Abstract
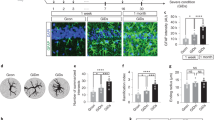
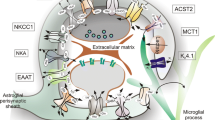
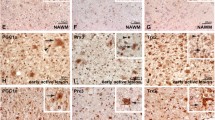
Ascorbic acid (AA), the reduced form of vitamin C, acts as a neuroprotector by eliminating free radicals in the brain. Sodium/vitamin C co-transporter isoform 2 (SVCT2) mediates uptake of AA by neurons. It has been reported that SVCT2 mRNA is induced in astrocytes under ischemic damage, suggesting that its expression is enhanced in pathological conditions. However, it remains to be established if SVCT expression is altered in the presence of reactive astrogliosis generated by different brain pathologies. In the present work, we demonstrate that SVCT2 expression is increased in astrocytes present at sites of neuroinflammation induced by intracerebroventricular injection of a GFP-adenovirus or the microbial enzyme, neuraminidase. A similar result was observed at 5 and 10 days after damage in a model of traumatic injury and in the hippocampus and cerebral cortex in the in vivo kindling model of epilepsy. Furthermore, we defined that cortical astrocytes maintained in culture for long periods acquire markers of reactive gliosis and express SVCT2, in a similar way as previously observed in situ. Finally, by means of second harmonic generation and 2-photon fluorescence imaging, we analyzed brain necropsied material from patients with Alzheimer’s disease (AD), which presented with an accumulation of amyloid plaques. Strikingly, although AD is characterized by focalized astrogliosis surrounding amyloid plaques, SVCT2 expression at the astroglial level was not detected. We conclude that SVCT2 is heterogeneously induced in reactive astrogliosis generated in different pathologies affecting the central nervous system (CNS).







Similar content being viewed by others
References
May JM, Qu ZC, Mendiratta S (1998) Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys 349(2):281–289. https://doi.org/10.1006/abbi.1997.0473
Nualart F, Mack L, Garcia A, Cisternas P, Bongarzone ER, Heitzer M, Jara N, Martinez F et al (2014) Vitamin C transporters, recycling and the bystander effect in the nervous system: SVCT2 versus gluts. J Stem Cell Res Ther 4(5):209. https://doi.org/10.4172/2157-7633.1000209
Rice ME, Lee EJ, Choy Y (1995) High levels of ascorbic acid, not glutathione, in the CNS of anoxia-tolerant reptiles contrasted with levels in anoxia-intolerant species. J Neurochem 64(4):1790–1799
Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, Vera JC (2003) Recycling of vitamin C by a bystander effect. J Biol Chem 278(12):10128–10133. https://doi.org/10.1074/jbc.M210686200
Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA (1999) A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 399(6731):70–75. https://doi.org/10.1038/19986
García L, Salazar K, Millan C, Rodriguez F, Montecinos H, Caprile T, Silva C, Cortes C et al (2005) Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50(1):32–47. https://doi.org/10.1002/glia.20133
Mun GH, Kim MJ, Lee JH, Kim HJ, Chung YH, Chung YB, Kang JS, Hwang YI et al (2006) Immunohistochemical study of the distribution of sodium-dependent vitamin C transporters in adult rat brain. J Neurosci Res 83(5):919–928. https://doi.org/10.1002/jnr.20751
Qiu S, Li L, Weeber EJ, May JM (2007) Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J Neurosci Res 85(5):1046–1056. https://doi.org/10.1002/jnr.21204
Salazar K, Cerda G, Martinez F, Sarmiento JM, Gonzalez C, Rodriguez F, Garcia-Robles M, Tapia JC et al (2014) SVCT2 transporter expression is post-natally induced in cortical neurons and its function is regulated by its short isoform. J Neurochem 130(5):693–706. https://doi.org/10.1111/jnc.12793
Garcia-Krauss A, Ferrada L, Astuya A, Salazar K, Cisternas P, Martinez F, Ramirez E, Nualart F (2016) Dehydroascorbic acid promotes cell death in neurons under oxidative stress: a protective role for astrocytes. Mol Neurobiol 53(9):5847–5863. https://doi.org/10.1007/s12035-015-9497-3
Caprile T, Salazar K, Astuya A, Cisternas P, Silva-Alvarez C, Montecinos H, Millan C, de Los Angeles Garcia M et al (2009) The Na+-dependent L-ascorbic acid transporter SVCT2 expressed in brainstem cells, neurons, and neuroblastoma cells is inhibited by flavonoids. J Neurochem 108(3):563–577. https://doi.org/10.1111/j.1471-4159.2008.05788.x
Silva-Alvarez C, Salazar K, Cisternas P, Martinez F, Liour S, Jara N, Bertinat R, Nualart F (2016) Apical polarization of SVCT2 in apical radial glial cells and progenitors during brain development. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0081-2
Oyarce K, Silva-Alvarez C, Ferrada L, Martinez F, Salazar K, Nualart F (2017) SVCT2 is expressed by cerebellar precursor cells, which differentiate into neurons in response to ascorbic acid. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0366-5
Meredith ME, Harrison FE, May JM (2011) Differential regulation of the ascorbic acid transporter SVCT2 during development and in response to ascorbic acid depletion. Biochem Biophys Res Commun 414(4):737–742. https://doi.org/10.1016/j.bbrc.2011.09.146
Portugal CC, Socodato R, Canedo T, Silva CM, Martins T, Coreixas VS, Loiola EC, Gess B et al (2017) Caveolin-1-mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci Signal 10(472). https://doi.org/10.1126/scisignal.aal2005
Angelow S, Haselbach M, Galla HJ (2003) Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res 988(1–2):105–113
Ulloa V, Garcia-Robles M, Martinez F, Salazar K, Reinicke K, Perez F, Godoy DF, Godoy AS et al (2013) Human choroid plexus papilloma cells efficiently transport glucose and vitamin C. J Neurochem. https://doi.org/10.1111/jnc.12295
Gess B, Lohmann C, Halfter H, Young P (2010) Sodium-dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L-ascorbic acid into Schwann cells. Glia 58(3):287–299. https://doi.org/10.1002/glia.20923
Nualart F, Salazar K, Oyarce K, Cisternas P, Jara N, Silva-Alvarez C, Pastor P, Martinez F et al (2012) Typical and atypical stem cells in the brain, vitamin C effect and neuropathology. Biol Res 45(3):243–256. https://doi.org/10.4067/S0716-97602012000300006
Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, Hediger MA (2003) Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem 86(4):896–906
Cisternas P, Silva-Alvarez C, Martinez F, Fernandez E, Ferrada L, Oyarce K, Salazar K, Bolanos JP et al (2014) The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J Neurochem 129(4):663–671. https://doi.org/10.1111/jnc.12663
Alvarez-Ferradas C, Morales JC, Wellmann M, Nualart F, Roncagliolo M, Fuenzalida M, Bonansco C (2015) Enhanced astroglial Ca2+ signaling increases excitatory synaptic strength in the epileptic brain. Glia 63(9):1507–1521. https://doi.org/10.1002/glia.22817
Steward O, Torre ER, Tomasulo R, Lothman E (1991) Neuronal activity up-regulates astroglial gene expression. Proc Natl Acad Sci U S A 88(15):6819–6823
Shi LH, Luo F, Woodward DJ, McIntyre DC, Chang JY (2007) Temporal sequence of ictal discharges propagation in the corticolimbic basal ganglia system during amygdala kindled seizures in freely moving rats. Epilepsy Res 73(1):85–97. https://doi.org/10.1016/j.eplepsyres.2006.08.008
Morales JC, Alvarez-Ferradas C, Roncagliolo M, Fuenzalida M, Wellmann M, Nualart FJ, Bonansco C (2014) A new rapid kindling variant for induction of cortical epileptogenesis in freely moving rats. Front Cell Neurosci 8:200. https://doi.org/10.3389/fncel.2014.00200
Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60(3):430–440. https://doi.org/10.1016/j.neuron.2008.10.013
Belanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11(3):281–295
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. https://doi.org/10.1007/s00401-009-0619-8
De Keyser J, Mostert JP, Koch MW (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 267(1–2):3–16. https://doi.org/10.1016/j.jns.2007.08.044
Savini I, Rossi A, Pierro C, Avigliano L, Catani MV (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34(3):347–355. https://doi.org/10.1007/s00726-007-0555-7
Burzle M, Hediger MA (2012) Functional and physiological role of vitamin C transporters. Curr Top Membr 70:357–375. https://doi.org/10.1016/B978-0-12-394316-3.00011-9
Rice ME (2000) Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23(5):209–216
Astuya A, Caprile T, Castro M, Salazar K, Garcia Mde L, Reinicke K, Rodriguez F, Vera JC et al (2005) Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J Neurosci Res 79(1–2):146–156. https://doi.org/10.1002/jnr.20326
Pastor P, Cisternas P, Salazar K, Silva-Alvarez C, Oyarce K, Jara N, Espinoza F, Martinez AD et al (2013) SVCT2 vitamin C transporter expression in progenitor cells of the postnatal neurogenic niche. Front Cell Neurosci 7:119. https://doi.org/10.3389/fncel.2013.00119
Allaman I, Belanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 34(2):76–87. https://doi.org/10.1016/j.tins.2010.12.001
Granados-Duran P, Lopez-Avalos MD, Grondona JM, Gomez-Roldan Mdel C, Cifuentes M, Perez-Martin M, Alvarez M, Rodriguez de Fonseca F et al (2015) Neuroinflammation induced by intracerebroventricular injection of microbial neuraminidase. Front Med 2:14. https://doi.org/10.3389/fmed.2015.00014
Cole-Edwards KK, Musto AE, Bazan NG (2006) c-Jun N-terminal kinase activation responses induced by hippocampal kindling are mediated by reactive astrocytes. J Neurosci : Off J Soc Neurosci 26(32):8295–8304. https://doi.org/10.1523/JNEUROSCI.1986-05.2006
Kandratavicius L, Balista PA, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Bueno-Junior LS, Leite JP (2014) Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat 10:1693–1705. https://doi.org/10.2147/NDT.S50371
Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9(4):453–457. https://doi.org/10.1038/nm838
Nagele RG, D'Andrea MR, Lee H, Venkataraman V, Wang HY (2003) Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res 971(2):197–209
Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M (2008) Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia 56(2):154–163. https://doi.org/10.1002/glia.20599
Abramov AY, Canevari L, Duchen MR (2004) Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 1742(1–3):81–87. https://doi.org/10.1016/j.bbamcr.2004.09.006
Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci : Off J Soc Neurosci 28(50):13574–13581. https://doi.org/10.1523/JNEUROSCI.4099-08.2008
de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J (2008) Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med 45(10):1375–1383. https://doi.org/10.1016/j.freeradbiomed.2008.09.001
Joshi G, Johnson JA (2012) The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov 7(3):218–229
Iwata N, Okazaki M, Xuan M, Kamiuchi S, Matsuzaki H, Hibino Y (2014) Orally administrated ascorbic acid suppresses neuronal damage and modifies expression of SVCT2 and GLUT1 in the brain of diabetic rats with cerebral ischemia-reperfusion. Nutrients 6(4):1554–1577. https://doi.org/10.3390/nu6041554
Portugal CC, da Encarnacao TG, Socodato R, Moreira SR, Brudzewsky D, Ambrosio AF, Paes-de-Carvalho R (2012) Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-kappaB (NF-kappaB). J Biol Chem 287(6):3860–3872. https://doi.org/10.1074/jbc.M111.260166
Gess B, Sevimli S, Strecker JK, Young P, Schabitz WR (2011) Sodium-dependent vitamin C transporter 2 (SVCT2) expression and activity in brain capillary endothelial cells after transient ischemia in mice. PLoS One 6(2):e17139. https://doi.org/10.1371/journal.pone.0017139
Savini I, Rossi A, Catani MV, Ceci R, Avigliano L (2007) Redox regulation of vitamin C transporter SVCT2 in C2C12 myotubes. Biochem Biophys Res Commun 361(2):385–390. https://doi.org/10.1016/j.bbrc.2007.07.007
Tian H, Ye X, Hou X, Yang X, Yang J, Wu C (2016) SVCT2, a potential therapeutic target, protects against oxidative stress during ethanol-induced neurotoxicity via JNK/p38 MAPKs, NF-kappaB and miRNA125a-5p. Free Radic Biol Med 96:362–373. https://doi.org/10.1016/j.freeradbiomed.2016.03.039
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Acknowledgements
This work was partially supported by a FONDECYT grant 1140477 and CONICYT PIA ECM-12 grant (both to Francisco Nualart), the National Institutes of Health grant (R01 NS065808) to Ernesto R. Bongarzone, grants 1130491 from FONDECYT, CONICYT-Chile, and CID 1/2006 from DIPUV to Christian Bonansco, Fondecyt grant 11140405 to Katterine Salazar and Fondecyt grant 11150678 to Fernando Martínez. The funders had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript. We thank Ms. Ximena Koch for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar, K., Martínez, F., Pérez-Martín, M. et al. SVCT2 Expression and Function in Reactive Astrocytes Is a Common Event in Different Brain Pathologies. Mol Neurobiol 55, 5439–5452 (2018). https://doi.org/10.1007/s12035-017-0762-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0762-5