Abstract
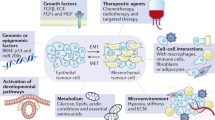
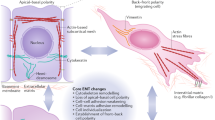
The conversion of cells with an epithelial phenotype into cells with a mesenchymal phenotype, referred to as epithelial–mesenchymal transition, is a critical process for embryonic development that also occurs in adult life, particularly during tumour progression. Tumour cells undergoing epithelial–mesenchymal transition acquire the capacity to disarm the body's antitumour defences, resist apoptosis and anticancer drugs, disseminate throughout the organism, and act as a reservoir that replenishes and expands the tumour cell population. Epithelial–mesenchymal transition is therefore becoming a target of prime interest for anticancer therapy. Here, we discuss the screening and classification of compounds that affect epithelial–mesenchymal transition, highlight some compounds of particular interest, and address issues related to their clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial−mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Kalluri, R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419 (2009).
Polyak, K. & Weinberg, R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009).
Vega, S. et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143 (2004).
Mejlvang, J. et al. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol. Biol. Cell 18, 4615–4624 (2007).
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S. & Yang, J. Spatiotemporal regulation of epithelial−mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 (2012).
Marcucci, F., Bellone, M., Caserta, C. A. & Corti, A. Pushing tumor cells towards a malignant phenotype: stimuli from the microenvironment, intercellular communications and alternative roads. Int. J. Cancer 135, 1265–1276 (2014).
Sahlgren, C., Gustafsson, M. V., Jin, S., Poellinger, L. & Lendahl, U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl Acad. Sci. USA 105, 6392–6397 (2008).
Martínez-Zaguilán, R. et al. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 14, 176–186 (1996).
Santisteban, M. et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 69, 2887–2895 (2009).
Gjorevski, N., Boghaert, E. & Nelson, C. M. Regulation of epithelial−mesenchymal transition by transmission of mechanical stress through epithelial tissues. Cancer Microenviron. 5, 29–38 (2012).
Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Sun, L. et al. miR-200b and miR-15b regulate chemotherapy-induced epithelial−mesenchymal transition in human tongue cancer cells by targeting BMI1. Oncogene 31, 432–445 (2012).
Piao, Y. et al. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin. Cancer Res. 19, 4392–4403 (2013).
Dumont, N. et al. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc. Natl Acad. Sci. USA 105, 14867–14872 (2008).
Wallin, J. J. et al. Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes. PLoS ONE 7, e36402 (2012).
Sunaga, N. et al. Oncogenic KRAS-induced interleukin-8 overexpression promotes cell growth and migration and contributes to aggressive phenotypes of non-small cell lung cancer. Int. J. Cancer 130, 1733–1744 (2012).
Chung, S. S., Giehl, N., Wu, Y. Y. & Vadgama, J. V. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial−mesenchymal transition and cancer stem cell traits. Int. J. Oncol. 44, 403–411 (2014).
Huang, L. E., Bindra, R. S., Glazer, P. M. & Harris, A. L. Hypoxia-induced genetic instability — a calculated mechanism underlying tumor progression. J. Mol. Med. (Berl.) 85, 139–148 (2007).
Creighton, C. J. et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl Acad. Sci. USA 106, 13820–13825 (2009).
Zhang, M., Atkinson, R. L. & Rosen, J. M. Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl Acad. Sci. USA 107, 3522–3527 (2010).
Tam, W. L. & Weinberg, R. A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 19, 1438–1449 (2013).
Scheel, C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 (2011).
Cruciat, C. M. & Niehrs, C. Secreted and transmembrane Wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 5, a015081 (2013).
Tate, C. M. et al. A BMP7 variant inhibits the tumorigenic potential of glioblastoma stem-like cells. Cell Death Differ. 19, 1644–1654 (2012).
Lombardo, Y. et al. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology 140, 297–309 (2011).
Whissell, G. et al. The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat. Cell Biol. 16, 695–707 (2014).
Todaro, M. et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 14, 342–356 (2014).
Chua, H. L. et al. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 26, 711–724 (2007).
Kumar, M. et al. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS ONE 8, e68597 (2013).
Xiong, H. et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial−mesenchymal transition. J. Biol. Chem. 287, 5819–5832 (2012).
Wong, C. C. L. et al. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J. Mol. Med. (Berl.) 90, 803–815 (2012).
Onder, T. T. et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68, 3645–3654 (2008).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Bartucci, M. et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 34, 681–690 (2015).
Ansieau, S. et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14, 79–89 (2008).
Zhang, Q. et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial−mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis 34, 962–973 (2013).
Byers, L. A. et al. An epithelial−mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290 (2013).
De Craene, B. & Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 13, 97–110 (2013).
Mani, S. A. et al. The epithelial−mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Cieply, B., Farris, J., Denvir, J., Ford, H. L. & Frisch, S. M. Epithelial−mesenchymal transition and tumor suppression are controlled by a reciprocal feedback loop between ZEB1 and Grainyhead-like-2. Cancer Res. 73, 6299–6309 (2013).
Ma, L. et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 12, 247–256 (2010).
Williams, L. V., Veliceasa, D., Vinokour, E. & Volpert, O. V. miR-200b inhibits prostate cancer EMT, growth and metastasis. PLoS ONE 8, e83991 (2013).
Bracken, C. P. et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial−mesenchymal transition. Cancer Res. 68, 7846–7854 (2008).
Pan, X. A., Wang, Z. X. & Wang, R. MicroRNA-21: a novel therapeutic target in human cancer. Cancer Biol. Ther. 10, 1224–1232 (2010).
Coppola, V. et al. BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial−mesenchymal transition. Oncogene 32, 1843–1853 (2013).
Gallardo-Pérez, J. C., Rivero-Segura, N. A., Marín-Hernández, A., Moreno-Sánchez, R. & Rodríguez-Enríquez, S. GPI/AMF inhibition blocks the development of the metastatic phenotype of mature multi-cellular tumor spheroids. Biochim. Biophys. Acta 1843, 1043–1053 (2014).
Yauch, R. L. et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin. Cancer Res. 11, 8686–8698 (2005).
McConkey, D. J. et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 28, 335–344 (2009).
Tam, W. L. et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell 24, 347–364 (2013).
Ashizawa, T. et al. Effect of the STAT3 inhibitor STX-0119 on the proliferation of a temozolomide-resistant glioblastoma cell line. Int. J. Oncol. 45, 411–418 (2014).
Wilson, C. et al. Overcoming EMT-associated resistance to anti-cancer drugs via Src/FAK pathway inhibition. Oncotarget 5, 7328–7341 (2014).
Huang, R. Y. J. et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis. 4, e915 (2013).
Aref, A. R. et al. Screening therapeutic EMT blocking agents in a three-dimensional microenvironment. Integr. Biol. (Camb.) 5, 381–389 (2013).
Vinci, M. et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 10, 29 (2012).
Zhang, Y. Q., Zhang, W. J. & Qin, L. D. Mesenchymal-mode migration assay and antimetastatic drug screening with high-throughput microfluidic channel networks. Angew. Chem. Int. Ed.Engl. 53, 2344–2348 (2014).
Buonato, J. M. & Lazzara, M. J. ERK1/2 blockade prevents epithelial−mesenchymal transition in lung cancer cells and promotes their sensitivity to EGFR inhibition. Cancer Res. 74, 309–319 (2014).
Chua, K. N. et al. A cell-based small molecule screening method for identifying inhibitors of epithelial−mesenchymal transition in carcinoma. PLoS ONE 7, e33183 (2012).
Gill, B. J. et al. A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model. Cancer Res. 72, 6013–6023 (2012).
Thomson, S., Petti, F., Sujka-Kwok, I., Epstein, D. & Haley, J. D. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin. Exp. Metastasis 25, 843–854 (2008).
Thomson, S. et al. A systems view of epithelial−mesenchymal transition signaling states. Clin. Exp. Metastasis 28, 137–155 (2011).
Shah, P., Gau, Y. & Sabnis, G. Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res. Treat. 143, 99–111 (2014).
Gurney, A. et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl Acad. Sci. USA 109, 11717–11722 (2012).
Kang, Y. et al. Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J. Natl Cancer Inst. 105, 1485–1495 (2013).
Wicki, A. et al. Tumor invasion in the absence of epithelial−mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9, 261–272 (2006).
Lu, X. & Kang, Y. B. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin. Cancer Res. 16, 5928–5935 (2010).
Rohwer, N. et al. Hypoxia-inducible factor 1α mediates anoikis resistance via suppression of α5 integrin. Cancer Res. 68, 10113–10120 (2008).
Lou, Y. et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 (2011).
Fiaschi, T. et al. Carbonic anhydrase IX from cancer-associated fibroblasts drives epithelial−mesenchymal transition in prostate carcinoma cells. Cell Cycle 12, 1791–1801 (2013).
Lock, F. E. et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32, 5210–5219 (2013).
De Milito, A. et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 127, 207–219 (2010).
Ferrari, S. et al. Proton pump inhibitor chemosensitization in human osteosarcoma: from the bench to the patients' bed. J. Transl. Med. 11, 268 (2013).
Erler, J. T. et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006).
El-Haibi, C. P. et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA 109, 17460–17465 (2012).
Taylor, M. A., Amin, J. D., Kirschmann, D. A. & Schiemann, W. P. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia 13, 406–418 (2011).
Yang, X. X. et al. Inactivation of lysyl oxidase by β-aminopropionitrile inhibits hypoxia-induced invasion and migration of cervical cancer cells. Oncol. Rep. 29, 541–548 (2013).
Pickup, M. W. et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res. 73, 5336–5346 (2013).
Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 12, 540–552 (2012).
Gilkes, D. M., Semenza, G. L. & Wirtz, D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 (2014).
Canadas, I. et al. Targeting epithelial-to-mesenchymal transition with Met inhibitors reverts chemoresistance in small cell lung cancer. Clin. Cancer Res. 20, 938–950 (2014).
Sun, S. Y. et al. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 74, 7546–7559 (2014).
Wu, Y. Y. et al. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 10, 1597–1606 (2012).
Guo, J. B. et al. PRRX1 promotes epithelial−mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer. Med. Oncol. 32, 393 (2015).
Bhola, N. E. et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Invest. 123, 1348–1358 (2013).
Philips, G. M. et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS ONE 6, e23943 (2011).
Wang, Z. W. et al. Acquisition of epithelial−mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the Notch signaling pathway. Cancer Res. 69, 2400–2407 (2009).
Li, Y. M. et al. Regulation of EMT by Notch signaling pathway in tumor progression. Curr. Cancer Drug Targets 13, 957–962 (2013).
Palagani, V. et al. Epithelial mesenchymal transition and pancreatic tumor initiating CD44+/EpCAM+ cells are inhibited by γ-secretase inhibitor IX. PLoS ONE 7, e46514 (2012).
Xie, G. Z. et al. IL-6-induced epithelial−mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int. J. Oncol. 40, 1171–1179 (2012).
Devarajan, E., Song, Y. H., Krishnappa, S. & Alt, E. Epithelial−mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells. Int. J. Cancer 131, 1023–1031 (2012).
Jechlinger, M. et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Invest. 116, 1561–1570 (2006).
Zhang, Z. C., Dong, Z. H., Lauxen, I. S., St'Ana, M. & Nor, J. E. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 74, 2869–2881 (2014).
Chen, J. et al. TGF-β1 and FGF2 stimulate the epithelial−mesenchymal transition of HERS cells through a MEK-dependent mechanism. J. Cell. Physiol. 229, 1647–1659 (2014).
Bhowmick, N. A., Zent, R., Ghiassi, M., McDonnell, M. & Moses, H. L. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 276, 46707–46713 (2001).
Bianchi, A., Gervasi, M. E. & Bakin, A. Role of β5-integrin in epithelial−mesenchymal transition in response to TGF-β. Cell Cycle 9, 1647–1659 (2010).
Parvani, J. G., Galliher-Beckley, A. J., Schiemann, B. J. & Schiemann, W. P. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Mol. Biol. Cell 24, 3449–3459 (2013).
Goyal, L., Muzumdar, M. D. & Zhu, A. X. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin. Cancer Res. 19, 2310–2318 (2013).
Lobry, C., Oh, P., Mansour, M. R., Look, A. T. & Aifantis, I. Notch signaling: switching an oncogene to a tumor suppressor. Blood 123, 2451–2459 (2014).
Rampias, T. et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 20, 1199–1205 (2014).
Anastas, J. N. & Moon, R. T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 (2013).
Chen, E. Y. et al. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/β-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc. Natl Acad. Sci. USA 111, 5349–5354 (2014).
Rudin, C. M. Vismodegib. Clin. Cancer Res. 18, 3218–3222 (2012).
Grygielewicz, P. et al. Epithelial−mesenchymal transition confers resistance to selective FGFR inhibitors in SNU-16 gastric cancer cells. Gastr. Cancer 19, 53–62 (2016).
Leight, J. L., Wozniak, M. A., Chen, S., Lynch, M. L. & Chen, C. S. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial−mesenchymal transition. Mol. Biol. Cell 23, 781–791 (2012).
Zhong, Z. J. et al. Anti-transforming growth factor β receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin. Cancer Res. 16, 1191–1205 (2010).
Liu, J. Q. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012).
Anderton, M. J. et al. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol. Pathol. 39, 916–924 (2011).
Fransvea, E., Angelotti, U., Antonaci, S. & Giannelli, G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology 47, 1557–1566 (2008).
Rodon, J. et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 21, 553–560 (2015).
Giannelli, G., Villa, E. & Lahn, M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 74, 1890–1894 (2014).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Sulzmaier, F. J., Jean, C. & Schlaepfer, D. D. FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598–610 (2014).
Fan, H., Zhao, X., Sun, S., Luo, M. & Guan, J. L. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial−mesenchymal transition and mammary cancer stem cell activities in vivo. J. Biol. Chem. 288, 3322–3333 (2013).
Bailey, K. M. & Liu, J. Caveolin-1 up-regulation during epithelial to mesenchymal transition is mediated by focal adhesion kinase. J. Biol. Chem. 283, 13714–13724 (2008).
Shapiro, I. M. et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci. Transl Med. 6, 237ra68 (2014).
Infante, J. R. et al. Safety, pharmacokinetic, and pharmacodynamic Phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J. Clin. Oncol. 30, 1527–1533 (2012).
Guarino, M. Src signaling in cancer invasion. J. Cell. Physiol. 223, 14–26 (2010).
Kim, L. C., Song, L. & Haura, E. B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 6, 587–595 (2009).
Zhong, L. et al. A preclinical evaluation of a novel multikinase inhibitor, SKLB-329, as a therapeutic agent against hepatocellular carcinoma. Int. J. Cancer 135, 2972–2983 (2014).
Vultur, A. et al. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 7, 1185–1194 (2008).
Lu, H. et al. IGFBP2/FAK pathway is causally associated with dasatinib resistance in non-small cell lung cancer cells. Mol. Cancer Ther. 12, 2864–2873 (2013).
Gucalp, A. et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin. Breast Cancer 11, 306–311 (2011).
Puls, L. N., Eadens, M. & Messersmith, W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist 16, 566–578 (2011).
Somlo, G. et al. Dasatinib plus capecitabine for advanced breast cancer: safety and efficacy in Phase I study CA180004. Clin. Cancer Res. 19, 1884–1893 (2013).
Chang, L. et al. Acquisition of epithelial−mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 4, e875 (2013).
Ning, J., Liu, W., Zhang, J., Lang, Y. & Xu, S. Ran GTPase induces EMT and enhances invasion in non-small cell lung cancer cells through activation of PI3K−AKT pathway. Oncol. Res. 21, 67–72 (2013).
Lin, G. et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 prevents epithelial−mesenchymal transition induced by hypoxia and TGF-β1. Eur. J. Pharmacol. 729, 45–53 (2014).
Chen, W., Wu, S., Zhang, G., Wang, W. & Shi, Y. Effect of AKT inhibition on epithelial−mesenchymal transition and ZEB1-potentiated radiotherapy in nasopharyngeal carcinoma. Oncol. Lett. 6, 1234–1240 (2013).
Gulhati, P. et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 71, 3246–3256 (2011).
Zeuner, A., Todaro, M., Stassi, G. & De Maria, R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell 15, 692–705 (2014).
Leconet, W. et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene 33, 5405–5414 (2014).
Wilson, C. et al. AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res. 74, 5878–5890 (2014).
Gjerdrum, C. et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl Acad. Sci. USA 107, 1124–1129 (2010).
Sheridan, C. First Axl inhibitor enters clinical trials. Nat. Biotechnol. 31, 775–776 (2013).
Bosurgi, L. et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc. Natl Acad. Sci. USA 110, 13091–13096 (2013).
Mulholland, D. J. et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 72, 1878–1889 (2012).
Arvizo, R. R. et al. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl Acad. Sci. USA 110, 6700–6705 (2013).
Holderfield, M., Deuker, M. M., McCormick, F. & McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer 14, 455–467 (2014).
Shimojo, Y. et al. Attenuation of reactive oxygen species by antioxidants suppresses hypoxia-induced epithelial−mesenchymal transition and metastasis of pancreatic cancer cells. Clin. Exp. Metastasis 30, 143–154 (2013).
Kim, Y. M. & Cho, M. Activation of NADPH oxidase subunit NCF4 induces ROS-mediated EMT signaling in HeLa cells. Cell. Signal. 26, 784–796 (2014).
Dong, C. et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 23, 316–331 (2013).
Libby, G. et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32, 1620–1625 (2009).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Li, L. et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin. Cancer Res. 20, 2714–2726 (2014).
Qu, C. et al. Metformin reverses multidrug resistance and epithelial−mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol. Cell. Biochem. 386, 63–71 (2014).
Zheng, L. et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin. Cancer Res. 19, 5372–5380 (2013).
Del Barco, S. et al. Metformin: multi-faceted protection against cancer. Oncotarget 2, 896–917 (2011).
Chou, C. C. et al. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt−MDM2−Foxo3a signaling axis. Cancer Res. 74, 4783–4795 (2014).
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Shin, S. R., Sanchez-Velar, N., Sherr, D. H. & Sonenshein, G. E. 7,12-dimethylbenz(a)anthracene treatment of a c-rel mouse mammary tumor cell line induces epithelial to mesenchymal transition via activation of nuclear factor-κB. Cancer Res. 66, 2570–2575 (2006).
Cheng, Z. X. et al. Nuclear factor-κB-dependent epithelial to mesenchymal transition induced by HIF-1α activation in pancreatic cancer cells under hypoxic conditions. PLoS ONE 6, e23752 (2011).
Fang, H. et al. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 286, 30393–30400 (2011).
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 11, 1314–1321 (2005).
Zhong, Z., Wen, Z. & Darnell, J. E. Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 (1994).
Bowman, T. et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl Acad. Sci. USA 98, 7319–7324 (2001).
Yu, C. L. et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269, 81–83 (1995).
Lee, H. et al. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 15, 283–293 (2009).
Colomiere, M. et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial−mesenchymal transition in ovarian carcinomas. Br. J. Cancer 100, 134–144 (2009).
Yue, P. et al. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene 31, 2309–2322 (2012).
Drake, J. M., Strohbehn, G., Bair, T. B., Moreland, J. G. & Henry, M. D. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol. Biol. Cell 20, 2207–2217 (2009).
Azmi, A. S. et al. Systems analysis reveals a transcriptional reversal of the mesenchymal phenotype induced by SNAIL-inhibitor GN-25. BMC Syst. Biol. 7, 85 (2013).
Shi, Z. D. et al. AC1MMYR2, an inhibitor of Dicer-mediated biogenesis of Oncomir mir-21, reverses epithelial−mesenchymal transition and suppresses tumor growth and progression. Cancer Res. 73, 5519–5531 (2013).
Song, X. et al. LBH589 inhibits proliferation and metastasis of hepatocellular carcinoma via inhibition of gankyrin/stat3/akt pathway. Mol. Cancer 12, 114 (2013).
Eades, G. et al. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J. Biol. Chem. 286, 25992–26002 (2011).
Jiang, G. M. et al. Histone deacetylase inhibitor induction of epithelial−mesenchymal transitions via up-regulation of Snail facilitates cancer progression. Biochim. Biophys. Acta 1833, 663–671 (2013).
Kong, D. J. et al. Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells. PLoS ONE 7, e45045 (2012).
Cui, B. et al. Targeting ROR1 inhibits epithelial−mesenchymal transition and metastasis. Cancer Res. 73, 3649–3660 (2013).
Zhang, S. P. et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc. Natl Acad. Sci. USA 111, 17266–17271 (2014).
Taube, J. H. et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc. Natl Acad. Sci. USA 107, 15449–15454 (2010).
Jeong, H., Ryu, Y. J., An, J., Lee, Y. & Kim, A. Epithelial−mesenchymal transition in breast cancer correlates with high histological grade and triple-negative phenotype. Histopathology 60, E87–E95 (2012).
Tan, T. Z. et al. Epithelial−mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 6, 1279–1293 (2014).
Kolev, V. N. et al. PI3K/mTOR dual inhibitor VS-5584 preferentially targets cancer stem cells. Cancer Res. 75, 446–455 (2015).
Smith, D. C. et al. A Phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clin. Cancer Res. 20, 6295–6303 (2014).
Yen, W. C. et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin. Cancer Res. 21, 2084–2095 (2015).
Li, Y. et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl Acad. Sci. USA 112, 1839–1844 (2015).
Fleuren, E. D. G. et al. The role of AXL and the in vitro activity of the receptor tyrosine kinase inhibitor BGB324 in Ewing sarcoma. Oncotarget 5, 12753–12768 (2014).
Yokobori, T. et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial−mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 73, 2059–2069 (2013).
Steinert, G. et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 74, 1694–1704 (2014).
Diaz, L. A. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Shiwarski, D. J. et al. To 'grow' or 'go': TMEM16A expression as a switch between tumor growth and metastasis in SCCHN. Clin. Cancer Res. 20, 4673–4688 (2014).
Veiseh, M. et al. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc. Natl Acad. Sci. USA 111, E1731–E1739 (2014).
Bhowmick, N. A., Neilson, E. G. & Moses, H. L. Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (2004).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Su, S. C. et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25, 605–620 (2014).
Toh, B. et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 9, e1001162 (2011).
Giannoni, E. et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial−mesenchymal transition and cancer stemness. Cancer Res. 70, 6945–6956 (2010).
Grosse-Steffen, T. et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin. Dev. Immunol. 2012, 720768 (2012).
Park, J. & Scherer, P. E. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Invest. 122, 4243–4256 (2012).
Comito, G. et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33, 2423–2431 (2014).
Yamashina, T. et al. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res. 74, 2698–2709 (2014).
Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014).
Casazza, A. et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell 24, 695–709 (2013).
Klug, F. et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 24, 589–602 (2013).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinomaassociated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Germano, G. et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23, 249–262 (2013).
Vanharanta, S. et al. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat. Med. 19, 50–56 (2013).
Pantuck, A. J., An, J. B., Liu, H. R. & Rettig, M. B. NF-κ-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel−Lindau inactivation in renal cell carcinomas. Cancer Res. 70, 752–761 (2010).
Salgia, R. et al. Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin. Cancer Res. 20, 1666–1675 (2014).
Ling, H., Fabbri, M. & Calin, G. A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847–865 (2013).
Fischer, K. R. et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 (2015).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015).
Acknowledgements
R.D.M. and G.S. are supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Marcucci, F., Stassi, G. & De Maria, R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov 15, 311–325 (2016). https://doi.org/10.1038/nrd.2015.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.13
This article is cited by
HepG2 exosomes coated luteolin nanoparticles remodeling hepatic stellate cells and combination with sorafenib for the treatment of hepatocellular carcinoma
Cancer Nanotechnology (2024)
Mesenchymal–epithelial transition and AXL inhibitor TP-0903 sensitise triple-negative breast cancer cells to the antimalarial compound, artesunate
Scientific Reports (2024)
Targeting the key players of phenotypic plasticity in cancer cells by phytochemicals
Cancer and Metastasis Reviews (2024)
Correlation between preoperative peripheral blood NLR, PLR, LMR and prognosis of patients with head and neck squamous cell carcinoma
BMC Cancer (2023)
EMT/MET plasticity in cancer and Go-or-Grow decisions in quiescence: the two sides of the same coin?
Molecular Cancer (2023)