Excess body weight is a significant risk factor for many cancers, especially breast cancer. Patients with breast cancer or those with a history of the disease who are overweight or obese have an increased risk of therapy-related morbidity, recurrence, and breast cancer-related mortality. Obesity may also affect quality-of-life factors for survivors, including sexual dysfunction, neuropathy, cardiotoxicity, chronic fatigue, and lymphedema. Most cancer guidelines recommend that breast cancer survivors who are overweight or obese lose weight and that those with a normal body mass index (BMI) maintain a stable body weight. The cornerstone of interventions to treat or prevent obesity is lifestyle modification with diet and exercise; however, integrating these things into clinical practice is challenging. This article will present feasible weight loss interventions, and will discuss practical implications of ongoing chemotherapy and endocrine therapy with regard to weight gain, and the impact of obesity on therapy-related conditions during breast cancer survivorship.
Introduction
Many epidemiologic studies have consistently demonstrated that excess body weight is a major risk factor for breast cancer.[1] With 268,670 new breast cancer cases and 41,400 breast cancer deaths projected in 2018 in the United States, breast cancer and obesity are both significant public health issues.[2] Obese patients with breast cancer have an increased relative risk of recurrence of 40% to 50% and a relative risk of breast cancer-related mortality of 53% to 60%.[3,4] Obese women with hormone receptor-positive operable breast cancer have inferior outcomes in terms of disease-free survival (DFS; hazard ratio [HR], 1.24; 95% CI, 1.06-1.46; P = .0008) and overall survival (OS; HR, 1.37; 95% CI, 1.13-1.67; P = .002), compared with non-obese women.[5] Overall, approximately 15% of breast cancer cases in postmenopausal women may be attributable to weight gain.[6] In premenopausal women, short-term weight gain may increase breast cancer risk, although data are mixed.[7]
Obesity Increases Inflammation, Cytokines, and Endogenous Hormones
The biological relationship between obesity and breast oncogenesis is likely mediated by several pathways. Obesity has been associated with increased levels of inflammatory cytokines, including interleukin-1β, interleukin-6, tumor necrosis factor α, and monocyte chemoattractant protein 1. Altered levels of these cytokines drive pro-proliferative pathways, such as angiogenesis, influx of macrophages, and antiapoptotic pathways.[8] Another untoward effect of obesity is increased synthesis of estrogens from androgens via augmented aromatization of androstenedione in peripheral adipose tissue in postmenopausal women. Breast carcinogenesis has also been associated with increased serum leptin levels and low adiponectin levels. Leptin is an adipocytokine synthesized by adipocytes; it acts as a satiety hormone at the hypothalamus level to reduce appetite and is paradoxically elevated in obese individuals.[9] High levels of leptin lead to concomitant activation of various oncogenic pathways, resulting in increased tumor growth, angiogenesis, and acquisition of a migratory and invasive mesenchymal phenotype.[10] Adiponectin counters obesity by modulating glucose metabolism, increasing fatty acid oxidation and insulin sensitivity, and decreasing production of inflammatory cytokines. Increased adiposity also increases circulating levels of insulin and insulin-like growth factor 1, which may also promote cell proliferation. Favorable changes in estrogens, sex hormone binding globulin, insulin, and leptin are observed to be associated with weight loss of at least 5%.[11]
Furthermore, weight loss of at least 10% has been associated with modulation of serum and tissue biomarkers, such as Ki-67, adiponectin, adiponectin-to-leptin ratio, sex hormone binding globulin, estradiol, testosterone, and insulin.[12] These biological data not only provide insight into how obesity can increase the risk of breast cancer, but suggest that weight loss may reverse some of these deleterious biological changes associated with obesity.
Concerns About Breast Cancer Therapy and Obesity
Chemotherapy can lead to weight gain
Chemotherapy-associated weight gain is experienced by most patients during the first year after diagnosis.[13] The Women's Healthy Eating and Living (WHEL) study found that women treated with chemotherapy were 65% more likely to gain weight compared with those not receiving chemotherapy.[1] Studies have shown that women have decreased levels of physical activity after the diagnosis of breast cancer, which may contribute to weight gain. Moreover, adjuvant chemotherapy may also decrease resting rates of metabolism.[14] Additionally, the hormonal changes of menopause can affect metabolism and lead to weight gain.[2,3]
Endocrine therapy does not significantly affect weight and is effective in obese patients
A common question asked in clinical practice is whether endocrine therapy causes weight gain. In the International Breast Cancer Intervention Study (IBIS)-I, which investigated tamoxifen vs placebo, the mean weight gain was 0.1 kg (standard deviation [SD], 0.1) vs 0.3 kg (SD, 0.1), respectively (P = .3). In the IBIS-II study, which compared anastrozole vs placebo, the median weight gain was 0.8 kg (SD, 5.3) vs 0.5 kg (SD, 7.4), respectively (P = .5). In the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial, which compared anastrozole vs tamoxifen, there was no significant difference between the two therapies (median weight gain, 1.4 kg [SD, 3.9] vs 1.5 kg [SD, 4.0], respectively; P = .4).[15] Recent data from the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial, however, suggest that patients receiving an aromatase inhibitor (AI) may be less likely to gain weight than those receiving a selective estrogen receptor modulator (odds ratio [OR], 0.54; 95% CI, 0.31-0.93).[16] While these data may be conflicting, and there are numerous other variables such as chemotherapy and menopausal status that can affect weight gain, there is insufficient evidence to suggest that, in general, endocrine agents cause a significant amount of weight gain. Efforts at weight management should be directed at lifestyle changes rather than therapy discontinuation.
An additional question that often comes up is whether AIs are as effective in obese patients. In obese patients, the total level of body aromatase is elevated, and studies suggest that circulating estradiol may not be fully suppressed by AIs; however, this has never been definitively linked to inferior outcomes.[17] One study found that obese women who received anastrozole had plasma drug concentration levels that exceeded those of women with normal body weight by 25%, as well as lower mean follicle-stimulating hormone levels, indicating higher estrogen activity.[18]
In the Anastrozole vs Letrozole: Investigation into Quality of Life and Tolerability (ALIQUOT) study, baseline estrogen levels were significantly correlated with body mass index (BMI) and three times higher in obese patients compared with patients of normal weight. Estrogen levels in patients receiving treatment with both anastrozole and letrozole were greater at higher BMI levels, but this was significant only for letrozole (P = .013 for estradiol and P = .035 for estrone sulfate). Suppression of both estrogen types was greater with letrozole across the full range of BMI.[19] Other studies, however, have shown that changes in estrone and estradiol levels were not associated with BMI.[20] While data on the pharmacodynamic effects of obesity on AI-induced estrogen reduction may be variable, there are no conclusive data that suggest that AIs are clinically inferior to other endocrine regimens, and these still remain the therapy of choice for postmenopausal women, irrespective of BMI.
In the Breast International Group (BIG) 1-98 study, increased mortality risk among obese women was similar regardless of the type of endocrine therapy administered (tamoxifen: HR, 1.18; 95% CI, 0.91-1.52 and letrozole: HR, 1.22; 95% CI, 0.93-1.60).[21] Thus, in patients with an indication for an AI, we do not recommend routinely changing endocrine therapy on the basis of BMI, or recommend one AI over another.
Effects of Obesity on Quality of Life in Survivors
Obesity at and following breast cancer diagnosis is significantly associated with poor health-related quality of life and functional health, and may increase the risk of adverse treatment effects.
Obesity can affect body image, sexual function, and genitourinary function in breast cancer survivors
Evaluation and treatment of female sexual dysfunction is often under-addressed in breast cancer survivors, in spite of the availability of safe and effective treatments.[22] While early menopause induced by chemotherapy and hormonal alterations associated with AIs can contribute to altered sexual function, surgery and radiation for breast cancer may also alter sexuality by changing body contour and sensation, decreasing arousal or lubrication, and reducing nipple sensation after nipple-sparing mastectomy.[23] A cross-sectional survey of 255 patients who were at least 1 year from surgery showed that obese and overweight women reported more appearance dissatisfaction (18.1% and 13.0%) compared with normal-weight women (4.1%; P = .02). A greater proportion of overweight women (94.7%) reported that their chest played an important role in intimacy before and after surgery, as compared with normal-weight women (80.6%), but a postoperative decline in the importance of this role was observed in all groups (overweight, P = .01; normal weight, P < .001).[24] Furthermore, being overweight or obese at baseline was associated with more problems with urinary incontinence and tendency to nap, and with poorer physical functioning and more bodily pain (vs BMI < 25 kg/m2).[25]
KEY POINTS
- Women with early-stage breast cancer who are overweight or obese at diagnosis and/or gain weight after diagnosis may have a higher risk of recurrence compared with women of normal weight.
- Obesity may increase the risk of treatment-related toxicities, such as sexual dysfunction, neuropathy, chronic fatigue, cardiotoxicity, and lymphedema.
- A standard-of-care approach and specific guidelines to manage weight loss and to improve physical activity levels among patients with a history of early-stage breast cancer are needed.
Neuropathy is more common in obese patients
Chemotherapy-induced peripheral neuropathy (CIPN) has been reported in up to 44% of patients receiving chemotherapy, particularly taxanes.[26] Higher BMI is associated with a higher incidence of neuropathy, with studies demonstrating a prevalence of 48.4% in participants with normal weight, 60.2% in overweight participants, and 66.7% in obese participants. Compared with women of normal weight, being obese was associated with an increased risk of CIPN (adjusted OR, 1.94; 95% CI, 1.03-3.65).[27] Neuropathy was significantly more likely to occur in overweight patients compared with normal-weight patients receiving taxane treatment at 24 months, and less likely to occur in patients with high moderate-to-vigorous physical activity levels compared with those with lower activity levels at 24 months.[28] While studies have not shown that weight loss can reverse neuropathy, these data suggest that physical activity may help decrease neuropathy. Furthermore, primary prevention with baseline normal weight may also be important.
Obesity is a risk factor for cardiotoxicity
Because obesity is already a strong risk factor for cardiovascular disease, cancer treatment in obese patients can further increase overall risk for adverse cardiac effects.[29] A recent meta-analysis suggests that being overweight or obese may be a risk factor for cardiotoxicity from anthracyclines, especially when these are administered in sequence with trastuzumab.[30] Other studies have found obesity to be associated with an increased risk of cardiac dysfunction in women using trastuzumab.[31] Additionally, in elderly patients (over 65 years of age), obesity is an independent risk factor for trastuzumab-related cardiac toxicity.[32]
Obesity can lead to chronic fatigue
About one-third of breast cancer survivors have chronic fatigue and about one-fourth will have persistent fatigue after 2 years.[33] Although the development of fatigue in breast cancer patients seems largely due to cancer therapy, the long-term persistence of fatigue is related to preexisting medical conditions and lifestyle factors. Higher BMI at baseline is significantly associated with increased physical fatigue during and after cancer treatment.[34] The Mammary Carcinoma Risk Factor Investigation (MARIE) study demonstrated that both a physically inactive lifestyle and obesity were associated with persistent physical fatigue, independent of chemotherapy and radiation therapy.[35] In a study of patients who were overweight or obese, fatigue after chemotherapy was lower in an exercise group than in a non-exercise group.[36] Higher BMI, associated with a greater tendency to catastrophize about fatigue and amplify physical symptoms, was predictive of fatigue beyond 9 months.[37] These studies demonstrate the importance of an ideal body weight, since chronic fatigue is associated with emotional distress and limits function and willingness to exercise.
Lymphedema is more common in obese patients
Cancer treatments such as lymph node dissection and radiation therapy can damage lymphatic drainage routes, leading to fluid build-up, discomfort, and reduced mobility and function. Excess adiposity may increase risk of lymphedema via increased inflammation, added stress on the lymphatic system, or slower healing times after surgery.[38] Data suggest that obesity increases the risk of lymphedema after treatment for breast cancer. Prospective studies have reported statistically significantly higher lymphedema risk for obese compared with normal-weight women (OR, 2.48; 95% CI, 1.05-5.84).[39] Breast cancer survivors who were obese at the time of treatment were approximately 3.6 times more likely to develop lymphedema at 6 months after diagnosis than those who were not obese.[40] In a pilot study among overweight breast cancer survivors, a 12-week diet intervention resulted in a significant reduction in BMI and swollen arm volume (reduced from 24% to 15%; P = .02).[41]
Weight Loss in Breast Cancer Survivors Is Feasible
Studies have demonstrated that modest weight loss is possible and improves survival outcomes in women with early-stage breast cancer.[42,43] Dietary guidelines suggest that patients with a history of early-stage breast cancer should receive a nutritional assessment immediately after diagnosis, since diet quality is an important component of maintaining a healthy body weight.[44]
Data from the Women's Health Initiative found that an intervention group that received teaching by dietitians 4 times a year (≥ 5 fruit and vegetable servings, ≥ 6 whole grain servings, and < 20% fat in their diet recommended) had a 26% decrease in risk of death by any cause and a 42% decrease in risk of death related to breast cancer compared with a control group.[45] In the Women's Intervention Nutrition Study (WINS), women participating in conventional treatment for early-stage breast cancer (N = 2,437) were randomly assigned either to a group that also received a dietary intervention (to reduce the percentage of calories from fat to 15%) or to a control group. Those in the dietary intervention group who experienced weight loss had a 24% lower risk of relapse at 5-year follow-up compared with those not receiving the dietary intervention. Of note, body weight was not an intervention target, and there was only a difference of 2.7 kg between groups through 5 years of observation (P = .005).[46]
However, the WHEL study randomized women with early-stage breast cancer to a dietary intervention (a telephone counseling program as well as cooking classes and newsletters that promoted 5 vegetable servings, 3 fruit servings, and 15% to 20% of energy intake from fat) or to printed "5-A-Day"� diet guidelines. The dietary intervention arm did not achieve significant weight loss and did not demonstrate a reduction in breast cancer, compared with the arm with printed materials.[47] These studies suggest that alteration in diet alone may be insufficient, and that weight loss is required to achieve improvements in breast cancer-related outcomes.[48]
In-person programs, which help with behavioral modification in the areas of diet and exercise, have been successful in achieving weight loss. A systematic review of overweight and obese patients in a primary-care setting shows that intensive, face-to-face behavioral counseling for at least 3 months, with at least 6 months' follow-up, can induce clinically meaningful weight loss.[49] The mean 6-month weight changes from baseline in the intervention groups ranged from a loss of 0.3 kg to 6.6 kg. In the control groups, the mean change ranged from a gain of 0.9 kg to a loss of 2.0 kg.[49]
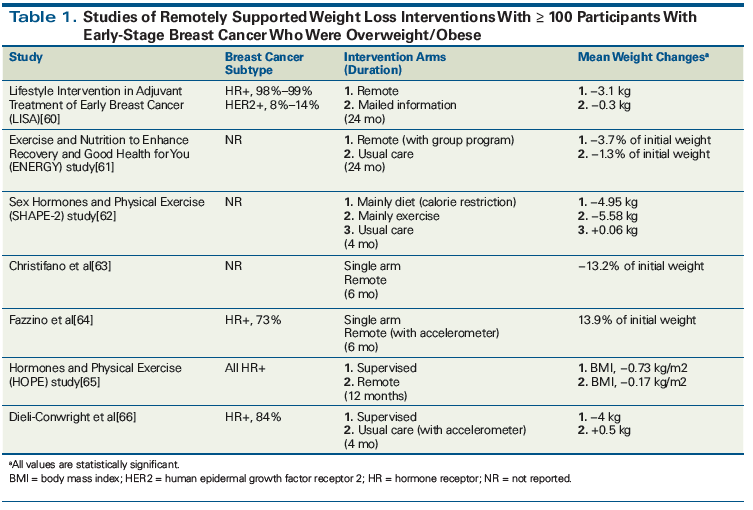
Since limitations on in-person weight loss interventions include time and cost constraints, remote interventions with similar efficacy may be more amenable to real-world integration. The Practice-Based Opportunities for Weight Reduction (POWER) trial demonstrated equivalent weight loss outcomes between in-person coaching and a remotely supported weight loss intervention (telephone calls by a health coach, accelerometers to assess physical activity, self-directed dietary and activity monitoring, and web-based learning modules).[50,51] This intervention has been adapted for patients with stage 0 to III breast cancer who have completed all local therapy and chemotherapy, and was found to have promising results in a randomized study.[51] Other studies have also suggested that remotely supported weight loss programs can produce results similar to those of in-person programs (Table 1). Some evidence supports the effectiveness of mobile technology interventions that incorporate self-monitoring, feedback on performance, and networks of social support, although success has been variable.[52] These experiences demonstrate that not only are weight loss interventions feasible, but that remotely supported approaches may be adaptable and ultimately integrated into clinical practice.
While definitive data are pending, initial data suggest that weight management for improved cancer-related outcomes is an important aspect of survivorship care. To confirm the positive impact of weight loss on survival outcomes (such as DFS), a large randomized study has been designed and is actively accruing.[53] The Alliance Breast Cancer Weight Loss trial (BWEL) is testing the 10-year impact of a telephone weight loss program on invasive disease-free survival in 3,136 women with a BMI ≥ 27 kg/m2 with a recent diagnosis of stage II to III estrogen receptor-positive or triple-negative breast cancer.[54] Patients will be randomized to a 2-year weight loss intervention consisting of a health education program that includes standardized mailings and twice-yearly conferences or to a program that also includes telephone calls from a trained coach.
While the aforementioned studies involved patients who had completed chemotherapy, remote lifestyle interventions at the time of diagnosis or early on during adjuvant treatment may provide an opportunity to prevent treatment-associated weight gain. Data suggest that healthy lifestyle programs can be integrated during chemotherapy for many patients; however, these programs may need to be adjusted for those patients who are less likely to participate. Patient participation is variable across different studies and can range from 11% to 65%.[55,56] In one study, patients who did not wish to exercise were less likely to be employed and had a lower level of education, more fatigue and lower quality of life, more negative attitudes about exercise, and less social support compared with participants who exercised.[57] Regardless of patients' inclination to participate in healthy lifestyle programs, given the possible benefits, we recommend that patients stay active during administration of chemotherapy for early-stage breast cancer, and that they discuss any limitations on types of physical exercise and activity levels with their healthcare providers.
Guidelines and Recommendations
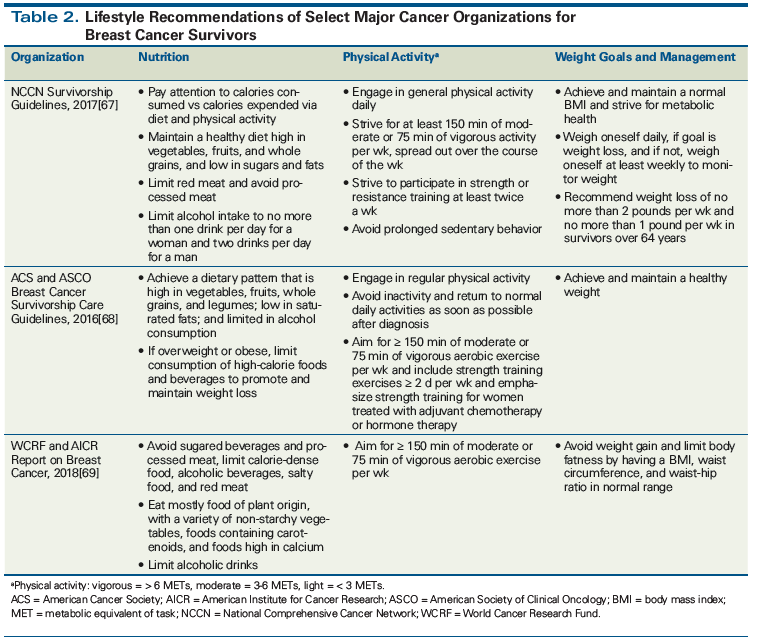
Cancer organizations and foundations recommend a healthy diet, adequate exercise, and a healthy body weight for all breast cancer survivors (Table 2). While cancer survivors often seek ways to minimize the risk of recurrence and death from breast cancer, many struggle with achieving sustainable weight loss.[58] An analysis of adherence to World Cancer Research Fund/American Institute for Cancer Research recommendations for breast cancer patients demonstrated that complying with five recommendations (being physically active as part of everyday life, limiting consumption of energy-dense food and avoiding sugary drinks, eating mostly foods of plant origin, limiting intake of red meat and avoiding processed meat, and limiting alcohol) vs complying with none or only one was associated with a 57% lower prevalence of metabolic syndrome.[59]
Conclusions
Obesity is highly prevalent in breast cancer patients and survivors, both prior to diagnosis and following completion of therapy. This excess body weight negatively impacts recurrence risk, therapy-related morbidity, and numerous quality-of-life factors. Recognition of the importance of weight management and implementation of feasible weight loss interventions are urgently needed. Furthermore, additional data are needed to define strategies that are effective, standardized, and scalable to real-world settings. As part of survivorship care, oncologists should recommend that all breast cancer survivors maintain an ideal body weight with healthy diet and regular exercise.
Financial Disclosure:Dr. Jerome, as a member of the Johns Hopkins faculty, receives a portion of the fees paid to Johns Hopkins for its faculty's monitoring of the Innergy weight-loss program. Dr. Santa-Maria receives research funding from MedImmune and Pfizer and is on the advisory board for Polyphor. The other authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Acknowledgment:We would like to thank Janelle Wilder Coughlin, PhD (Associate Professor, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine), for offering her expertise in developing this manuscript.
References:
1. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer - viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794-8.
2. American Cancer Society. Cancer facts & figures 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. Accessed July 12, 2018.
3. Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370-8.
4. Litton JK, Gonzalez-Angulo AM, Warneke CL, et al. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26:4072-7.
5. Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937-46.
6. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193-201.
7. Rosner B, Eliassen AH, Toriola AT, et al. Short-term weight gain and breast cancer risk by hormone receptor classification among pre- and postmenopausal women. Breast Cancer Res Treat. 2015;150:643-53.
8. Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann NY Acad Sci. 2011;1229:45-52.
9. Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537-56.
10. Saxena NK, Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:309-20.
11. Rock CL, Pande C, Flatt SW, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188-95.
12. Fabian CJ, Kimler BF, Donnelly JE, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with > 10% weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142:119-32.
13. Irwin ML, McTiernan A, Baumgartner RN, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774-82.
14. Demark-Wahnefried W, Hars V, Conaway MR, et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. Am J Clin Nutr. 1997;65:1495-501.
15. Sestak I, Harvie M, Howell A, et al. Weight change associated with anastrozole and tamoxifen treatment in postmenopausal women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2012;134:727-34.
16. Sedjo RL, Byers T, Ganz PA, et al. Weight gain prior to entry into a weight-loss intervention study among overweight and obese breast cancer survivors. J Cancer Surviv. 2014;8:410-8.
17. SÅowik A, Fraczek PA, Krzemieniecki K. Body mass index and aromatase inhibitors: a step forward in individualizing therapy for breast cancer patients? Expert Rev Anticancer Ther. 2016;16:759-66.
18. Hubalek M, Oberguggenberger A, Beer B, et al. Does obesity interfere with anastrozole treatment? Positive association between body mass index and anastrozole plasma levels. Clin Breast Cancer. 2014;14:291-6.
19. Folkerd EJ, Dixon JM, Renshaw L, et al. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012;30:2977-80.
20. Coscia EB, Sabha M, Gerenutti M, et al. Estrone and estradiol levels in breast cancer patients using anastrozole are not related to body mass index. Rev Bras Ginecol Obstet. 2017;39:14-20.
21. Ewertz M, Gray KP, Regan MM, et al. Obesity and risk of recurrence or death after adjuvant endocrine therapy with letrozole or tamoxifen in the Breast International Group 1-98 trial. J Clin Oncol. 2012;30:3967-75.
22. Streicher L, Simon JA. Sexual function post-breast cancer. Cancer Treat Res. 2018;173:167-89.
23. Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol. 2012;30:3712-9.
24. Rojas KE, Matthews N, Raker C, et al. Body mass index (BMI), postoperative appearance satisfaction, and sexual function in breast cancer survivorship. J Cancer Surviv. 2018;12:127-33.
25. Imayama I, Alfano CM, Neuhouser ML, et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140:159-76.
26. Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. 2017;166:519-26.
27. Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159:327-33.
28. Greenlee H, Hershman DL, Shi Z, et al. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: the Pathways Study. J Natl Cancer Inst. 2016;109:djw206.
29. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231-47.
30. Guenancia C, Lefebvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2016;34:3157-65.
31. Gunaldi M, Duman BB, Afsar CU, et al. Risk factors for developing cardiotoxicity of trastuzumab in breast cancer patients: an observational single-centre study. J Oncol Pharm Pract. 2016;22:242-7.
32. Wang HY, Yin BB, Jia DY, Hou YL. Association between obesity and trastuzumab-related cardiac toxicity in elderly patients with breast cancer. Oncotarget. 2017;8:79289-97.
33. Reinertsen KV, Cvancarova M, Loge JH, et al. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405-14.
34. Schmidt ME, Wiskemann J, Schneeweiss A, et al. Determinants of physical, affective, and cognitive fatigue during breast cancer therapy and 12 months follow-up. Int J Cancer. 2018;142:1148-57.
35. Schmidt ME, Chang-Claude J, Seibold P, et al. Determinants of long-term fatigue in breast cancer survivors: results of a prospective patient cohort study. Psychooncology. 2015;24:40-6.
36. Herath K, Peswani N, Chitambar CR. Impact of obesity and exercise on chemotherapy-related fatigue. Support Care Cancer. 2016;24:4257-62.
37. Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740-8.
38. Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors: the Iowa Women's Health Study. Breast Cancer Res Treat. 2011;130:981-91.
39. Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in black women and white women. Breast Cancer Res Treat. 2009;113:383-91.
40. Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19:853-7.
41. Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110:1868-74.
42. Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244-59.
43. Michaud TL, You W, Wilson KE, et al. Cost effectiveness and return on investment of a scalable community weight loss intervention. Prev Med. 2017;105:295-303.
44. Limon-Miro AT, Lopez-Teros V, Astiazaran-Garcia H. Dietary guidelines for breast cancer patients: a critical review. Adv Nutr. 2017;8:613-23.
45. George SM, Ballard-Barbash R, Shikany JM, et al. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the Women's Health Initiative. Cancer Epidemiol Biomarkers Prev. 2014;23:575-83.
46. Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767-76.
47. Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289-98.
48. Stearns V. A diet low in fat and high in vegetables, fruit, and fiber following breast cancer treatment did not reduce new breast cancer events. ACP J Club. 2008;148:8.
49. Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312:1779-91.
50. Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959-68.
51. Santa-Maria C, Coughlin J, Blackford A. POWER-remote: a randomized study evaluating the effect of a remote-based weight loss program in women with early stage breast cancer. Presented at the 2016 San Antonio Breast Cancer Symposium; December 6-10, 2016; San Antonio, Texas. Abstract P4-14-01.
52. Cox M, Basen-Engquist K, Carmack CL, et al. Comparison of internet and telephone interventions for weight loss among cancer survivors: randomized controlled trial and feasibility study. JMIR Cancer. 2017;3:e16.
53. Rack B, Andergassen U, Neugebauer J, et al. The German SUCCESS C Study: the first European lifestyle study on breast cancer. Breast Care (Basel). 2010;5:395-400.
54. Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3:37.
55. Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334:517.
56. Villarini A, Pasanisi P, Raimondi M, et al. Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast Cancer Res Treat. 2012;135:581-9.
57. van Waart H, van Harten WH, Buffart LM, et al. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2016;25:964-70.
58. Massetti GM, Dietz WH, Richardson LC. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA. 2017;318:1975-6.
59. Bruno E, Gargano G, Villarini A, et al. Adherence to WCRF/AICR cancer prevention recommendations and metabolic syndrome in breast cancer patients. Int J Cancer. 2016;138:237-44.
60. Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231-9.
61. Rock CL, Flatt SW, Byers TE, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33:3169-76.
62. van Gemert WA, Schuit AJ, van der Palen J, et al. Effect of weight loss, with or without exercise, on body composition and sex hormones in postmenopausal women: the SHAPE-2 trial. Breast Cancer Res. 2015;17:120.
63. Christifano DN, Fazzino TL, Sullivan DA, Befort CA. Diet quality of breast cancer survivors after a six-month weight management intervention: improvements and association with weight loss. Nutr Cancer. 2016;68:1301-8.
64. Fazzino TL, Fabian C, Befort CA. Change in physical activity during a weight management intervention for breast cancer survivors: association with weight outcomes. Obesity (Silver Spring). 2017;25(suppl 2):S109-S115.
65. Thomas GA, Cartmel B, Harrigan M, et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity (Silver Spring). 2017;25:346-51.
66. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol. 2018;36:875-83.
67. Denlinger CS, Sanft T, Baker KS, et al. Survivorship, version 2.2017. NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1140-63.
68. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611-35.
69. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. https://www.wcrf.org/dietandcancer. Accessed July 19, 2018.