-
PDF
- Split View
-
Views
-
Cite
Cite
Qing Lv, Qinghua Xia, Jiang Li, Zhiyong Wang, Allicin suppresses growth and metastasis of gastric carcinoma: the key role of microRNA-383-5p-mediated inhibition of ERBB4 signaling, Bioscience, Biotechnology, and Biochemistry, Volume 84, Issue 10, 2 October 2020, Pages 1997–2004, https://doi.org/10.1080/09168451.2020.1780903
Close - Share Icon Share
Abstract
Allicin is a natural product suppressing the progression of gastric carcinoma (GC). In the current study, the mechanism underlying the anti-GC effect of allicin was explored by focusing on the role of miR-383-5p/ERBB4 signaling. Two GC cell lines were treated with allicin and the effects on viability, apoptosis, migration, invasion, and miR-383-5p/ERBB4 activity in the cells were assessed. The interaction between allicin and miR-383-5p was further explored by inhibiting the miR-383-5p level. Allicin suppressed cell viability and induced apoptosis in both GC cell lines. The compound also inhibited migration and invasion of GC cells, which was associated with the up-regulation miR-383-5p and down-regulation of ERBB4. The inhibition of miR-383-5p by specific inhibitor blocked the anti-GC effect of allicin. Our results demonstrated that allicin contributed to the suppressed growth and metastasis potentials in GC cell lines. The effect was accompanied by an increased level of miR-383-5p and subsequent inhibition of ERBB4.
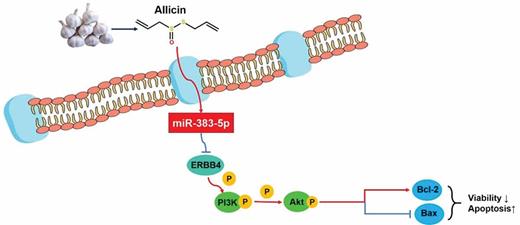
Graphical abstract
Allicin antagonizes the oncogenesis of stomach by inducing the level of miR-383-5p, which then inhibits ERBB-mediated pro-tumor signaling.
Gastric carcinoma (GC) ranks fourth in the most common malignancies worldwide and is the second leading causable factor of cancer-related death [1]. Although the developments in early detection and extensive surgery of GC have lowered the overall incidence of GC in recent years, more than 50% of all the GC patients still die from recurrence and metastasis several years after the treatments [2]. Generally, the lymph node metastasis has been recognized as the most important prognostic feature for GC: most clinicians even believe that D2 lymphadenectomy is the only standard and optimal surgical procedure for GC patients [3]. However, even numerous studies have attempted to reveal the underlying mechanisms driving the lymph node metastasis associated with GC, few achievements have been made and the key factors involved in the metastasis have not been identified. Given the fact that lymph node metastasis determines the prognosis and survival of GC patients, the further exploration of the indicators that can contribute to the early diagnosis and the effective management of GC is not only necessary but also prompting.
In the previous investigations regarding the factors differentially functioning between the health people and GC patients, the involvement of microRNAs (miRs) has attracted increasing attentions. For example, miR-212 is a tumor suppressor and can antagonize GC by targeting RBP2 [4]. In another study performed by Jiang et al., the authors showed that miR-874 can inhibit cell proliferation and metastasis of GC cells by targeting AQP3 [5]. Except for acting as tumor suppressors of GC, miRs such as miR-145, miR-22, and miR-19a also contributes to the progression of GC [6–8]. Thus, the proper modulation of miRs can serve as a promising strategy for handling GC in the clinic.
Allicin is a natural organosulfur compound abundant in garlic (Allium sativum L.) and is characterized by anti-oxidant, anti-microbial, and anticarcinogenic activities [9,10]. Regarding its effect on GC, allicin induces apoptosis in SGC-7901 cells by inducing G2/M phase cell cycle arrest [11] and suppressed the viability of MGC-803 cells by activating MAPK pathway [12]. Moreover, the function of allicin to antagonize GC is also related to its inhibition effect on Helicobacter pylori [13]. Even though the anti-GC effects of allicin have been previously proved, few studies have explored the possible role of miRs in the anti-GC function of allicin. Given the universal involvement of miRs in the development of GC, it is reasonable to explore the relation between miRs and the anti-GC function of allicin, which might expand the application of the compound in treating cancers.
Based on the previous studies, the current study was the first to explore the interaction between allicin and miRs in handling GC. The GC cells were firstly treated with allicin to detect its effects on cell proliferation and metastasis potentials in reference to cisplatin. Thereafter, the effect of allicin on miR-383-5p/ERBB4 pathway was assessed. According to the report of Wei et al., the inhibition of miR-383-5p was associated with the poor prognosis of GC patients [14]. Moreover, the downstream effector of miR-383-5p, ERBB4 is a well-characterized oncogene in the progression of GC [15]. Thereafter, the level of miR-383-5p was inhibited under the treatment of allicin to confirm its indispensable role in the anti-GC function of allicin.
Materials and methods
Cell cultures and treatment of allicin
Human GC cell lines HGC27 and AGS were provided by the genetics laboratory of Harbin medical university and maintained in RPMI1640 medium supplemented with 15% (v/v) FBS (fetal bovine serum) (Sigma-Aldrich, USA) at 37°C with 5% CO2 in a humidified chamber. Afterward, GC cells were seeded in 24-well plates (3 × 104 per well) and were incubated with allicin (A543500, the BSZH Scientific Inc., China) (10 µg/mL, dissolved in DMSO) for 48 h. Cisplatin (10 μM) was employed as a positive control. Both cell lines were authenticated using STR method and mycoplasma contamination was excluded using MycAwayTM-Color One-Step Mycoplasma Detection Kit (40611ES25, Yeasen, China)
Brdu (bromodeoxyuridine) assay
Cell viabilities after different treatments were assessed using BrdU ELISA kits (Maibio, China) according to the manufacturer’s instructions and represented by the optical density (OD) value at 450 nm measured with a Microplate Reader (ELX-800, BIOTEK, USA).
Flow cytometry
The effect of allicin on the apoptosis of GC cells was assessed using flow cytometry technique with an Apoptosis Detection Kit (JingMei Biotech, Beijing, China). The apoptotic rates were analyzed using a FACScan flow cytometer (Accuri C6, BD, USA): the total apoptotic rate was equal to the sum of the late and early apoptotic rates.
Scratch assay
The migration potential of GC cells was assessed using scratch assays. GC cells (2 × 104) were seeded in one well of a 24-well plate. Then, a reference point was marked on the medium to guarantee the same area of image acquisition and the cells were then allowed to grow for two days to form a confluent monolayer. The layer was scratched to generate a cell-free straight line and debris at the edges of the scratch was removed. Afterward, the gap width of the scratch at 24 h of the culture was recorded in reference to the reference point by the ImageJ software (US National Institutes of Health).
Transwell assay
The invasion potential of GC cells was measured using transwell assay. 2 × 104 cells were inoculated in the upper chamber of a transwell system (BSA-coated porous polycarbonate membrane with a pore size of 8 μm, Corning star, Cambridge, MA) pre-coated with 40 μL matrigel (1.5 mg ⁄mL; BD Biosciences, San Jose, CA, USA). The cells were allowed to grow for 2 h to form a reconstituted basement membrane. After another 4 h for letting the cells penetrate the porous membrane, the cells in the upper surface of the membrane were removed, and the cells on the lower surface of the membrane were stained with 1% (w/v) crystal violet for 30 s. The cell number penetrating the porous membrane was determined using the Image-Pro Plus 6.0 software (Nikon).
Reverse transcription-quantitative PCR (RT-qPCR)
The total RNA was extracted using RNA Purified Total RNA Extraction Kit (BioTeke, Beijing, China) and cDNA templates were obtained by the reverse transcription PCR of RNA using Super M-MLV reverse transcriptase (BioTeke, Beijing, China). β-actin was selected as the internal reference gene. The final qPCR reaction mix contained 10 μL SYBR GREEN Master Mix, 0.5 μL of each primer (miR-383-5p, forward: 5ʹ-GGGAGATCAGAAGGTGATTGTGGCT-3ʹ; reverse: 5ʹ-CAGTGCGTGTCGTGGAGT-3ʹ. U6, forward: 5ʹ-CTCGCTTCGGCAGCACA-3ʹ; reverse: 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ), 1 μL cDNA template, and 8 μL Rnase-free H2O. Amplification was performed routinely and the relative expression level of miR-383-5p was calculated by the Data Assist Software (Applied Biosystems/Life Technologies, version 3.0) according to the expression of 2−ΔΔCt.
Western blotting
The total protein was extracted using the Total Protein Extraction Kit (WLA019, Wanleibio, China) and β-actin was used as the internal reference protein. Forty micrograms protein was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Then, the proteins were transferred onto polyvinylidene difluoride (PVDF) membrane and blocked with skimmed milk solution for 1 h. The membranes were incubated with the primary antibody against ERBB4 (1:5000) (Cell Signaling Technology, USA), Akt (1:500) (Bioss, China), p-Akt (1:200) (Santa Cruz Biotechnology, USA), PI3 K (1:500) (Bioss, China), p-PI3 K (1:200) (Bioss, China), Bcl-2 (1:5000) (Boster, USA), Bax (1:5000) (Boster, USA), or β-actin (1:1000) (Santa Cruz, USA) at 4°C overnight. Afterward, the membranes were incubated with the secondary HRP-conjugated IgG antibody (1:5000) (Abcam, USA) for 45 min at 37°C and the protein blots were developed using the Beyo ECL Plus reagent. The results were imaged in the Gel Imaging System and the relative expression levels of proteins were calculated by the Gel-Pro-Analyzer (Media Cybernetics, USA) in the reference to the control group.
Determination of the effect of miR-383-5p inhibition on the anti-GC effects of allicin
The specific inhibitor of miR-383-5p (5ʹ-AGCCACAAUCACCUUCUGAUCU-3ʹ) [16] was synthesized by GenePharma Co., Ltd (Shanghai, China) and transfected into AGS cells using LipofectamineTM 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Cells were collected for subsequent assays 48 h post-transfection and the inhibition efficacy of miR-383-5p was detected with RT-qPCR assay. The effects on miR-383-5p inhibition on the anti-GC function of allicin were assessed by detecting cell viability, migration, and invasion potentials in AGS cells as described above.
Statistical analysis
All the data were represented as mean ± standard deviation (SD) and each assay was performed by three replicates. Differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Duncan test. All the statistical analyses and graph plotting were conducted using GraphPad Prism 6 (GraphPad Software, San Diego, CA) with a significant level of 0.05 (two-tailed P value).
Results
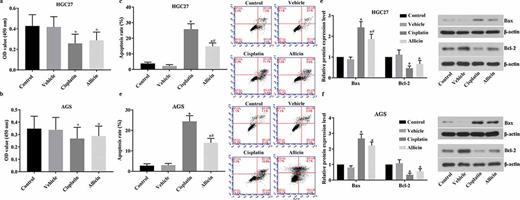
Allicin suppressed viability and induced apoptosis in GC cells
Detection results of cell viability and apoptosis in GC cells after allicin administration. 3 × 104/well GC cells were treated with allicin 10 µg/mL for 48 h or 10 µM cisplatin for 48 h. Cell viability was detected with BrdU and cell apoptosis was detected with flow cytometry. (a) Results of BrdU detection in HCG27 cell line. (b) Results of BrdU detection in AGS cell line. (c) Results of flow cytometry detection in HGC27 cell line. (d) Results of flow cytometry detection in AGS cell line. (e) Results of western blotting detection in HGC27 cell line. (f) Results of western blotting detection in AGS cell line. Control group, parental cells. Vehicle group, cells treated with DMSO. Cisplatin group, cells treated with 10 µg/mL cisplatin for 48 h. Allicin group, cells treated with 10 µg/mL allicin for 48 h. “*” represents statistically significant from Vehicle group, P < 0.05. “#” represents statistically significant from Cisplatin group, P < 0.05.
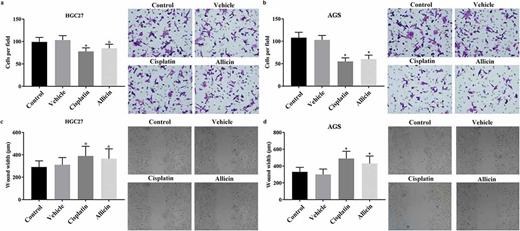
Allicin inhibited the migration and invasion abilities of GC cells
Detection results of migration and invasion in GC cells after allicin administration. 2 × 104 GC cells were pre-treated with allicin 10 µg/mL for 48 h or 10 µM cisplatin for 48 h and then subjected to transwell system for 4 h or scratch assay GC cells for two days, respectively. (a) Results of scratch detection in HGC27 cell line. (b) Results of scratch detection in AGS cell line. (c) Results of transwell detection in HGC27 cell line. (d) Results of transwell detection in AGS cell line. Control group, parental cells. Vehicle group, cells treated with DMSO. Cisplatin group, cells treated with 10 µg/mL cisplatin for 48 h. Allicin group, cells treated with 10 µg/mL allicin for 48 h. “*” represents statistically significant from Vehicle group, P < 0.05.
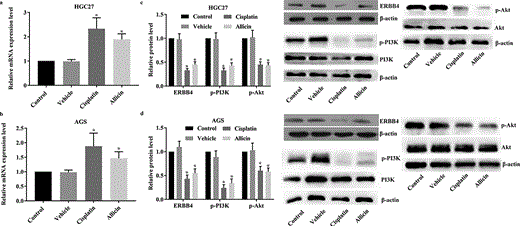
Allicin induced the level of miR-383-5p but suppressed the expressions of ERBB4, p-PI3 K, and p-Akt
Detection results of miR-383-5p and ERBB4/PI3 K/Akt pathway in GC cells after allicin administration. 3 × 104/well GC cells were treated with allicin 10 µg/mL for 48 h or 10 µM cisplatin for 48 h. The expression of miR-383-5p was detected with RT-qPCR assay and the activity of ERBB4/PI3 K/Akt pathway was detected with western blotting assay. (a) Results of RT-qPCR detection of miR-383-5p level in HGC27 cells. (b) Results of RT-qPCR detection of miR-383-5p level in AGS cells. (c) Results of western blotting detection of ERBB4/PI3 K/Akt pathway in HGC27 cells. (d) Results of western blotting detection of ERBB4/PI3 K/Akt pathway in AGS cells. Control group, parental cells. Vehicle group, cells treated with DMSO. Cisplatin group, cells treated with 10 µg/mL cisplatin for 48 h. Allicin group, cells treated with 10 µg/mL allicin for 48 h. “*” represents statistically significant from Vehicle group, P < 0.05.
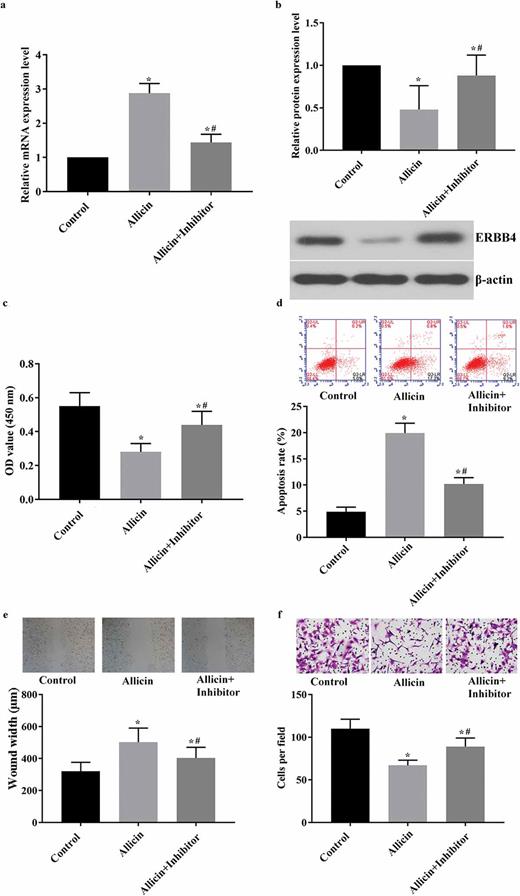
Anti-GC function of allicin was associated with the induced level of miR-383-5p
Detection results of viability, apoptosis, migration, and invasion in miR-383-5p-inhibited AGS cells after allicin administration. 3 × 104/well GC cells were transfected with miR-383-5p inhibitor and then treated with 10 µM allicin for 48 h. The cell viability, apoptosis, migration, invasion, and expressions of miR-383-5p and ERBB4 were detected routinely. (a) Results of RT-qPCR detection of miR-383-5p level in HGC27 cells. (b) Results of western blotting detection of ERBB4. (c) Results of BrdU detection in AGS cell line. (d) Results of flow cytometry detection in AGS cell line. (e) Results of scratch detection in AGS cell line. (f) Results of transwell detection in AGS cell line. Control group, parental cells. Allicin group, cells treated with 10 µg/mL allicin for 48 h. Allicin + Inhibitor group, cells were transfected with miR-383-5p inhibitor and then treated with 10 µg/mL allicin for 48 h. “*” represents statistically significant from Control group, P < 0.05. “#” represents statistically significant from Allicin group, P < 0.05.
Discussion
Allicin, also known as diallyl thiosulfinate, is the major bioactive component of garlic [17]. The compound has been long characterized by its strong anti-bacterial and anti-inflammatory activities [18]. With emerging attentions paid to the anti-cancer function of natural products, the potential of allicin in handling cancers has also been revealed: different studies demonstrated that allicin is capable of inhibiting the survival of numerous cancers, including those occurred in colon, lung, cervix, and breast [19–22]. Regarding its effects on GC, previous studies also reported that allicin induces apoptosis in different GC cell lines [12,18]. However, the mechanism underlying the anti-GC function of allicin stays partially revealed even with its evident effects to antagonize GC cells. Thus, in the current study, we attempted to provide additional information on the pathways mediating its anti-GC effects by exploring the interaction between allicin and miRs for the first time.
The suppressive effect of allicin on GC was firstly affirmed by subjecting two GC cell lines to the treatment of allicin. Then, the changes in cell viability, apoptosis, migration, and invasion were assessed. Allicin suppressed the proliferation, while induced apoptosis in GC cell lines. For migration and invasion abilities, both cell lines responded to allicin treatment and showed lower metastasis potential. Moreover, the effects of allicin were compared with cisplatin: the results showed that the suppressive effects of allicin were weaker than that of cisplatin, but the difference was statistically insignificant for some parameters. Given the fact that allicin is a natural product easily isolated from garlic and its mildness to human bodies [23], the compound can serve as a promising adjuvant strategy along with chemotherapies in the future clinic handling of GC.
In recent years, the regulation of GC progression by miRs has become a hot subject. Thus, the current study focused on the influence of allicin on the expression of miR-383-5p, which is a typical tumor suppressor identified in GC [7,24]. It was shown by our data that allicin contributed to the increased expression of miR-383-5p in GC cells, which was associated with the suppressed proliferation and metastasis potentials. The anti-cancer effects of miR-383-5p were also previously verified in ovarian, lung, and hepatic cancers [25–27]. Thus, the inducing effect of allicin on miR-383-5p level might represent the possible involvement of the miR in the anti-GC function of allicin. To further confirm the possibility, the level of miR-383-5p was inhibited in AGS cells before incubated with allicin. After the inhibition of miR-383-5p, the effect of allicin on GC cells was dramatically impaired: the weaker suppressive effects on the proliferation and metastasis potentials of GC cells were observed. The results inferred that the induced level of miR-383-5p was a pre-requisite for the effects of allicin on GC cells. Moreover, we also detected the changes in the downstream effectors of miR-383-5p: allicin suppressed the expression of ERBB4 and activity of PI3 K/Akt pathway, and the changes in the ERBB4/PI3 K/Akt signaling were restored in miR-383-5p-inhibited AGS cells even under the treatment of allicin. ERBB4 is a well-characterized oncogene in different cancer types [28] and its promotive function in GC development was also verified by previous studies [15,29]. Thus, the current results depicted a regulation sequence that allicin induced the expression of miR-383-5p, which then led to the inhibition of ERBB4/PI3 K/Akt signaling, indicating that the anti-GC function of allicin is in close relation to the expression status of miRs and their downstream pathways. However, even with the inhibition of miR-383-5p, it seemed that the anti-GC function of allicin was not completely impaired: the proliferation and metastasis potentials of GC cells in Allicin + Inhibitor group were still lower than those in Control group. The results reminded that the anti-GC function of allicin may be exerted in multi-pronged mechanisms. As discussed in the introduction part, the compound can inhibit GC by inducing G2/M phase cell cycle arrest and MAPK pathway [11,12]. Moreover, allicin can interfere with the H. pylori-induced oxidative and inflammatory responses in gastric epithelial tissues, which is critical to the initiation of oncogenesis of stomach [13]. Even though the anti-GC signaling transduction mediated by miR-383-5p was inhibited by a specific inhibitor, allicin might also exert its effects via other mechanisms beyond miR-383-5p. This hypothesis is important to the application of allicin in that the interactions between different pathways will finally influence the anti-GC function of allicin, and thus, more comprehensive work is needed to elaborate the co-modulation effects of multiple pathways on the function of allicin.
Conclusions
Collectively, the findings outlined in the current study demonstrated that the negative impact of allicin on the proliferation and metastasis of GC was exerted by inducing the expression of miR-383-5p, which then suppressed the activity of ERBB4/PI3 K/Akt signaling. However, the current study only provided a preliminary explanation of the interaction between allicin and miRs, the comprehensive investigation of the miR members influenced by allicin needs further exploration. We believed that with more comprehensive work in the future, allicin can be developed as a promising adjuvant for the treatment of GC.
Authors’ contributions
QL designed the study, performed lab experiments, and wrote the draft. QX and JL collected data and analyzed the data. ZW designed the experiments, wrote the draft, and approved the submission.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors disclose no conflict of interest.
References