pdf
“Recent preclinical evaluation studies of inactivated vaccine candidates on SARS-CoV-2 in mice, rats and non-human primates demonstrated the induction of protective IgG responses, without evidence for IgG-mediated pathology or increased susceptibility to VAERD. Although… animal models of SARS-CoV-2 infection have been described, sequence variability in FcγR-coding genes — as well as substantial interspecies differences in FcγR structure and function — limits our ability to interpret data….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7418887/pdf/41577_2020_Article_410.pdf
“The most important pathomechanism in COVID-19, therefore, could be ADE in which CD32a plays the central role. CD32a is expressed on the surfaces of monocytes and macrophages among other cells such as alveolar macrophages….” ” Infection of alveolar macrophages through ADE may explain their excessive activation and generation of local hyperinflammatory environment and the resultant systemic cytokine storm….” “Furthermore, the course of COVID-19 is in general more severe in those with underlying conditions such as hypertension, poorly controlled diabetes mellitus, and cardiovascular disease among others. This may be attributed to the known increased
expression of CD32a on monocytes and macrophages in these patients.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7178552/pdf/mSphere.00344-20.pdf
“Here, we discuss the various mechanisms of SARS-CoV-mediated inflammation. We also assume that SARS-CoV-2 likely shares similar inflammatory responses. Potential therapeutic tools to reduce SARS-CoV-2-induced inflammatory responses include various methods to block FcR activation.” “ADE occurs when antiviral neutralizing antibodies cannot completely neutralize the
virus. Instead, the virus-NAb complex attaches to the Fc receptor (FcR), leading to viral endocytosis and infection of the target cells. The outcome is an increase in the overall replication of the virus and greater disease severity.” “Virus-NAb complex binding to FcR can also activate proinflammatory signaling, skewing macrophage responses to the accumulation of proinflammatory (M1 or classically activated) macrophages in lungs. The M1 macrophages secrete
inflammatory cytokines such as MCP-1 and IL-8, leading to lung injury.”
https://link.springer.com/content/pdf/10.1007/s12250-020-00207-4.pdf
“A vaccine created based on one variant of the S protein of SARS-CoV2 might induce the production of antibodies with high affinity to the vaccine antigen, but lower affinity for a circulating strain that has already undergone antigenic drift, which changed this protein. Therefore, that vaccine might lose its protective power. Moreover, the antibody–virus complex might take on the role of a “Trojan horse” making it easier for the virus to infect the host’s monocytes or macrophages and other CD32+ immune cells.” “Antibodies are produced slower in elderly people due to immunosenescence, and by the time the antibody titer reaches the level necessary to neutralize the virus, antigenic determinants of the pathogen have time to evolve. This can occur due to either direct mutations or activation of a new quasispecies different from the original dominant one. In this case, the neutralizing antibodies developed towards original antigenic determinants might start forming unstable complexes with the changed antigens and “drag in” the virus into monocytes and macrophages, where it can start to replicate. As a result, a generalized infection and a cytokine storm might develop. Indeed,… older patients are more likely to have higher antibody titers and more serious illness.” “Immunization against SARS-CoV-2 can aggravate subsequent infection, and this possibility must be considered…. Attracting attention to the ADE phenomenon, its mechanism and modeling is very important now that trials of vaccines against COVID-19 are already going on with full speed ahead. High variability of S-protein glycosylation patterns along with this protein conformational mobility all promote antigenic diversity of SARS-CoV-2 isolates. This diversity makes this protein a non-optimal vaccine target antigen because it can promote ADE or/and short-lived vaccine related protection.”
https://link.springer.com/content/pdf/10.1134/S0026893320060151.pdf
“We hypothesized that antibodies developed for the SARS-CoV-2 variant of the virus with one conformation of S-protein may lose their ability to neutralize the virus when the conformation of this protein changes and, as a result, cause ADE.” “Since the affinity of antibodies to antigenic epitopes of the S1 subunit and RBD domain can change along with the conformation of the S-protein’s change, we believe that antibodies to these epitopes are more likely to trigger ADE. Therefore, a vaccine targeting these epitopes in the S1 subunit is more likely to trigger the production of antibodies that can induce ADE following natural viral infection.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7569100/pdf/11439_2020_Article_9303.pdf
“Non-neutralizing antibodies to variable S domains may enable an alternative infection pathway via Fc receptor-mediated uptake. This may be a gating event for the immune response dysregulation observed in more severe COVID-19 disease.” “The S protein S1 extended domain shows the highest number of exposed surface highly variable residues….” “…evolutionary selection for mutations to these residues may facilitate antigenic drift to escape immune responses.”
https://www.preprints.org/manuscript/202003.0138/v1
“SARS-CoV2 infection is asymptomatic in about 80% of the infected individuals at the population level.” “The titer of IgG antiSARS-CoV2 antibodies appear to be higher in patients with severe form
of the disease….” “Antibodies can exacerbate the lung pathology by activating macrophages even though it may not necessarily enhance viral load and dissemination, as seen in classical ADE.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7439999/pdf/main.pdf
“In SARS-CoV-2, ADE occurs most likely via enhanced immune activation. Here, sub-optimal antibodies form immune complexes with the virus that deposit into airway tissues and activate cytokine and complement pathways. This triggers inflammation, airway obstruction, and even acute respiratory distress syndrome. By this mechanism, vaccines could potentially result in more severe symptoms upon infection with SARS-CoV-2.” “For a vaccine with high effectiveness, the impact of ADE-induced increased mortality is marginal. If effectiveness is low, the effect is visible. This is not surprising, because the occurrence of ADE and vaccine effectiveness are not independent, namely, ADE occurs only if the vaccine fails to immunize properly.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8061987/pdf/pone.0245417.pdf
“…CoV-2 employs the angiotensin converting enzyme 2 (ACE2) receptor and the serine protease TMPRSS2 to infect lung epithelial cells. CoV-2 also infects lung endothelial cells and macrophages and monocytes. Intriguingly, macrophages do not express appreciable levels of ACE2 or TMPRSS2, suggesting that this virus might use an entirely different receptor and a serine protease other than TMPRSS2 to infect these immune cells. An alternative, and possibly more likely explanation, based on a plethora of past studies with other coronaviruses, is that Cov-2 employs antibody-dependent enhancement (ADE) to infect immune cells.” “Interestingly, in cats infected with FIPV [Feline Infectious Peritonitis], the expression of ORF7 has been found to be required for macrophage
infectivity and ADE, suggesting that coronaviruses have evolved molecular mechanisms to modulate macrophages. Indeed, ADE has been observed to date in several coronaviruses… and, notably, Orf7 is present in the genomes of both Cov-1 and Cov-2, ultimately suggesting that macrophages are involved in the basic biology of coronaviruses and ADE.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7291966/pdf/main.pdf
“The mechanism of ADE-FcγRII can also be responsible for lymphopenia in COVID-19 patients. Also, our hypotheses for high mortality in the older patient are the high amounts of serum immunoglobins from other types of Coronaviridae family, with a wide spectrum of affinities, would trigger the ADE mechanism, cytokine release syndrome, and elevated IL-6, which leads to multi-organ failure….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7351975/pdf/dddt-14-2607.pdf
In Vitro and In Vivo:
*”We found that in vitro infection of whole PBMCs [peripheral blood mononuclear cells] from healthy donors was productive of virus progeny. Results revealed that monocytes, as well as B and T lymphocytes, are susceptible to SARS-CoV-2 active infection and viral replication was indicated by detection of double-stranded RNA. Moreover, flow cytometry and immunofluorescence analysis revealed that SARS-CoV-2 was frequently detected in monocytes and B lymphocytes from COVID-19 patients, and less frequently in CD4+ 49 T lymphocytes.” “The predominance of B lymphocytes as target cells of SARS-CoV-2 infection in vivo, in contrast to what was seen in PBMCs infected in vitro, suggests that the susceptibility of different lymphocyte subsets in natural SARS-CoV-2 infection may depend on ACE2-independent alternative virus entry mechanisms. These findings corroborate previous observations that SARS-CoV enters B lymphocytes and monocyte-derived cells via a FcγRII492 dependent pathway, which is facilitated by the presence of antibodies.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8132220/pdf/nihpp-2020.07.28.225912v2.pdf
*”Select RBD NAbs also demonstrated Fc receptor-γ (FcγR)-mediated enhancement of virus infection in vitro, while five non-neutralizing NTD antibodies mediated 46 FcγR-independent in vitro infection enhancement. However, both types of infection-enhancing antibodies protected from SARS-CoV-2 replication in monkeys and mice. Nonetheless, three of 31 monkeys infused with enhancing antibodies had higher lung inflammation scores compared to controls. One monkey had alveolar edema and elevated bronchoalveolar lavage inflammatory cytokines. Thus, while in vitro antibody-enhanced infection does not necessarily herald enhanced infection in vivo, increased lung inflammation can occur in SARS-CoV-2 antibody-infused macaques.” “5 non-neutralizing NTD antibodies enhanced SARS-CoV-2 pseudovirus infection in 293T/ACE2 cells by 56% to 148%.”
https://www.biorxiv.org/content/10.1101/2020.12.31.424729v2.full.pdf
“Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–specific afucosylated IgG were also found in critically ill COVID-19 patients but not in individuals with mild symptoms…. Afucosylated IgG promoted interleukin-6 (IL-6) release in macrophages cultured in vitro, which
is in line with an observed association of SARSCoV-2–specific IgG afucosylation with IL-6 and
C-reactive protein (CRP) in these patients.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7919849/pdf/371_abc8378.pdf
Does Not Occur:
In vitro:
*”None of the sera enhanced infection of human cells with SARS-CoV-2 at any dilution, arguing against antibody-dependent enhancement of infection in our system.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8544894/pdf/msphere.01145-20.pdf
*”We found that SARS2-PV [pseudovirus] entry into the three FcR-expressing cell lines was minimal
(< 0.02%) whereas the same amount of SARS2-PV yielded an infection rate of ~7% in VeroE6-hACE2 cells. Moreover, treatment with serially diluted control sera or antiRBD sera did not significantly affect SARS2-PV entry of the three cell lines (Fig. 1m–o), indicating that anti-RBD
sera do not promote ADE of SARS2-PV.” “…anti-RBD antibodies do not promote ADE, at least not in the assay system we used. It remains to be determined whether antibodies targeting other regions of S protein could mediate ADE of SARS-CoV-2.”
https://www.nature.com/articles/s41421-020-00199-1.pdf
*”Here we demonstrate that SARS-CoV-2 and SARS-CoV neither infect human monocyte-derived macrophages (hMDM) nor induce inflammatory cytokines in these cells…. These results support the view that ADE may not be involved in the immunopathological processes associated with
COVID-19, however, more studies are necessary to understand the potential contribution of antibodies-virus complexes with other cells expressing FcR receptors.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8072125/pdf/fcimb-11-644574.pdf
“…our data showed that antiSARS-CoV-2 mAbs and convalescent plasma from COVID-19 patients
did not facilitate SARS-CoV-2 D614 infection in human blood monocyte-derived macrophages that readily promote Zika virus ADE, providing no evidence of SARS-CoV-2 D614 ADE.” Note that increases in intracellular viral genomic RNA and infectious viral particles were increased in the presence of convalescent plasma, but this increase was considered negligible.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8498781/pdf/main.pdf
In vitro with Convalescent Plasma:
*”CP [convalescent plasma] from a patient with COVID-19 blocked the entry of SARSCoV-2 into MDMs [Monocyte-derived macrophages] and MDDCs [monocyte-derived dendritic cells]. Furthermore, a lower dose of CP (final concentration: 1%) did not enhance viral entry, cytokine expression, or cell death, suggesting that antibody-dependent enhancement of macrophage infection did not occur. Although we observed no antibody-dependent enhancement, it will still be important to carefully evaluate patients for disease enhancement in clinical trials of vaccination….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7799009/pdf/jiaa753.pdf
“The strategy employed was to assay neutralization of MLV particles pseudotyped with SARS-CoV-2 S protein in cells expressing the ACE2 receptor alone or in combination with Fc receptors, FcαR or FcγRII. Each sample was tested against the three cell lines simultaneously and the titer inhibiting virus infectivity to 50% (IC50) of untreated samples was calculated in each case. If the IC50 value for a particular sample is increased in the presence of an antibody receptor, then the sample will be identified as providing ADE for the pseudovirions. However, the IC50 values determined from each of the cell lines were compared and found to be no different for each individual sample. These results indicate the absence of ADE for S-protein pseudotyped retroviral particles at least in the presence of FcαR and FcγRII.” “Conceivably, individuals previously infected with non-VOC [variant of concern] could experience ADE if subsequently infected by a VOC and should be considered when designing future studies. With the accumulation of further mutations in the virus and the rise of newer mutants with fitness and transmissibility, later iterations of the virus may gain ADE function requiring reevaluation of the studies we report here.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8452094/pdf/nihpp-2021.09.14.460394v1.pdf
In Vitro with Sera from Rats Vaccinated with an RBD (Receptor Binding Domain) Based Vaccine:
“Importantly, anti-sera from immunized animals did not mediate antibody-dependent enhancement (ADE) of S-protein-mediated entry under conditions in which Zika virus ADE was readily observed. These data suggest that an RBD-based vaccine for SARSCoV-2 could be safe and effective.”
https://www.biorxiv.org/content/10.1101/2020.04.10.036418v1.full.pdf
In Vitro with Sera from Mice Vaccinated with an mRNA-LNP Based Vaccine:
*”SARS-CoV-2 pseudovirus infection of hACE2/mFcgR1-293T cells was efficiently neutralized as expected by sera derived from mice vaccinated with the full-length Dfurin or RBD mRNAs at low dilutions… and there was no enhanced infection observed at any serum dilution.” “Thus, we reason that ADE may not be a critical safety issue for SARS-CoV-2 mRNA-based vaccines, although this assumption needs to be directly investigated in future in vivo (animal) experiments and may differ between animals and humans.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7392193/pdf/main.pdf
In Vitro with Sera from Mice Vaccinated with an RBD Based Vaccine:
“…we found that the anti-RBD sera did not promote antibody-dependent enhancement of
either SARS-CoV-2 pseudovirus entry or authentic virus infection of Fc receptor-bearing cells.”
https://www.biorxiv.org/content/10.1101/2020.05.21.107565v1.full.pdf
In Vitro with Sera from Mice Vaccinated with an saRNA LNP Vaccine:
“The saRNA LNP vaccine presented in these studies elicited robust antibody and cellular responses, with a Th1 bias that we hypothesize will enable immunogenicity in humans. We did not observe any antibody-dependent enhancement (ADE) of SARSCoV-2 in our in vitro studies….” https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7347890/pdf/41467_2020_Article_17409.pdf
https://static-content.springer.com/esm/art%3A10.1038%2Fs41467-020-17409-9/MediaObjects/41467_2020_17409_MOESM1_ESM.pdf
In Vitro with Sera from Mice Vaccinated with SK-01 and OZG-3861 Vaccines:
“…the antibodies formed neutralized the virus without causing ADE.” Note that the experiment was performed with only 1 dilution.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7970959/pdf/41598_2021_Article_83930.pdf
In Vitro with Monoclonal Antibody Treatment:
“…the in vitro assay indicated no CT-P59-mediated increase in authentic viral infections in FcR-bearing cells and permissive cells, in line with no worsening of symptoms in CT-P59-treated animals as described above.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7803729/pdf/41467_2020_Article_20602.pdf
In Vitro with Polyclonal Antibody Treatment Derived from Pigs:
“…pig GH-pAb Fc domains fail to interact with human Fc receptors, thereby avoiding
macrophage-dependent exacerbated inflammatory responses and a possible antibody-dependent enhancement.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8014652/pdf/EJI-9999-0.pdf
In Vitro with antibody PR1077:
“The antibody-dependent entry of SARS-CoV-2 pseudovirus in the presence of PR1077 was measured, and no ADE effect was detected at various antibody concentrations.”
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001209
In Hamsters with Monoclonal Antibody Treatment with Engineered Fc Regions:
“The second limit of mAbs in the field of infectious diseases is the risk of antibody-dependent enhancement (ADE) of disease, which is usually mediated by the binding of the fragment crystallizable (Fc) region portion of the antibody to Fc gamma receptors (FcgRs) expressed by immune cells….” “…we mitigated the risk of ADE by engineering their Fc region.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7901298/pdf/main.pdf
In Hamsters Vaccinated with CVnCoV (CureVac):
“Hamsters vaccinated with a suboptimal dose of CVnCoV leading to breakthrough viral replication exhibited no evidence of vaccine-enhanced disease.” Note that the hamsters were euthanized only either 4 or 7 days post-challenge.
https://www.nature.com/articles/s41541-021-00311-w.pdf
In Hamsters Vaccinated with an mRNA Vaccine Candidate:
“Histopathological examination of samples of the brain, liver, kidney, spleen, thymus, heart, and adrenal gland revealed no notable vaccine- or virus-related changes in SARS-CoV-2 SAM self-amplifying messenger RNA vaccinated animals following virus challenge (data not shown).” “No evidence of enhanced respiratory disease was found in any of the vaccinated animals.” Note that many of the study’s authors “are current or former employees of the GSK group of companies and may own GSK shares….” Also note that biodistribution analysis in rats found that “At day 2, SAM RNA was detected with relatively high levels in muscle, lymph nodes, and spleen, and relatively lower levels in heart, liver, gonads, lungs, gonads, and blood. RNA levels progressively decreased in quantity in all tissues by day 60, but remained detectable in lymph nodes, spleen, and muscle…. RNA was detectable in the testes only on day 2 and in the ovaries on day 2 and day 8.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8721936/pdf/main.pdf
In Mice Vaccinated with OZG-38.61.3:
“No difference was observed between the groups in the lung X-ray imaging analysis of the mice groups taken in our study. Also, it is observed that both vaccine doses do not cause antibody-dependent enhancement (ADE) side effects in the lung in histopathology analysis.”
https://www.biorxiv.org/content/10.1101/2020.10.28.356667v3.full.pdf
In Macaques Vaccinated with BNT162b (Pfizer/BioNTech):
“…there was no evidence of vaccine-mediated enhancement of viral replication, disease or pathology.” Note that this conclusion is based on findings from only 12 macaques that were necropsied between only 7-16 days post-challenge. Only 6/12 were inoculated with the formula that would become the Pfizer/BioNTech shot, and these 6 were necropsied between only 7-8 days post-challenge. Additionally, “None of the challenged macaques—whether immunized or not—
showed clinical signs of illness,” which suggests that this may be a poor experimental model for evaluating signs of ADE. The authors are comprised of many BioNTech and Pfizer employees.
https://www.nature.com/articles/s41586-021-03275-y.pdf
In Macaques Vaccinated with mRNA-1273 (Moderna):
“Here, we show that mRNA-1273 induces high levels of neutralizing activity… and no pathologic changes in the lung in either of the mRNA1273 vaccine groups 1 week after challenge.” Note that the animals were sacrificed between only 7-15 days post-challenge.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7449230/pdf/NEJMoa2024671.pdf
In Macaques Vaccinated with ChAdOx1 nCoV-19 (Astrazeneca):
“Notably, we found no evidence of immune-enhanced disease after viral challenge in vaccinated SARS-CoV-2-infected animals.” Note that all animals were euthanized only 7 days post-infection and two of the authors have relevant patents and/or patent applications.
https://www.nature.com/articles/s41586-020-2608-y.pdf
In Macaques Vaccinated with PiCoVacc (Sinovac):
“No antibody-dependent enhancement (ADE) of infection was observed for the vaccinated macaques despite the observation that a relatively low NAb titer existed within the medium-dose group before infection, offering partial protection. The possibility of manifestation of ADE after antibody titers wane could not be ruled out in this study. Further studies involving observation of challenged animals at longer periods of time after vaccination are warranted to address this.”
https://www.science.org/doi/epdf/10.1126/science.abc1932
https://www.biorxiv.org/content/10.1101/2020.04.17.046375v1.full.pdf
In Macaques Vaccinated with BBIBP-CorV:
“…low-dose and high-dose BBIBP-CorV conferred highly efficient protection against SARS-CoV-2 in macaques without observed antibody-dependent enhancement of infection.” Note that the animals were euthanized for pathological examination only 7 days post-inoculation.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7275151/pdf/main.pdf
In Macaques Vaccinated with CVnCoV (CureVac):
“Comprehensive analyses of pathological changes in challenged animals via lung histopathology and Computed Tomography (CT) scans gave no indication of enhanced disease upon CVnCoV vaccination. ” Note that “Post-challenge, abnormalities in the lung were detected in 6 of 6 animals in the 0.5 μg [low dose] CVnCoV group, and 5 of 6 in the unvaccinated control group.” Further, the low dose group had a higher cumulative score of lung pathology compared to the control group. While this difference was not found to be statistically significant, it must be considered that it could have been if the study had been conducted with a larger sample size and that the study’s findings suggest the possibility of ADE occuring with suboptimal antibody levels such as those elicited by a suboptimal dose as in this study or those which may result from waning antibody levels over time.
https://www.biorxiv.org/content/10.1101/2020.12.23.424138v1.full.pdf
In Macaques Vaccinated with Ad26 (Johnson & Johnson):
“A limitation of our study is that we did not evaluate the durability of neutralizing antibody responses elicited by these vaccines…. Our studies were also not specifically designed to assess safety or the possibility of vaccine-associated enhanced respiratory disease or antibody-dependent enhancement of infection. However, it is worth noting that… macaques with sub-protective neutralizing antibody titres did not demonstrate enhanced viral replication or clinical disease.” Note that multiple authors are Johnson and Johnson employees and stock holders.
https://www.nature.com/articles/s41586-020-2607-z.pdf
In Macaques Vaccinated with NVX-CoV2373 (Nuvaxovid):
“…there was little, or no inflammation observed in the lungs of macaques immunized with NVX-CoV2373 vaccine 7 days post challenge….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7584426/pdf/main.pdf
In Macaques Vaccinated with ARCoV:
“…no evidence of ADE was observed through the study. All ARCoV-vaccinated animals showed no sign of enhanced viral replication or diseases.” Note that the macaques were sacrificed only 7 days post-challenge and that the authors consist of “co-inventors on pending patent applications related to the ARCoV mRNA vaccine” and employees of Suzhou Abogen Biosciences.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8703211/pdf/41392_2021_Article_861.pdf
In Macaques Vaccinated with DNA Vaccine Candidates:
“Future studies should also address the question of enhanced respiratory disease, which may result from antibody-dependent enhancement. Although our study was not designed to examine safety issues, it is worth noting that the DNA vaccines induced TH1 rather than TH2 responses, and we did not observe enhanced clinical disease even with the suboptimal vaccine constructs that failed to protect against infection.” Note that 2 of the study’s authors are employees of Janssen Vaccines.
In Macaques Vaccinated with ReCovR+N:
“In addition, no antibody-dependent enhancement of infection (ADE) was observed for any vaccinated macaques.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8083607/pdf/nwab053.pdf
In Macaques Injected with Antibodies from Macaques Vaccinated with a Formaldehyde and β-propiolactone Double-inactivated SARS-CoV-2 Vaccine:
“Although ADE has not been found in several completed phase III clinical trials, the decline in
neutralizing antibody levels within a year of vaccination has been demonstrated in several studies. Therefore, whether the nonneutralizing or subneutralizing antibody levels resulting from the decline in neutralizing antibody levels after initial infection or vaccination will result in ADE and promote viral infection or pulmonary immunopathology poses a challenge to the long-term safety of vaccines.” “The anti-SARS-CoV-2 IgG-infused group and control group showed similar, mild to moderate pulmonary immunopathology during the acute phase of virus infection, and no evidence of vaccine-related pulmonary immunopathology enhancement was found…. Although more evidence is needed to confirm or deny the existence of ADE in SARSCoV-2 infection and the immune damage caused by it, the available evidence has further supported the safety of the SARS-CoV-2 vaccine.” Note that although the Macaques in the experimental group had lower viral loads and experimental and control groups showed a similar degree of pulmonary immunopathology, the experimental group did show increased levels levels of MCP-1, IL-8 and IL-33 than the control group. Also note that there were only 6 Macaques in the study.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8635581/pdf/TEMI_10_2002670.pdf
In Macaques with Monoclonal Antibody Treatment with Engineered FC regions:
“Consistent with its isotype and Fc engineering, P4A1–2A displayed 4–81-fold decrease in binding affinity to different FcγRs….” “…no treatment-related adverse effects were observed in any of the tests performed….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8113581/pdf/41467_2021_Article_22926.pdf
In Macaques Rechallenged After Viral Clearance of Initital Infection:
“Low but detectable levels of sgmRNA [i.e., new RNA resulting from viral replication] were still observed in four of nine animals in NS [nasal swabs] on day 1 after rechallenge, but sgmRNA levels declined quickly and median peak sgmRNA levels in NS were >4.8 log10 lower after rechallenge compared with after the primary challenge…. Moreover, little or no clinical disease was observed in the animals after rechallenge.”
Note that the animals were rechallenged only 35 days following initial infection.
https://www.science.org/doi/10.1126/science.abc4776
In Macaques, Hamsters, or Mice Vaccinated with MRT5500:
“…MRT5500… protected against SARS-CoV-2-induced weight loss and lung pathology in hamsters. In addition, MRT5500 elicited TH1-biased responses in both mouse and non-human primate (NHP), thus alleviating a hypothetical concern of potential vaccine-associated enhanced respiratory diseases known associated with TH2-biased responses.” Note that this research was funded by Translate Bio and Sanofi Pasteur and the authors were comprised of their employers and stockholders.
https://www.nature.com/articles/s41541-021-00324-5.pdf
In Patients Treated with Convalescent Plasma:
“No such pulmonary injury and infection enhancement were observed in our patients, probably owing to high levels of neutralizing antibodies, timely transfusion, and appropriate plasma volume.” Note that this study had a brief post-intervention follow up period.
https://www.pnas.org/content/pnas/117/17/9490.full.pdf
“We present the first report of CP in children with life-threatening coronavirus disease 2019…. Infusion of CP was not associated with antibody-dependent enhancement….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7734626/pdf/nihms-1652161.pdf
In People Recovering from SARS-CoV-2:
“There have been concerns regarding vaccine enhancement of disease by certain candidate COVID-19 vaccine approaches, via antibody-dependent enhancement (ADE) or development of a
TH2 responses. Herein, we saw predominant TH1 responses in convalescing COVID-19 cases, with little to no TH2 cytokines.”
https://www.cell.com/action/showPdf?pii=S0092-8674%2820%2930610-3
In People Generally:
“With others, we conclude that the differences in clinical, epidemiological and pathological features of SARS and DENV diseases suggest that iADE [intrinsic ADE] does not contribute to the severity of natural human coronavirus infections. Because myeloid cells are not major
targets of infection, vaccine derived non-protective coronavirus antibodies are not expected to produce iADE infections in humans.” “VAH [vaccine hypersensitivity reactions] is a post-vaccination outcome that may be associated with non-protective antibodies…. the full force of worldwide investigative resources should be directed at unraveling the pathogenesis of VAH.” Note that this paper distinguishes between iADE, specifically referring to virus antibody complexes utilizing FcRs to infect cells, and VAH, referring to increased pathology as a result of vaccination. While the authors accept the possibility of the latter, they reject the probability of the former based on questionable reasoning. They essentially argue that because coronaviruses infect ACE2 or DPP4 expressing cells and not FcR expressing cells, iADE should not be expected to occur. However, as has been shown in multiple studies including those listed above and in our previous article, coronavirus infecting FcR expressing cells is precisely what occurs during ADE.
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7454712/pdf/jiaa518.pdf
“Serum antibody levels were not correlated with clinical severity.”
https://www.thelancet.com/action/showPdf?pii=S1473-3099%2820%2930196-1
“Based on developing scientific data, ADE concerns with COVID-19 have significantly lessened.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7832065/pdf/main.pdf
“They [live unattenuated vaccines] could circumvent antibody-dependent enhancement….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7461232/pdf/JMV-9999-na.pdf
In a Predictive Model:
“…the reduction in mortality due to immunization achieved through vaccination always outweighs increased mortality due to ADE. This is an encouraging result that justifies neglecting ADE in future models.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8061987/pdf/pone.0245417.pdf
With Use of Aptamers Instead of Antibodies:
“…ADE and immunogenicity are potential problems for SARS-CoV-2 neutralizing antibodies, but aptamers developed in this work and reported in previous researches have exhibited no ADE
or little immune response.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8014204/pdf/ANIE-9999-0.pdf
With Use of Monoclonal Antibodies:
“In addition, because of the ability to control dosing and composition, mAb therapy has improved efficacy over convalescent plasma treatment and prevents the potential risks of antibody-dependent enhancement (ADE) from non-neutralizing or poorly neutralizing Abs present in plasma that consists of a polyclonal mixture.”
https://www.science.org/doi/epdf/10.1126/science.abc5902
With Use of Engineered Monoclonal Antibodies:
“Engineering the Fc region of mAbs to abolish its affinity for the Fcg receptor is a feasible solution to mitigate the risk of ADE.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7458058/pdf/main.pdf
With Use of IgY Antibodies:
“IgY does not react with the human complement system or Fc receptor like IgG derived from mammals does, thereby reducing the risk of antibody-dependent enhancement (ADE).”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8133490/pdf/main.pdf
With Use of SC31 Antibodies:
“To investigate the risk of ADE, SC31 and its LALA variant were tested in vitro at sub-neutralizing concentrations using SARS-CoV-2 pseudovirus and THP-1 and Raji cell lines. ADE has been previously been modelled for SARS-CoV pseudovirus in these same cell lines. Importantly, no pseudovirus infection was observed in both THP-1 and Raji cells for either antibody at all the concentrations tested. This indicates that, despite its potent Fc-mediated effector functions, SC31 is unlikely to mediate ADE.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8221499/pdf/pone.0253487.pdf
With Use of HIV-1 Antibodies:
“We were unable to observe an enhancement of pseudoviral entry into K562 cells under a wide range of antibody concentrations, suggesting these antibodies are unlikely to mediate ADE via FcγRII receptor engagement.”
https://www.biorxiv.org/content/10.1101/2021.01.03.425141v1.full.pdf
With Use of Aptamers:
“…the circulating SARS-COV-2 variant of concerns, reduced antibody sensitivity and/or neutralization, and possible antibody-dependent enhancement (ADE) have warranted the search for alternative potent therapeutics. Aptamers, which are single-stranded oligonucleotides,… may offer the capacity to generate high-affinity neutralizers….”
https://wires.onlinelibrary.wiley.com/doi/pdf/10.1002/wnan.1785
May or May not Occur:
In Hamsters with Monoclonal Antibody Treatment:
“We note these animals showed a trend for greater weight loss than control animals but this did not achieve statistical significance. Given concerns about antibody-mediated enhanced disease in
SARS-CoV-2 infection, this observation merits further attention using larger animal group sizes. The
weight loss data are further corroborated by quantification of lung viral load measured by real-time PCR and showed a moderate correlation to weight loss.”
https://www.biorxiv.org/content/10.1101/2020.05.11.088674v2.full.pdf
In Vitro with Monoclonal Antibody Treatment:
“…mAb MW05/IgG1 enhanced the infection of SARS-CoV-2 on both Raji and Daudi cells. Instead, no ADE of SARS-CoV-2 or SARS-CoV infection in immune cells was observed for MW06. As ADE is one of the main concerns for the development of anti-SARS-CoV-2 neutralizing monoclonal antibodies, these results support that MW06 is a good therapeutic antibody candidate for further clinical development.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8317929/pdf/KMAB_13_1953683.pdf
With Monoclonal Antibody Treatment:
“The development of neutralizing antibodies against SARS-CoV-2 has some challenges, such as mutations in the less conservative region of the S1 subunit and the induction of ADE by non-neutralizing antibodies.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8079842/pdf/11684_2021_Article_847.pdf
With Antibody Treatment:
“Considering that the ADE effect has been shown at the cellular level mediated by SARSCoV-2-specifc antibodies, the potential for an ADE efect should be considered when using neutralizing antibodies to treat COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8614633/pdf/12951_2021_Article_1148.pdf
With Convalescent Plasma Treatment:
“Current studies of convalescent plasma are limited by lack of representation of patients in the early phase of infection, as well as confounding from multiple concurrent therapies and small patient numbers. It is possible that ADE could result in more severe disease only in a subset of patients who are genetically susceptible, therefore, studies of convalescent plasma with small numbers of patients may underestimate the risks of paradoxical worsening in selected populations.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7187833/pdf/main.pdf
“Furthermore, the use of CP therapy may cause antibody dependent enhancement (ADE) or immune enhancement…. Employment of ADE mechanism may be one of the reasons behind the high severity of SARS-CoV-2 among the older population. Age and production of antibodies are inversely proportional.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7718590/pdf/main.pdf
“Another major challenge for CPT [convalescent plasma therapy] is the antibody-dependent enhancement (ADE) of viral infection mediated by preexisting enhancing, non-neutralizing, or sub-neutralizing levels of antibodies from the convalescent plasma administered…. As expected, the latest study has shown that anti-SARS-CoV-2 antibody can also cause ADE in COVID-19 experiments.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7490573/pdf/41392_2020_Article_310.pdf
In People Treated with Convalescent Plasma Hospitalized with Severe or Life-threatening COVID-19:
“The incidence of all serious adverse events (SAEs) in the first four hours after transfusion was <1%, including mortality rate (0.3%)…. The seven-day mortality rate was 14.9%…. Given the deadly nature of COVID-19 and the large population of critically ill patients included in these analyses, the mortality rate does not appear excessive. These early indicators suggest that transfusion of convalescent plasma is safe in hospitalized patients with COVID-19.”
https://www.medrxiv.org/content/10.1101/2020.05.12.20099879v1.full.pdf
In People Previously Exposed to Coronaviruses:
“…the recurrent virus exposure over a short time-lapse might result in the Antibody Dependent Enhancement, triggering the violent immune reaction responsible for the severe clinical outcomes observed in the Hubei province.”
https://www.sciencedirect.com/science/article/pii/S1286457920300484
“One of the most perplexing questions regarding the current COVID-19 coronavirus epidemic is the discrepancy between the severity of cases observed in the Hubei province of China and those occurring elsewhere in the world. One possible answer is antibody dependent enhancement (ADE) of SARS-CoV-2 due to prior exposure to other coronaviruses.” “Should ADE be proven to be a mechanism of pathogenesis, both treatment regimens and vaccine development will need to take this phenomenon into consideration to ensure it is mitigated and in the case of a vaccine, avoided altogether.”
https://www.sciencedirect.com/science/article/pii/S1286457920300344?via%3Dihub
“Prior infections with SARS-CoV-2 (or other viruses/ coronaviruses) may arguably predispose to more severe forms of the disease following re-infection with SARS-CoV-2, with an immunological mechanism known as Antibody-Dependent-Enhancement….” “If confirmed by in vivo studies… the possibility to produce an effective vaccine against SARS-CoV-2 might be hampered.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7295427/pdf/bmjgh-2020-002564.pdf
“Secondary infection by related virus strains might lead to an exacerbated illness due to the presence of pre-existing cross-reactive nonneutralizing antibodies…. Binding of virions complexed with antibodies and/or complement fragments to FcγRII and/or CR [complement receptors] on target cells initiates receptor-mediated signaling events, leading to enhanced expression of inflammatory cytokines and suppression of intracellular antiviral responses at the transcriptome level, followed by endocytosis of the virus and subsequent activation of immune cells. The activated immune cells might accumulate in the lung and promote cytokine storm and lymphopenia. Furthermore, the formation of immune complexes can promote complement activation and subsequent tissue damage.” “Further thoughtful and rigorous research is
needed to understand whether there is an association between ADE and rates of severity and mortality attributable to COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8339023/pdf/mpp-0030-0422.pdf
“The higher prevalence of common CoVs in previous years in some regions in the world, might explain the higher pathogenicity of SARS-CoV-2 infection in these affected regions based on the assumption of ADE with pre-existing enhancing antibodies against those common CoVs. For example, the link between early response with higher titers and older age may indicate a priming effect from existing antibodies against other endemic strains. The HCoV circulate continuously, therefore, it is sensible to assume that CoVs antibodies are higher in older people compared to children, including enhancing antibodies. Children infected by SARS-CoV2 usually have milder presentation of the disease. However, we do not have enough evidence to conclude such role of ADE.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8757655/pdf/main.pdf
“Finally, although it is currently unclear whether ADE influences the pathogenesis of SARS-CoV-2, it has been hypothesized that severe COVID-19 cases may arise from the presence of nonneutralizing antibodies from prior coronavirus infections”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7857410/pdf/370_811.pdf
In Relation to MIS-C [Multisystem Inflammatory Syndrome in Children] Pathogenesis:
“ADE could explain… why some patients, developing high titers of virus-specific antibodies, have a worse clinical outcome…. Patients with MIS-C carry higher anti-spike antibodies, compared to children infected by SARS-CoV2 but not developing MIS-C. It has also been speculated that, in infants, ADE deriving from maternally acquired SARS-CoV-2 antibodies bound to mast cells can be the triggering mechanism of MIS-C.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8581204/pdf/fmed-08-747190.pdf
“Moreover, the higher concentration of serum antibodies in pediatric patients portrays the possible operation of antibody-dependent enhancement mechanism in provoking MIS-C, which is more certain to arise as an outcome of acquired immune response and not due to enhanced multiplication of virus…. Non-neutralizing antibodies or inadequate quantities of neutralizing antibodies bound to the epitopes of SARS-CoV-2, in the patient’s blood, promote its intake inside the host tissue which is described as ADE…. This interaction activates macrophages, natural killer cells, lymphocytes, and monocytes…. inducing a surge of pro-inflammatory cytokines….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8810427/pdf/main.pdf
“One potential explanation for the progression to severe MIS-C disease despite the presence of readily detectable anti-SARS-CoV-2 antibodies could be due to the potential role of antibody-dependent enhancement (ADE). We reason that the incidence of the ADE phenomenon whereby the pathogen-specific antibodies can promote pathology should be considered in vaccine development against SARS-CoV-2.” “We hypothesize that the initial exposure of children to the SARSCoV-2 induces both neutralizing and non-neutralizing antibodies production by immune cells…. However, a select number of those that shift to producing predominantly non-neutralizing antibodies progress to severe disease due to ADE.” “A growing body of evidence suggests the host’s innate immune response to SARS-CoV-2 infection triggers the inflammation cascade that causes severe tissue damage…. The virus-specific antibodies increase the uptake of virus by macrophages in the tissues that lead to the synthesis of high levels of pro-inflammatory cytokines, also known ‘cytokine storm….’ Binding of virus-antibody complex to FcR induces cellular endocytosis. The existing SARS-CoV-2-specific antibodies in MIS-C patients may thus promote viral entry into immune cells resulting in immune cell activation and subsequent acute inflammation.”
https://onlinelibrary.wiley.com/doi/epdf/10.1111/pai.13361
“Here, we evaluated the peripheral blood immune profiles of nine MIS-C cases. Despite the absence of clinically apparent upper respiratory infection, all children harbored antibodies against SARS-CoV-2. This antibody response demonstrated typical IgG class switching, absence of circulating IgM but elevated IgA, and effective virus neutralization, resembling, but not identical to, serologies from convalescent COVID-19 adults. Their peripheral blood secretome exhibited drastic elevations of inflammatory mediators, indicative of lymphocyte and myeloid cell activation…. Importantly, we identified IgG and IgA autoantibody repertoires against endothelial, mucosal, and immune antigens, together with strong neutrophil and monocyte upregulation of CD54 and CD64. The latter marker, also known as the high-affinity FcgR1, can engage autoantibodies and immune complexes to trigger potent inflammation and tissue injury. These results suggest that autoreactivity secondary to SARS-CoV-2 infection and the inflammatory innate immune response may be critical to the pathogenesis of MIS-C.”
https://www.cell.com/action/showPdf?pii=S0092-8674%2820%2931231-9
“Antibodies to SARS-CoV might accentuate disease through antibody-dependent enhancement of viral entry and amplification of viral replication…. or by triggering a host inflammatory response through the formation of immune complexes or direct anti-tissue antibody activation or cellular activation, or both. Similar mechanisms might be involved in the inflammatory disorder associated with SARS-CoV-2. SARS-CoV-2 is not usually detected in patients with MIS-C; thus the antibody-dependent enhancement of inflammation is more likely to occur through an acquired immune response rather than increased viral replication. Anti-spike antibodies against SARS-CoV have been shown to accentuate inflammation in primates and in human macrophages; therefore, the anti-spike antibodies against SARS-CoV-2 might also be able to trigger inflammation through a similar mechanism.”
https://www.thelancet.com/action/showPdf?pii=S1473-3099%2820%2930651-4
“Immunoglobulin M (IgM) and Immunoglobulin G (IgG) to the receptor binding domain of the SARS-CoV-2 spike protein were increased in severe MIS-C (P < .001), with dysregulated humoral responses observed.”
https://www.jpeds.com/action/showPdf?pii=S0022-3476%2820%2931023-4
“Moreover, serum neutralizing antibody titers and antibody-dependent cellular phagocytosis were higher in adults compared to pediatric patients with COVID-19.” “…the pediatric patients with MIS-C [multisystem inflammatory syndrome in children] (group 2) had a greater proportion of IgG1 versus IgG3 spike-specific antibodies and more ADCP [antibody-dependent cellular phagocytosis] activity compared to non–MIS-C pediatric patients.” “Our studies suggest that early immune responses… resulted in more rapid resolution of the viral infection and may have mitigated against the progressive cytokine release and tissue pathology that occurs with more robust adaptive immune responses.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7658796/pdf/abd5487.pdf
“Compared to the two previous years, we found a 13-fold increased incidence in KD [Kawasaki Disease] in children hospitalised in… a large university hospital centre in Paris. The temporal association with the onset of the SARS-CoV-2 epidemic in France and the results of RT-PCR and IgG testing in our patients suggest a causal link. Furthermore, all but one of our patients had no suggestive symptoms of acute Covid-19 disease and most had positive serum IgG responses, suggesting that the development of KD in these patients is more likely to be the result of a post-viral immunological reaction.”
https://www.medrxiv.org/content/10.1101/2020.05.10.20097394v1.full.pdf
“The first ADE risk is mediated by antibody-dependent infection of macrophages via Fc receptors. The second ADE risk is related to activation and degranulation of mast cells with Fc receptor-bound SARS-CoV-2 antibodies, which leads to increased histamine release; this model is consistent with multisystem inflammatory syndrome in infants with maternally transferred antibodies to SARS-CoV-2. These two ADE risks have critical implications for B-cell-based vaccines for subsets of the population; these are based on age, pregnancy, cross-reactive antibodies, and variabilities in antibody levels over time.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8459929/pdf/KHVI_17_1969855.pdf
In People as Discussed in Relation to Antibody Features, Behaviors, and Correlations:
“With regard to SARS-CoV-2, titers of IgM and IgG are significantly higher in patients with severe disease than in nonsevere disease (P < .05). The weak responders for IgG had a significantly higher
viral clearance rate than that of strong responders.”
https://academic.oup.com/jid/article/222/2/183/5828055
“…we found that antibody levels were higher after severe infection than after mild infections….”
https://wwwnc.cdc.gov/eid/article/26/7/20-0841_article
“…antibodies against different epitopes of spike glycoprotein either protect or enhance SARS-CoV infections in a Vero E6 cell line as well as in vivo in macaques. Antibodies produced to the epitopes S597–603 and S604–625 strongly aggravated lung damage in macaques. Sera of 64% out of 470 COVID patients contained antibodies that bind in this region of the spike glycoprotein.” “…the development of IgG against SARSCoV-2 in the course of COVID-19 might not be a simple sign of viral clearance and developing protection against the virus. On the contrary, …[it] might be crucial to the severity of the course of the current infection with SARSCoV-2….”
https://www.frontiersin.org/articles/10.3389/fimmu.2020.01120/full
“Children with COVID-19 have lower neutralising antibody levels and less ADE than adults. Higher levels of non-neutralising cross-reactive HCoVs antibodies might partly explain increased susceptibility to severe COVID-19 in older adults. ADE is a phenomenon that also needs to be considered in the development of vaccines….”
https://adc.bmj.com/content/archdischild/106/5/429.full.pdf
“Anti-S antibodies, including neutralizing antibodies, may contribute to the precipitous “crash” that many patients with SARS/COVID-19 experience 7-14 days after the onset of symptoms when virus-specific antibodies appear… vaccines need to be scrutinized to make sure their benefits exceed their risks in people of all ages and with a range of underlying conditions.” “Children have more frequent colds than adults, as noted previously, and thus may have higher titers of protective cross-reactive antibodies. On the other hand, cross-reactive antibodies might have a role in the pediatric multisystem inflammatory syndrome and/or in ADE in adults.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7706293/pdf/HEP4-4-1864.pdf
“Determining the affinity and concentration of these potentially cross-reactive antibodies to the new SARS-CoV-2 antigens is therefore particularly important when assessing both existing immunity against common HCoVs and adverse effects like antibody-dependent enhancement (ADE) in COVID-19…. All 12 sera contained low concentrations of high-affinity antibodies against spike antigens of HCoV-NL63 and HCoV-HKU1, indicative of past exposure to these pathogens, while the affinity against the SARS-CoV-2 spike protein was lower.”
https://pubs.acs.org/doi/pdf/10.1021/acsinfecdis.1c00486
“The results suggest that a high Ab titer may be considered as a risk factor for critical illness…. Furthermore, it might be evidence for the possibility of antibody-dependent enhancement effects….”
https://academic.oup.com/cid/article/71/16/2027/5812996
“…several studies on the early serological response to SARS-CoV-2 have revealed unconventional patterns of seroconversion similar to those of secondary immune responses and an unexpected association between early and potent antibody responses and disease severity. This seroconversion pattern is suggestive of the presence of some degree of cross-reactive immunity that could be induced by previous encounters with common human coronaviruses.” “…the immunopathogenesis potential of cross-reactive immunity should be considered in current efforts to develop safe and effective vaccines. Careful selection of antigenic B cell epitopes will be necessary to avoid the potential induction of antibody dependent enhancement of disease.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7594548/pdf/fimmu-11-567710.pdf
“Viral-specific IgG was present in urine (7/10) and sputum (7/10) in 10 severely ill patients. In contrast, no antibody was detected in the mildly ill group, indicating that severe infection might result in tissue damage, including to the airways in the lung and to the kidneys.”
https://www.jci.org/articles/view/138759/pdf
“COVID-19 severity is associated with increased IgG response….” “…high levels of IgG were frequently found in patients with severe disease. These results indicated that beside the antiviral efficacy, the antibody response might be associated with secondary antibody-mediated organ damage.”
https://www.medrxiv.org/content/10.1101/2020.03.12.20035048v1.full.pdf
“The analysis of 166 severe and 167 mild cases from hospitals in Spain, Italy and Portugal revealed statistically significant differences in the composition of the IgG N-glycome. The most notable difference was the decrease in bisecting Nacetylglucosamine (GlcNAc) in severe patients from all three cohorts. IgG galactosylation was also lower in severe cases in all cohorts, but the difference in galactosylation was not statistically significant after correction for multiple testing.” “Consistent changes in the levels of sialylated and fucosylated IgG glycan structures between mild and severe COVID-19 cases were not detected.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7717252/pdf/cwaa102.pdf
“We observed that elderly patients were more likely to induce higher titers of NAbs than younger patients.”
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3566211
“Fc functional antibodies may… enhance infection or pathology through Ab-dependent enhancement (ADE)…. ADE has the potential to turn mild infections into life-threatening conditions….” “Overall, these findings… support our hypothesis that differences in Ab signatures between children and elderly, primed by their prior exposure(s) to circulating hCoV, may contribute to their differential clinical outcomes to COVID-19….” “…upon infection with SARS-CoV-2, the elderly may preferentially induce skewed Ab responses targeting prior cross-reactive hCoV anitgens and as observed in this study, COVID-19-positive elderly induced elevated IgG and IgA antibodies to the more cross-reactive antigens of SARS-CoV-2… compared to children.”
https://www.nature.com/articles/s41467-021-22236-7.pdf
“IgG and IgM titers in the severe group were higher than those in the non-severe group, although a significant difference was only observed in IgG titer in the 2-week post-symptom onset group.”
https://www.nature.com/articles/s41591-020-0897-1.pdf
“SARS-COV-2 neutralizing antibody titers were positively correlated with severity of COVID-19 pneumonia, and the possible contribution of ADE to disease severity needs much more research in the context of COVID-19 pneumonia.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7425713/pdf/main.pdf
“…increased levels of IgG1 antibodies still indicate a potential risk of ADE….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7404424/pdf/JMV-9999-na.pdf
In Pregnant Mothers and Babies:
“…the extent to which antibody-dependent enhancement may be a factor in pregnant and lactating women and newborns is unknown.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7810027/pdf/main.pdf
“SARS-CoV-2 may escape the antibody–virus complex at sub-neutralizing concentrations of antibodies progressing towards replication process that may be abortive without producing viable virus particles or nonabortive, in either case, massive death of immune cells can happen that can result in infammation cascade and a cytokine storm.” “Maternally acquired SARS-CoV-2 antibodies bound to mast cells could also trigger ADE in children along with the development of MIS (multisystem infammatory syndrome) via placental transport of these antibodies.” “But not just the virus variants; even vaccines against COVID-19 could initiate ADE response.” “…the possibilities for ADE poses a theoretical risk and need to be addressed with utmost care.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8814804/pdf/41745_2021_Article_268.pdf
In People Generally/Miscellaneous:
“…the worse clinical picture seen in this patient with COVID-19 after a prior asymptomatic infection may be the result of developing antibody dependent enhancement (ADE).”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7474827/pdf/main.pdf
“…past investigations of ADE mechanism have identified a range of cellular pathways manipulated by viruses that may alter future cellular function, potentially leading to a long-term disturbance of homeostasis, which can lead to chronic alterations in mitochondrial function and energy regulation, ultimately manifesting as multisystem disease…. The emergence of long-COVID brings a new urgency to these questions.” “Of course, viruses do not need ADE entry to manipulate host immune-inflammatory responses to infection…, but the impact associated with the expansion of cellular range to Fc-Receptor (FcR) or Complement-Receptor (CR) bearing cells, not normally
permissive to infection, requires consideration.” “ADE currently has renewed interest in relation to potential COVID vaccine safety, but in a more general context also raises questions on ME [Myalgic Encephalomyelitis] pathogenesis.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8024622/pdf/fmed-08-662513.pdf
“…altered FcγR or subneutralized IgG antibodies might induce antibody-dependent enhancement (ADE) of immune reaction, and autoantibodies to form immune complex or to bind endothelial cells and induce abnormal hyperinflammation.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7959769/pdf/fimmu-12-632890.pdf
“ADE of the disease… is often associated with the infection of immune cells and leads to immune cell apoptosis. Severe forms of COVID-19 are associated with substantially decreased levels of
immune cells.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8128251/pdf/fimmu-12-629102.pdf
“Upon seroconversion, the antibodies may bind to the virus, and then via attachment of virus-antibody complex to FcR an FcR mediated ADE response may occur. The ADE response in FcR possessing cells, such as macrophages, monocytes, and myeloid cells, leads to the virus endocytosis and activation of myeloid cells. Also increased ADE response may induce and exacerbate cytokine and chemokine storm, leading to a secondary inflammatory phase, severe lymphopenia, and lung injury.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7832464/pdf/main.pdf
“In the context of SARS-CoV-2, it is plausible to think that ADE occurs…. lung epithelial cells express high levels of FcgRIIa. Moreover, immune cells, including monocytes and dendritic cells, highly express this receptor. These populations, especially monocytes, greatly account for the inflammatory infiltrate in the lungs during pneumonia, which is consistent with the transient lymphopenia observed in patients as circulating cells may migrate to the lungs.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7213670/pdf/cln-75-1912.pdf
“ADE… could well explain the «cytokine storm», which has been described in the fatal cases of COVID-19.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7204740/pdf/main.pdf
“It is possible that antibody-dependent enhancement, a phenomenon seen in previous coronaviruses, may play a role in advancing the cytokine storm in COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8859668/pdf/10.1177_11795565221075319.pdf
“Collectively, the current knowledge of CoVs and SARS-CoV-2 infection, in particular, points to an inadvertent collusion involving myeloid cells in COVID-19 pathogenesis, despite their critical role in early sensing and antiviral responses.”
https://www.cell.com/action/showPdf?pii=S1074-7613%2820%2930183-7
“…questions remain about the relative impact that IgG makes… and to what degree it contributes to the immunopathology of antibody-dependent enhancement (ADE).” “…there is great concern that the accelerated pace to develop a vaccine against SARS-CoV-2 will result in a detrimental immune response, i.e., an antibody-dependent enhancement (ADE) of the infection…. prior sensitization to conserved epitopes could lead to the production of non- neutralizing or sub-neutralizing binding antibodies, principally of the IgG isotype, and form antigenantibody complexes. These immune complexes (IC) act as molecular bridges between a virus and immune cells expressing either a complement receptor, IgG Fc receptor (FcgR) on the surface and neonatal Fc receptor (FcRn) intracellularly. The FcgR can function as a mimic for the ACE2 receptor that is not expressed on all immune cells and allows for neutralizing antibodies to gain access to the reproductive machinery of those cells…. When the IC binds to an activating FcgR on APCs it also results in the production of proinflammatory cytokines and chemokines that lead to lung and other organ injury. This hypercytokinemia causes an increased transudate and production of hyaluronan in the alveoli that absorbs up to 1,000 times its molecular weight with water resulting in the severe acute respiratory syndrome (SARS) and death.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7561361/pdf/fimmu-11-591897.pdf
“While sarbecoviruses and merbecoviruses are associated with severe, potentially lethal diseases and are known for their epidemic potential in humans and animals, several years of research did not allow for the development of effective and safe vaccines. In addition to the high variability and ability to elude immune recognition…. the antibody-dependent enhancement (ADE) of the infection was previously reported for some coronaviruses, including sarbecoviruses.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8027494/pdf/fgene-12-602196.pdf
“In some cases, use of antibodies alone is proposed to cause antibody-dependent enhancement (ADE) of infection of immune cells like macrophages. This can induce a severe immune response and damage to different organs.”
https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/cbic.202000728
“‘It’s important to talk about it [ADE],’ says Gregory Glenn, president of R&D at Novavax, which launched its COVID-19 vaccine trial in May. But ‘we can’t be overly cautious. People are dying. So we need to be aggressive here.'”
https://www.nature.com/articles/d41587-020-00016-w
Calls for Caution:
“ADE may occur if a patient has pre-existing antibodies to a virus that cross-react, do not neutralize, and enhance infection against another virus, or another serotype of the same virus.” “Although vaccine development and a robust therapeutic pipeline are of critical importance currently, it is equally important that the emerging data is critically analyzed, and the sense of urgency does not avert clinicians from their Hippocratic Oath of “first do no harm.” The race against COVID-19 must not extract but the best out of us!”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7290157/pdf/fimmu-11-01294.pdf
“The development of an effective vaccine without considering the adverse immune reactions derived from antibody-dependent or cell-based immune enhancement may threaten vaccinated people’s lives and long-term side effects must be considered.”
https://www.eurekaselect.com/article/110879
“There are huge theoretical concerns of vaccine-induced ADE which can occur in several scenarios. It is possible that vaccine-induced antibodies against SARS-CoV-2 may bind to the virus without neutralizing it. Currently mutations on the viral Spike protein have been found in circulating clinical SARS-CoV-2 strains. If this happen, the non-neutralizing antibodies could produce ADE effect and enhance the viral entry into cells…. Furthermore, insufficient concentration of neutralizing Ab may also cause ADE.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7443330/pdf/main.pdf
“It would be a public health and ‘‘trust-in-medicine” nightmare with potential repercussions for years – including a boost to antivaccine forces – if immune protection wears off or antibody-dependant enhancement develops and we face recurrent threats from COVID-19 among the immunized.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7452821/pdf/main.pdf
“ADE as a complication of COVID-19 should be at the forefront while developing SARS-COV-2 vaccines….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7466534/pdf/fimmu-11-01880.pdf
“If the first-tested vaccines fail to protect most recipients but prime or trigger an antibody or other immune response that exacerbates COVID-19 disease in people who become infected, there will be a ferocious public backlash against vaccines in general…. The stakes are high. A powerful Opinion piece in the New York Times argues strongly for the need to obtain the most comprehensive data set possible on the potential risks of SARSCoV-2 vaccines and urges the FDA to not issue an emergency-use approval based solely on immunogenicity data (https://www.nytimes.com/2020/06/08/opinion/trump-coronavirus-vaccine.html). We wholeheartedly agree.”
https://journals.asm.org/doi/epdf/10.1128/JVI.01083-20
“Clearly, vaccine administration without time to fully understand resultant health implications would cause an even greater global setback to the current pandemic…. ADE should be given full consideration when evaluating the safety of any candidate SARS-CoV-2 vaccine.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7311339/pdf/main.pdf
“Given that ADE has been demonstrated to occur during vaccine development for other coronaviruses, it is a concern that must be considered closely during SARSCoV-2 vaccine development.” “It is imperative to stress the need for long-term testing and follow-up of vaccinated individuals for any adverse effects.”
https://cdnsciencepub.com/doi/pdf/10.1139/cjm-2020-0465
“Antigen-experienced older individuals are more susceptible to the phenomenon of ADE, while less antigen-experienced younger individuals mount more targeted antibody responses to viral neoantigens. This may in part account for the greater immunopathology of COVID-19 in older individuals.” “…pre-existing CoV-specific T cell memory may be enhanced by immunization
with a SARS-CoV-2 vaccine leading to either suboptimal T cell responses or immunopathology.” “Comprehensive safety studies are particularly critical because some candidate vaccines use platform technologies that have not been examined extensively in human subjects to date, including some of the viral vectors, mRNA and nanoparticle constructs, and because of the potential for enhanced disease and adverse events related to aberrant immune responses to be seen upon infection pre- and post-licensure.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7566192/pdf/fimmu-11-579250.pdf
“…structural proteins (S, E, M, and N proteins) are of particular interest as vaccine antigens. However, they can induce inflammatory responses (S-protein), inhibit IFN-1 production (M-proteins), or both (E- and N-proteins). The inhibition of IFN-1 and upregulation of pro-inflammatory responses continues with release/translation of the protein antigen; this could last longer for DNA- and RNA-based vaccines compared to protein vaccines. Moreover, the effect
would be more locally enhanced at the site of injection…. Thus, it is worth mentioning that immunopathology is not associated only with local immune responses, in the same way that vaccines are not limited only to the injection site. Therefore, whole SARS proteins should not be used in their native full-length forms as vaccine antigens.” “ADE-triggering epitopes should be identified and excluded from SARS-2 vaccines or, as a minimum precaution, vaccines should be tested for potential ADE-related side effects.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8373118/pdf/ADVS-8-2100985.pdf
“…due to the taxonomic and structural similarities between SARS-CoV, MERS-CoV, and SARS-COV-2, ADE is an issue that should be considered seriously in designing MERS-CoV and SARS-CoV-2 vaccines, particularly those with a full-length S protein.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7785583/pdf/fimmu-11-602256.pdf
“The high sequence homology and the similarity in structure shared among SARS-CoV, MERS-CoV, and SARS-CoV2 S glycoproteins raises reasonable concerns about the development of COVID-19
vaccines based on the S protein.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7494754/pdf/fimmu-11-02130.pdf
“ADE could explain some peculiarities of COVID-19 immunopathology and may seriously hamper vaccination attempts. Caution is thus required when aiming at provoking an antibody immune response toward coronaviruses, as this response could either block the infection or enhance it.” “…using the spike RBD for vaccination may lead to unwanted ADE effects and is limited to strain specificity.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7490329/pdf/fmolb-07-00226.pdf
“ADE has been reported in the evaluation of vaccine candidates directed to S protein for SARS-CoV. Because of their similarities, these findings enable us to foresee the high risks of ADE in SARSCoV-2 vaccines directed to S protein.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8092843/pdf/JVI.01837-20.pdf
“Conceptually, many S2 subunit-specific antibodies are non- or weakly neutralizing and potentially responsible for ADE. Thus, vaccine design to minimize the elicitation of S2 subunit-specific antibodies should be considered, mainly when/if ADE will be observed for SARS-CoV-2.
Although ADE has yet to be fully observed for SARS-CoV-2 infection or vaccination, previous coronavirus vaccine candidates were reported to be complicated by ADE.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8567933/pdf/TEMI_10_1994354.pdf
“One of the most perplexing questions regarding the current COVID-19 coronavirus epidemic is the possible worsening of the disease by immunotherapies, as the consequence of an antibody dependent enhancement (ADE) of infection with SARS-CoV-2.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7268185/pdf/12967_2020_Article_2392.pdf
“…the development of SARS-CoV-2 vaccines has to incorporate approaches to
develop the vaccine with minimum or no risk for ADE….”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7536968/pdf/RMV-9999-e2161.pdf
“This warrants for extensive studies to investigate if this antibody mediated entry of the virus enhances the coronavirus replication in the host cells.”
https://www.infezmed.it/media/journal/Vol_29_2_2021_1.pdf
“Releasing into 1 million people a vaccine that will have been tested only in a few dozen challenge participants carries some risk of previously undetected toxicity or enhanced COVID-19.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8084609/pdf/main.pdf
“If the adverse reaction rate of a COVID-19 vaccine is only 1%, about 78 million individuals will be affected if the whole world population is vaccinated. The adverse reaction rate of a COVID-19 vaccine should be kept extremely low if it is distributed globally. The comprehensive safety evaluation in different animal models and clinical trials and rational design of
antigens and adjuvants will contribute to lower incidence of VADE [vaccine- associated disease enhancement].”
https://www.nature.com/articles/s41579-020-00462-y.pdf
“…the COVID-19-induced immunopathological changes such as antibody-dependent enhancement (ADE) and Th2 immunopathology have significant implications in developing suitable antiviral agents. Hence, these aspects should be considered while designing a potent vaccine.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8168478/pdf/BMRI2021-8160860.pdf
“Safety is a primary goal for vaccines that are given to otherwise healthy people, and there is a risk that vaccination could make subsequent SARS-CoV-2 infection more severe.”
https://www.science.org/doi/epdf/10.1126/science.abb8923
“…in some cases, ADE causes uptake of these antibody-bound antigen molecules into the efector cells promoting their enhanced replication and infammation. Therefore, ADE should be an important aspect to be considered before proceeding for clinical trials of any drugs for therapeutic and vaccine development.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7563911/pdf/11010_2020_Article_3935.pdf
“…antibodies could potentially trigger immunopathogenic events in SARS-CoV-2-infected patients or enhance infection.” “These topics will, no doubt, be investigated thoroughly as much-needed SARS-CoV-2 vaccines undergo pre-clinical and clinical testing.”
https://elifesciences.org/articles/57877
“We argue that ADE should be given full consideration in the safety evaluation of emerging candidate vaccines for SARS-CoV-2.”
https://www.nature.com/articles/s41577-020-0321-6.pdf
“Since… vaccine is under development, due consideration has to be provided on ADE to prevent untoward reactions.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7484565/pdf/KHVI_16_1796425.pdf
“Whether ADE has a role in SARS-CoV-2 infection will need to be carefully examined because of its potential adverse effect in vaccination.”
https://www.cell.com/cell-reports/pdfExtended/S2211-1247(20)30702-6
“Current SARS-CoV-2 vaccines appear to be providing protection with high antibody titers; the possibility of ADE risks associated with waning titers of antibodies over time remains unknown.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7943455/pdf/fimmu-12-640093.pdf
“…clinical data has not yet fully established a role for ADE in human COVID-19 pathology…. Such evidence is sorely needed to ensure product safety….”
https://www.nature.com/articles/s41564-020-00789-5.pdf
“There remains a need for more detailed assessment of antibody kinetics to help determine the impact of antibody-dependent enhancement in COVID-19 pathogenesis….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7499509/pdf/ciaa1280.pdf
“The clinical relevance of the ADE mechanism in SARS-CoV-2 pathogenesis, if confirmed, would be the greatest concern in COVID-19 vaccine development….” “Considering our limited knowledge in this regard, further thoughtful and rigorous research works are urgently required to explore the possible role of ADE in the severity and mortality of COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8223819/pdf/WJCC-9-4480.pdf
“…because ADE of disease cannot be reliably predicted after either vaccination or treatment with antibodies-regardless of what virus is the causative agent-it will be essential to depend on careful analysis of safety in humans as immune interventions for COVID-19 move forward.”
https://www.nature.com/articles/s41586-020-2538-8.pdf
“The existence of ADE in SARS-CoV-2 might aggravate the COVID-19 pandemic with higher morbidity and mortality….” ” ADE development should also be considered as possible adverse effects of… vaccine development.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7300451/pdf/CYTO-9999-na.pdf
“Vaccines may cause adverse events that are harmful to the host due to unwanted immune enhancement responses, so careful and complete tests are required before the approval of a global vaccine for COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8127526/pdf/main.pdf
“The imminent risk that may be triggered by a vaccine-mediated antibody response is that the mechanism of ADE occurs and places vaccinated individuals at greater risk of a more severe disease phenotype compared to unvaccinated individuals.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8512237/pdf/10.1177_20587384211050199.pdf
“Unlike conventionally developed vaccines, Biovacc-19’s method of operation is upon nonhuman-like (NHL) epitopes in 21.6% of the composition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)’s spike protein….” “The possibility of inducing autoimmune responses or antibody-dependent enhancements, needs to be carefully guarded against….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7468800/pdf/S2633289220000083a.pdf
“The binding of CR3022 antibody with SARS-CoV-2 may lead to virus-antibody complexes, which instead of neutralizing the virus, it enhances its pathogenicity by allowing the virus to bind to ACE2 and/or leading to ADE.” “..studies need to elucidate whether ADE is a mechanism of pathogenesis of SARSCoV-2. Moreover, this phenomenon needs to be taken into account when developing new treatments.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7955763/pdf/itt-10-63.pdf
“Whether such non-neutralizing antibodies can lead to an ‘ADE’ like response in the future cannot be ruled out and hence needs to be evaluated carefully in the clinic.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7754925/pdf/KHVI_17_1845524.pdf
“…antibody therapy for SARS-CoV-2 still faces potential challenges, including… the potential for antibody-mediated enhancement of infection or inflammation.”
https://www.annualreviews.org/doi/pdf/10.1146/annurev-med-042420-113838
“Going forwards, it will be crucial to evaluate animal and clinical datasets for signs of ADE, and to balance ADE-related safety risks against intervention efficacy if clinical ADE is observed.”
https://www.nature.com/articles/s41564-020-00789-5.pdf
“…’Antibody-Dependent Enhancement…’ could be linked to the severity of coronavirus infections
and could possibly create difficulties with new vaccines.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7308593/pdf/fimmu-11-01441.pdf
“…the existence of only low-quality and low-concentration antibodies can enhance the infection… and inflammation…. Given that SARS-CoV-2 specific IgG avidity is strong in ICU patients after 1 month, these negative effects may not play a role in COVID-19 disease progression and severity. A recent study suggests that the SARS-CoV-2 IgG concentrations can wane over time, however, it is unclear if the antibodies that persist are of high enough quality to neutralize the virus. Further studies are needed to determine if both the antibody concentration and antibody avidity correlate with virus neutralization and persist over time.”
https://academic.oup.com/cid/article/73/9/e3095/5905578
“As of today, there are no reports of ADE with the use of COVID-19 candidate vaccines in nonhuman primates and humans. However, it is an early period in the development of these vaccines, and as the matter is of major importance in the success of such a vaccine, we need to be vigilant.” “Finally, vaccine development is a risky process, and one critical issue in the COVID-19 vaccine would be the occurrence of ADE which may be disastrous for those receiving the vaccine.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7286271/pdf/main.pdf
“Whether ADE can help SARSCoV-2 infect immune cells through a non-ACE2-dependent pathway and participate in SARS-CoV-2 hepatic injury is an issue of concern.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8326263/pdf/WJG-27-4504.pdf
“The potential pathogenic consequences of using antibodies targeting COVID-19 virus present an important and concerning challenge to the development of vaccines and antibody-based treatments. Further studies should be conducted on large-scale cohorts to prove or reject this possibility.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7384506/pdf/MEDJ-35-151.pdf
“Therefore, if a candidate vaccine for SARS-CoV-2 is specific for any particular strain, then subsequent infection by other strain in the vaccinated person can potentially cause ADE…. Researchers must take note of what is known about ADE and coronaviruses and proceed with extreme caution in the development of the SARS-CoV-2 vaccine.”
https://www.degruyter.com/document/doi/10.1515/jbcpp-2021-0264/html
“…the risk of ADE in COVID-19 vaccination should not be ignored.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8513558/pdf/41423_2021_Article_774.pdf
“…studies are also warranted to overcome ADE responses, and Th-2 immunopathology for the development of safe and efficacious vaccines against SARS-CoV2.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7531039/pdf/fimmu-11-552925.pdf
“Given the concern about ADE, and the remarkably broad additional potential immune-protective function of antibodies in controlling inflammation, it is critically important that vaccines will be evaluated for their pro-inflammatory and anti-inflammatory antiviral Fc functional profiles to maximize the true potential of the humoral immune system.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7278217/pdf/41577_2020_Article_359.pdf
“We also discuss the challenges of NAbs therapeutics such as ADE and escape mutations. Such evidence is urgently needed to the future development of antibody therapeutic interventions that are required to reduce the global burden of COVID-19.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7799234/pdf/tbaa028.pdf
“If occurring in COVID-19 patients, ADE may account for some severe outcomes occurring later during the natural course of the disease. The protean clinical features that seem to be associated with autopsy reports should probably be investigated, since the expression of Fc receptors is widespread in nonimmune cells, including intestinal epithelial, kidney and endothelial cells. On the other hand, ADE may affect safety and efficacy of passive and active immunisation schedules.”
https://smw.ch/article/doi/smw.2020.20249
“Whether these non-neutralizing cross-reactive antibody responses have effector functions with a protective effect or lead to antibody-dependent disease enhancement of SARS-CoV-2 infection remains to be evaluated in further studies.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8318684/pdf/main.pdf
“…data raise the possibility of ADE by antibodies in COVID-19, and close attention should be paid to the matter.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7572790/pdf/main.pdf
“Granted, the occurrence of ADE has only largely been limited to in vitro demonstrations and little to no evidence exists to suggest whether SARS-CoV-2 can also induce cytokine and chemokine release through ADE. However, since ADE has been reported in the other highly pathogenic hCoV infections, it might be worth investigating whether ADE could increase antigen presentation on ADE-permissive cells and, if so, whether this too could cause an aberrant cytokine release that could contribute to the cytokine storm in COVID-19”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8402835/pdf/viruses-13-01457.pdf
“ADE response studies should be included for all candidate vaccines as a major safety parameter for approval of the vaccine.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8461057/pdf/fcimb-11-690621.pdf
“Although there is no direct evidence that there is ADE in COVID-19, this potential risk is a huge challenge for prevention and vaccine development.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8438590/pdf/main.pdf
“There is the potential for ADE, but the bigger problem is probably Th2 immunopathology….”
– Ralph S. Baric
https://www.pnas.org/content/pnas/117/15/8218.full.pdf
“Careful studies will be necessary to understand whether ADE pathologies might be associated with vaccine and antibody candidates against SARSCoV-2.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7498231/pdf/main.pdf
“…antibody-dependent enhancement (ADE) of diseases is perhaps one of the leading concerns to vaccine developers and regulatory agencies…. Since SARS-CoV-2 and SARS-CoV belong to the same family virus, the potential of ADE is a special concern in COVID-19 vaccine development.” “Additional efforts are also needed in the development of innovative animal models suitable for screening the efficacy and safety (particularly the ADE) of vaccine candidates to provide a better bridging to human clinical trials. “
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7733917/pdf/KHVI_16_1787067.pdf
“…the potential risk of ADE for monoclonal antibodies and vaccines, where antibody Fc interactions can promote inflammation in respiratory mucosa, is often associated with poorly neutralizing antibodies…. Although therapy using convalescent plasma containing antibodies from recovered COVID-19 patients has revealed no substantial ADE burdens, novel mutations of SARS-CoV-2 may lead to neutralization loss and increase the ADE risk.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8326117/pdf/ijbsv17p2957.pdf
“…caution and vigilance to identify any evidence of enhanced coronavirus infection by ADE will be required.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7270518/pdf/12929_2020_Article_663.pdf
“…preclinical experience with vaccine candidates for SARS and the Middle East respiratory syndrome (MERS) have raised concerns about exacerbating lung disease, either directly or as a result of antibody-dependent enhancement. Such an adverse effect may be associated with a type 2 helper T-cell (Th2) response. Hence, testing in a suitable animal model and rigorous safety monitoring in clinical trials will be critical.”
https://www.nejm.org/doi/pdf/10.1056/NEJMp2005630?articleTools=true
“We also speculate that ADE may exist in SARS-CoV-2.” “Understanding of ADE in SARS-CoV and MERS-CoV infections has taken several years… From this, we speculate that studies on ADE in this newly emerged coronavirus will take some time, but the elucidation of this effect is of great importance.”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7483033/pdf/main.pdf
“Past experience has demonstrated that vaccine development needs to be performed cautiously to avoid potential exacerbation of disease (i.e. ADE).”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7211658/pdf/main.pdf
“Whether SARS-CoV-2 can cause ADE effects remains an open question. Epidemiological studies investigating ADE in individuals with multiple SARS-CoV-2 infections or cross-reactivity to common-cold-causing CoVs will likely take several years. However, given that ADE has been observed with the closely related SARS-CoV, we believe that the question of ADE effects in SARS-CoV-2 should be urgently resolved using experimental immunology.” “Although the development of vaccines and therapeutics for SARS-CoV-2 remains urgent, we must proceed with caution… to rationally minimize the risk of ADE.”
https://www.nature.com/articles/s41587-020-0577-1.pdf
“Although vaccine research and development technology has made great progress, the possibility of obtaining a safe and effective vaccine that can control the global epidemic in a short period of time is still low due to the antibody-dependent enhancement effect (ADE) of the vaccine and the mutation of the virus.”
https://pubmed.ncbi.nlm.nih.gov/33210600/
“But some viruses become more potent when they infect organisms previously treated with inactivated vaccines, in a poorly understood phenomenon known as antibody-dependent enhancement (ADE)…. but the risk will be closely monitored in all the inactivated-vaccine phase III trials…”
https://www.nature.com/articles/d41586-020-02244-1
“The probability of ADE varies with antibody concentration, suggesting that its effects might appear late after vaccination and highlighting the need for long-term follow-up in clinical studies.” “Early studies should be performed in healthy adults who are fully informed about areas of uncertainty regarding risks, and human challenge studies are an intriguing but controversial approach to assessing efficacy and safety. These considerations are especially important since many candidate vaccine platforms are novel and have not yet yielded licensed vaccines.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7239154/pdf/jiaa234.pdf
“Vaccine trials will need to consider the possibility of antibody-driven pathology upon antigen rechallenge….”
https://www.cell.com/action/showPdf?pii=S1074-7613%2820%2930183-7
“…there is also a concern about the induction of ADE syndrome in humans vaccinated with SARS-CoV-2 vaccine candidates.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7479216/pdf/fimmu-11-02173.pdf
“…vaccines against SARS-CoV-2 may also generate non-neutralizing antibodies that facilitate viral entry into cells that express Fc receptor, a phenomenon known as antibody-dependent enhancement (ADE).”
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC7302209/pdf/nihpp-2020.06.04.135004.pdf
“Indeed, development of antibodies to SARS-CoV-2 might have undesirable consequences…. ADE may, or may not, be pertinent to SARS-CoV-2 vaccine development.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7212970/pdf/main.pdf
“To date, the exact role of ADE in SARS-CoV-2 infection remains unclear.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7372093/pdf/fimmu-11-01660.pdf
“More research is necessary on… antibody-dependent enhancement….”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7985173/pdf/fimmu-12-637651.pdf

2 thoughts on “ADE Update”