-
PDF
- Split View
-
Views
-
Cite
Cite
Kimie Nakagawa, Yuko Sasaki, Shigeaki Kato, Noboru Kubodera, Toshio Okano, 22-Oxa-1α,25-dihydroxyvitamin D 3 inhibits metastasis and angiogenesis in lung cancer , Carcinogenesis, Volume 26, Issue 6, June 2005, Pages 1044–1054, https://doi.org/10.1093/carcin/bgi049
Close - Share Icon Share
Abstract
1α,25-Dihydroxyvitamin D 3 (1α,25-D 3 ) has potent antiproliferative and anti-invasive properties in vitro in cancer cells. However, its calcemic effect in vivo limits its therapeutic applications. Here, we report the efficacy of 22-oxa-1α,25-dihydroxyvitamin D 3 (22-oxa-1α,25-D 3 ), a low calcemic analog of vitamin D, against the development of metastatic lung carcinoma after an intravenous injection of green fluorescent protein-transfected Lewis lung carcinoma (LLC-GFP) cells in C57BL/6 mice. The mice injected with tumor cells were implanted simultaneously with osmotic minipumps containing either 1α,25-D 3 , 22-oxa-1α,25-D 3 or vehicle. The 1α,25-D 3 treatment group had been hypercalcemic, but the 22-oxa-1α,25-D 3 and vehicle treatment groups remained normocalcemic for the duration of the experiment. The total number of lung metastases, lung weight and the expression of GFP mRNA in the lung were markedly decreased in 1α,25-D 3 and 22-oxa-1α,25-D 3 -treated mice. In the in vitro experiment, 1α,25-D 3 and 22-oxa-1α,25-D 3 reduced the expression of matrix metalloproteinase (MMP)-2, MMP-9, vascular endothelial growth factor and parathyroid hormone-related protein in LLC-GFP cells. Furthermore, in the angiogenesis assay, the number of tumor cells or basic fibroblast growth factor-induced angiogenesis was reduced in 1α,25-D 3 and 22-oxa-1α,25-D 3 -treated mice. Moreover, using a new experimental model of vitamin D receptor (VDR) null mutant ( VDR−/− ) mice with corrected hypocalcemia and hypervitaminosis D, we examine the anti-cancer effect of 22-oxa-1α,25-D 3 without other functions induced by 22-oxa-1α,25-D 3 in the host. In the VDR−/− mice, 22-oxa-1α,25-D 3 directly inhibited the metastatic activity of LLC-GFP cells in a dose-dependent manner without exerting a direct influence on the calcemic activity or other actions regulated by 22-oxa-1α,25-D 3 in the host. These results indicate that the inhibition of metastasis and angiogenesis-inducing activity in cancer cells seemed to be a major mechanism responsible for the anti-cancer effects of 22-oxa-1α,25-D 3 . Our findings show that 22-oxa-1α,25-D 3 is beneficial for the prevention of metastasis in lung carcinoma.
Introduction
Lung cancer is the most common cause of cancer death in the world. Lung cancer frequently metastasizes to the systemic lymph nodes and distant organs, including the liver, lung, kidney and bone, and >90% of deaths from lung cancer can be attributed to metastases ( 1 ). In Japan, an estimated 90 000 new cases of lung cancer were diagnosed in 2003, and lung cancer will remain the leading cause of cancer death. Therefore, metastasis to multiple organs is a critical problem for patients with lung cancer. The prevention and treatment of the cancer metastases are clinically important.
The active form of vitamin D 3 , 1α,25-dihydroxyvitamin D 3 (1α,25-D 3 ), is a major regulator of calcium homeostasis, and is critically important for the normal mineralization of bone. In addition to the small intestine, bone and kidney, a multitude of other sites of action for this steroid hormone have been discovered. This insight resulted mainly from the observation that the vitamin D receptor (VDR), which mediates the hormone's genomic activity, is expressed in almost all tissues of the human body. Previous studies have shown that 1α,25-D 3 and its analogs are able to reduce the invasiveness of metastatic cancer cells in vitro ( 2 , 3 ). Using different animal models, it has now been confirmed that 1α,25-D 3 and its analogs suppressed invasion and metastasis and exerted an anti-angiogenic activity in vivo as well. In melanoma, lung, prostate, colon and breast cancer models, a reduction in the number and size of metastatic nodules has been observed in animals treated with 1α,25-D 3 and its analogs compared with untreated animals ( 4 – 7 ). However, the hypercalcemic activity of 1α,25-D 3 has precluded its application as a pharmacological agent. For this reason, various synthetic vitamin D 3 compounds with reduced calcemic activity have been developed. 22-Oxa-1α,25-dihydroxyvitamin D 3 (22-oxa-1α,25-D 3 ) is an analog of vitamin D that has reduced calcemic effects but demonstrates a strong action on cell differentiation ( 8 , 9 ). The weaker calcemic effect of 22-oxa-1α,25-D 3 has been mainly attributed to its short half-life in the blood stream ( 10 ). Previous studies have shown that 22-oxa-1α,25-D 3 reduced tumor size and tumor weight significantly, without increasing the serum calcium concentration in a number of different breast cancer cell in vivo models, including nude mice implanted with human breast cancer cells and rats carrying DMBA-induced breast tumors ( 9 , 11 ). However, despite the promising effects of 22-oxa-1α,25-D 3 in animal models of cancer, the molecular mechanisms behind these anti-cancer effects have not been clarified.
In the present study, we examined the effects of 22-oxa-1α,25-D 3 on the metastasis of lung cancer in mice. We have generated a highly metastatic model using Lewis lung carcinoma (LLC) cells expressing green fluorescent protein (GFP). LLC has been an important tumor model for studies of metastasis, angiogenesis and neoadjuvant chemotherapy. A major advantage of GFP-expressing LLC (LLC-GFP) cells is that the imaging requires no preparative procedures and therefore, the method is uniquely suited for visualizing live tissue during tumor progression ( 12 , 13 ). In addition, GFP labeling is extremely effective for measuring the number and volume of metastatic nodules in target organs.
Moreover, LLC-GFP cells expressed the VDR and their proliferative and invasive activity were significantly inhibited by 1α,25-D 3 treatment. The mechanism of this inhibition in response to 1α,25-D 3 has not been made clear. In this study, we observed in vitro the secretion and mRNA expression levels of matrix metalloproteinase (MMP)-2, MMP-9, vascular endothelial growth factor (VEGF) and parathyroid hormone-related protein (PTHrP), which were the most important factors for tumor invasion and tumor-induced angiogenesis. The secretion of these factors and mRNA expression were significantly inhibited by 22-oxa-1α,25-D 3 treatment. These findings suggest that 22-oxa-1α,25-D 3 may play a critical role in the expression and secretion of MMP-2, MMP-9, VEGF and PTHrP in metastatic cancer cells.
Furthermore, we reported previously that VDR−/− mice with corrected hypocalcemia and hypervitaminosis D being fed a high calcium and vitamin D-deficient diet could be used as an experimental model to screen the anti-cancer effects of new vitamin D analogs in vivo . Here, using this new experimental model, we examined the anti-cancer effects of 22-oxa-1α,25-D 3 without calcemic activity and other actions regulated by 22-oxa-1α,25-D 3 in the host. We investigate the activity of 22-oxa-1α,25-D 3 in the LLC-GFP metastasis model in vitro and in vivo , and explore its mechanisms of action.
Materials and methods
Animals
Female C57BL/6 mice (Clea Japan, Inc., Tokyo, Japan), 8 weeks of age, were used for all experiments. Mice were then fed distilled water and a chow diet ad libitum (F-2, Clea Japan, Inc., Tokyo, Japan; ingredients: 1.2% calcium, 0.6% phosphorus and 1.08 IU vitamin D 3 /g). The mice were maintained under specific pathogen-free conditions with a 12-h light/dark cycle. This study was conducted in accordance with the standards established by the Guidelines for the Care and Use of Laboratory Animals of Kobe Pharmaceutical University.
Cell culture
Cloned metastatic variant cells of LLC were kindly supplied by the Cell Resource Center for Biomedical Research, Tohoku University. These cells, which were cloned from metastatic lung nodule of a mouse with a s.c. parental LLC tumor, are locally invasive and metastasize to the lungs but not to other sites. For GFP gene transduction, LLC cells were stable transfected into the pEGFP-1 vector (Clontech, Palo Alto, CA) by electroporation. Transfected LLC cells (LLC-GFP cells) were cultured into a selective medium that contained 300 µg/ml of G418 (Geneticin, Roche, Diagnostics, Mannheim, Germany) for 6 days. The brightest fluorescing cells above the 95 percentile were sorted and cloned using flow cytometry. Cells were maintained by selection with 500 µg/ml of G418. The medium used for culturing the tumor cells and for all assays was RPMI1640 medium (Life Technologies, Grand Island, NY) supplemented with l -glutamine (0.29 mg/ml), kanamycin (0.06 mg/ml) and 10% heat-inactive fetal bovine serum (FBS).
Assessment of cell growth in vitro
The effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on the proliferation of LLC-GFP cells in vitro were assessed by cell counting and cell cycle analysis. Cells were seeded at a density of 2 × 10 5 cells/well in 6-well culture plates in RPMI1640 medium containing 10% FBS for 24 h. After 24 h, fresh medium containing 10% charcoal-stripped FBS with vehicle (ethanol), 1α,25-D 3 or 22-oxa-1α,25-D 3 (10 −7 M) was added to the cultured cells, and incubation continued for 1–3 days. 1α,25-D 3 and 22-oxa-1α,25-D 3 were dissolved in ethanol, the final concentration of ethanol in all cultures not exceeding 0.1%. Cells were trypsinized at specific time points, and an aliquot of cells was counted and the cell cycle analyzed. For the analysis of the cell cycle, each group of cells was collected and washed with phosphate-buffered saline (PBS) (−) once. Then, the cells were resuspended in PBS(−) containing 0.2% Triton X-100 and 100 µg of RNase and incubated at 37°C for 30 min. Cells were washed with PBS(−) and incubated with 0.5 ml of DNA-attaining solution containing propidium iodide (50 µg/ml) at 4°C for 20 min. The cells were analyzed with a flow cytometer equipped with an argon laser (488 nm, Becton Dickinson FACScan™) and the cell cycle distribution was analyzed using ModiFit LT (Verity).
In vitro invasion of LLC-GFP cells through a Matrigel-coated membrane
The capacity of tumor cells to traverse a basement membrane-matrix-coated filter has been shown to be representative of their invasiveness. Therefore, the capacity of tumor cells to migrate through Matrigel-coated membranes was measured. Nucleopore filters (8 µm pore size) were coated with 100 µl of a 1:20 dilution of Matrigel (Becton Dickinson, Bedford, MA) and allowed to dry. Representative filters were stained with crystal violet and, when examined microscopically, appeared to be evenly coated with Matrigel. Medium was added to the lower compartment of each blind-well chemotactic chamber. A dried, coated filter was placed over the lower compartment. After reconstitution of Matrigel, 5 × 10 4 cells were added to the upper compartment along with the same concentration of 1α,25-D 3 or 22-oxa-1α,25-D 3 with which the cells had been preincubated, with the vehicle, 1α,25-D 3 or 22-oxa-1α,25-D 3 (10 −8 or 10 −7 M) for 3 days. Chambers were incubated for 24 h, after which the filters were removed, wiped clean on the upper surface and fixed in 10% formalin. The number of cells on the lower surface of the filter was counted under the fluorescent microscope. Data were reported as the number of cells/low power field enumerated from triplicate chambers from each of the four experiments.
Intravenous injection of LLC-GFP cells in mice and administration of 1α,25-D 3 and 22-oxa-1α,25-D 3
Mice were injected i.v. with a single dose of 5 × 10 5 LLC-GFP cells. Cells were first harvested by trypsinization and washed two times with PBS(−) and then injected in a total volume of 0.2 ml in RPMI1640 medium containing 10% FBS. A preventative protocol was designed in which 1α,25-D 3 or 22-oxa-1α,25-D 3 was administered continuously using an osmotic minipump (model 2 ML4 Alzet; Alza Corp., Palo Alto, CA) implanted s.c. on the same day as the inoculation of LLC-GFP cells. In preliminary experiments, we used increasing concentrations of 22-oxa-1α,25-D 3 (0.5, 1 and 10 µg/kg/24 h) to determine the minimal effective dosage that will not cause hypercalcemia in non-tumor-bearing mice. An infusion rate of 1 µg/kg/24 h was chosen, and each minipump contained 22-oxa-1α,25-D 3 dissolved in 0.1% Tween-20 and 10% ethanol, to deliver a continuous dose for up to 3 weeks at a rate of 2.5 µl/h. Untreated animals were implanted with a minipump containing the vehicle alone. Mice with tumor implants were killed 3 weeks later. The lungs were removed and the weight, number of lung nodules and GFP mRNA expression were measured.
Analysis of serum calcium level
The blood samples were centrifuged at 3000 g for 10 min and the supernatants were employed as serum samples. The serum calcium level was determined by micro colorimetric assay (Wako, Japan).
Imaging and tumor scoring
A Leica fluorescence stereo microscope model MZ FL III (Leica Microsystem, Inc., USA) equipped with a mercury 50 W lamp power supply was used. Selective excitation of GFP was produced through a D480/40 band-pass filter and 510 DCXR dichroic mirror. Emitted fluorescence was collected through a long-pass filter DC300F and digital camera system (Leica Microsystem, Inc., USA). The numbers of metastatic nodules on the surface of the lung were counted under the fluorescence stereo microscope. The GFP-expressing spots on the lungs were enumerated to quantify the lung metastasis.
Real-time quantitative PCR
Lung tissues and LLC-GFP cells were homogenized in ISOGEN (Nippon Gene, Tokyo Japan). Total RNA was isolated as specified by the manufacturer. The purified RNA was reverse-transcribed with AMV reverse transcriptase (TaKaRa, Japan). Quantitative analyses of gene expression were conducted using GFP (5′-CTGCTCTCTTGGGTCCACTGG-3′ and 5′-CACCGCCTTGGCTTGTCACAT-3′), MMP-2 (5′-GATCATTGGTTACACACCTG-3′ and 5′-TGCTCCCATCGACCAAAGT-3′), MMP-9 (5′-TGTACCGCTATGGTTACAC-3′ and 5′-TCCAGCTCACCAGTCTGGGGCA-3′), mouse PTHrP (5′-ATGAATTCTGCTCAGCTACTCCGTG-3′ and 5′-TACCTAGGGAGGTCCTGGAGGTGTG-3′), and β-actin (5′-AGGCCCAGAGCAAGAGAGGTAT-3′ and 5′-CATGTCGTCCCAGTTGGTAACA-3′)-specific primer sets, a GeneAmp 5700 Sequence Detection System (PE Biosystems, Foster City, CA) and the SYBR Green core reagent kit (PE Biosystems, Foster City, CA).
Semiquantitative RT–PCR
Total RNA prepared from the LLC-GFP cells was reverse-transcribed and PCR-amplified at 95°C for 40 s, 62°C for 40 s and 72°C for 1 min for 20–35 cycles using VEGF [5′-CTGCTCTCTTGGGTCCACTGG-3′ and 5′-CACCGCCTTGGCTTGTCACAT-3′; ( 14 )]-specific primers (amplification sizes were VEGF188, 635 bp; VEGF164, 563 bp; VEGF120, 431 bp). Specific β-actin primers were used for the internal control to normalize the sample amounts. Agarose gels (2%) were stained with ethidium bromide and visualized under UV light. All assays were performed in triplicate. Gel images were analyzed with the NIH Image analysis system.
Preparation of conditioned medium
LLC-GFP cells (1 × 10 5 ) were cultured in 6-well tissue culture plates with 2 ml of culture medium/well for 24 h. After 24 h, fresh medium containing 10% charcoal-stripped FBS with vehicle, 1α,25-D 3 or 22-oxa-1α,25-D 3 was added to the cultured cells, and incubation continued for 3 days. The culture medium was changed to a serum-free medium containing 0.1% bovine serum albumin and 1α,25-D 3 or 22-oxa-1α,25-D 3 . After changing the culture medium, conditioned medium was collected at 6, 12 and 24 h, cleared by centrifugation and then stored at −80°C until evaluation. After removal of the conditioned medium, LLC-GFP cells were harvested by trypsinization and the cells in each well were enumerated. The conditioned medium was analyzed by gelatin zymography and enzyme immunoassay for mouse VEGF.
Gelatin zymography
The conditioned medium from LLC-GFP cells was analyzed for MMP-2 and MMP-9 by gelatin zymography ( 15 ). Proteins were separated by SDS–PAGE under non-reducing conditions on 10% polyacrylamide gels containing 1 mg/ml of gelatin. SDS was extracted with Triton X-100 from the gels, which were then incubated overnight at 37°C in 50 mM Tris–HCl, pH 7.8, containing 150 mM NaCl and 5 mM CaCl 2 . The gels were stained with Coomassie Brilliant Blue R-250 and destained in 45% methanol and 10% acetic acid. All assays were performed in triplicate. Gel images were analyzed with the NIH Image analysis system.
Enzyme immunoassay for mouse VEGF
The VEGF level in serum or conditioned medium of LLC-GFP cells was determined using a VEGF EIA kit (Quantikine M, R&D system, USA) based on a sandwich enzyme immunoassay, according to the manufacturer's instructions. The concentration of VEGF in 100 µl of sample was estimated from a standard curve obtained using standard mouse VEGF serially diluted. The assay was carried out according to the manufacturer's specifications.
Mouse dorsal air sac model
The mouse dorsal air sac model was established according to Sakamoto et al . ( 16 ). Briefly, a suspension of LLC-GFP cells (1 × 10 6 cells/200 µl) in PBS(−), free of Mg 2+ and Ca 2+ , was injected into a Millipore chamber consisting of a Millipore filter ring (PR0001401) and two Millipore filters (HAWP29323, 45 µm), which were attached to the ring with MF cement (XX7000000). This chamber was implanted into a dorsal air sac produced in each strain of mouse by injecting 10 ml of air through a 25-gauge needle. At the same time, an osmotic minipump with 1α,25-D 3 or 22-oxa-1α,25-D 3 was implanted on the other side of the chamber ring in the mice. Mice implanted with a chamber ring were killed 10 days later. The chamber ring was removed and the neovascular vessels under the skin covering the implanted chamber ring were enumerated.
In vivo chamber angiogenesis assay
This assay was reported previously ( 17 ). Chambers were constructed from a Millipore chamber ring. A 13 µm diameter 30 µm nylon net filter (Millipore Corporation, MA) was glued to the ring. About 0.2 ml of liquid 4°C growth factor-reduced Matrigel matrix (BD Biosciences, MA) containing 300 ng of basic fibroblast growth factor (bFGF) (ICN Pharmaceuticals, CA) was injected into the chamber. The 4°C Matrigel matrix rapidly formed a gel at room temperature. The chambers were finally sealed with a 180 µm nylon net filter (Millipore Corporation, MA). All chambers and filters were disinfected in 70% ethanol before use. This chamber was implanted into a dorsal air sac produced in each strain of mouse by injecting 10 ml of air through a 25-gauge needle. At the same time, an osmotic minipump with vehicle, 1α,25-D 3 or 22-oxa-1α,25-D 3 was implanted on the other side of the chamber ring in the mouse. Mice with a implanted chamber ring were killed 10 days later. After removal of the chamber from the anesthetized mice on day 10, tissues attached to the chamber were cleaned off and the chamber was gently washed with PBS(−). Subsequently, the chamber content was resuspended in 1 ml of PBS(−) and centrifuged at 134 g for 1 min. Finally, an optical density reading at 415 nm (OD 415 nm) of 200 µl of supernatant was analyzed using a microplate reader (BIO-RAD). OD 620 nm was used as a reference wavelength and data were shown as OD 415–620 nm. The 415 nm wavelength was chosen because a spectrum scan showed that mouse and rat blood solutions were strong absorbers of 400–425 nm light.
Intravenous injection of LLC-GFP cells in VDR −/− mice with corrected hypocalcemia and hypervitaminosis D and administration of 22-oxa-1α,25-D 3
VDR−/− mice were generated by homologous gene targeting as described previously ( 18 ). Null mutant mice were obtained by crossbreeding a heterozygous VDR knockout female with a heterozygous male. Mice were weaned at 3 weeks of age, then fed a high calcium and vitamin D-deficient diet (Clea Japan, Inc.; ingredients: 2% calcium, 1.25% phosphorus, 0 IU vitamin D 3 /100 g and 20% lactose) ad libitum . We used wild-type ( VDR+/+ ) and VDR−/− mice, 10 weeks of age, which were fed a high calcium and vitamin D-deficient diet, to completely eliminate endogenous 1α,25-D 3 and to correct the serum calcium levels. First, we confirmed that VDR−/− mice showed extreme hypervitaminosis D (18.54 ± 2.11 pg/ml and 10 452.41 ± 849.81 pg/ml in VDR+/+ and VDR−/− mice, respectively) along with severe hypocalcemia (9.54 ± 0.08 mg/dl and 6.18 ± 0.13 mg/dl in VDR+/+ and VDR−/− mice, respectively) when compared with VDR+/+ mice. Feeding of the high calcium and vitamin D-deficient diet resulted in the complete elimination of 1α,25-D 3 (below the detection limit: 1 pg/ml and 39.6 ± 14.6 pg/ml in VDR+/+ and VDR−/− mice, respectively) both before and after 22-oxa-1α,25-D 3 administration in VDR+/+ and VDR−/− mice. Mice were injected i.v. with a single dose of 5 × 10 5 LLC-GFP cells in a total volume of 0.2 ml in RPMI1640 medium containing 10% FBS. Thereafter, 22-oxa-1α,25-D 3 was administered continuously using an osmotic minipump implanted s.c. on the same day as the inoculation with LLC-GFP cells. An infusion rate of 1 or 10 µg/kg/24 h was chosen, and each minipump contained 22-oxa-1α,25-D 3 dissolved in 0.1% Tween-20 and 10% ethanol to deliver a continuous dose for up to 3 weeks at a rate of 2.5 µl/h. Untreated animals were implanted with a minipump containing the vehicle alone. Mice with tumor implants were killed 3 weeks later and serum Ca level, lung weight, number of lung nodules and GFP mRNA expression in the lung were measured.
Statistical analyses
Data are presented as the mean ± SE. Student's t -test was used for group analyses. Correlation coefficients were calculated for possible interrelations between variables. P -values <0.05 were considered as statistically significant.
Results
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on cell growth and metastatic activity of LLC-GFP cells in vitro
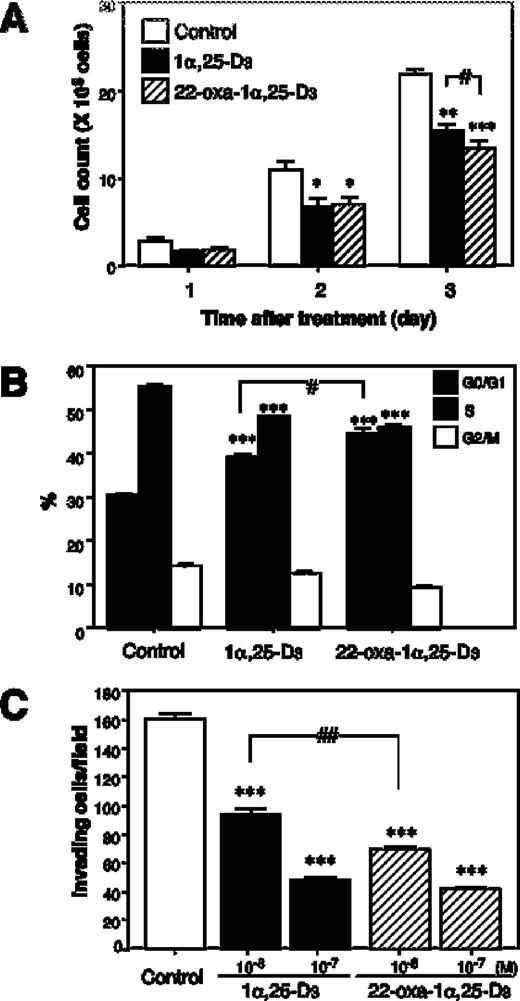
We examined the effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on the proliferation of lung cancer LLC-GFP cells in vitro . Cells were grown as described in ‘Materials and methods’ and treated with 1α,25-D 3 or 22-oxa-1α,25-D 3 . As shown in Figure 1 , treatment with 10 −7 M of 1α,25-D 3 and 22-oxa-1α,25-D 3 for 1–3 days resulted in a time-dependent decrease in the cell number ( Figure 1A ). Moreover, the cell cycle of LLC-GFP cells treated with 10 −7 M of 1α,25-D 3 and 22-oxa-1α,25-D 3 for 3 days was analyzed by flow cytometry. In vehicle (ethanol)-treated cultures, 31% of the cells were in the G 0 /G 1 -phase population and 55% of the cells in the S-phase population. In 1α,25-D 3 -treated cultures, 40% of the cells were in the G 0 /G 1 -phase population and 48% of the cells in the S-phase population. In the 22-oxa-1α,25-D 3 -treated cultures, 44% of the cells were in the G 0 /G 1 -phase population and 46% of the cells in the S-phase population. Addition of 1α,25-D 3 and 22-oxa-1α,25-D 3 to the culture medium caused a significant arrest of the cell cycle at G 0 /G 1 ( Figure 1B ). These effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 were observed in a dose-dependent manner. The minimal dosage producing a significant inhibition of cell growth was 10 −8 M for 1α,25-D 3 and 22-oxa-1α,25-D 3 (data not shown).
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on LLC-GFP cell growth and metastasis in vitro . ( A ) Cell number at 72 h with 10 −7 M of 1α,25-D 3 or 22-oxa-1α,25-D 3 . ( B ) Cell cycle phase distribution in 1α,25-D 3 or 22-oxa-1α,25-D 3 treated-LLC-GFP cells at 72 h. ( C ) The capacity of 1α,25-D 3 or 22-oxa-1α,25-D 3 treated-LLC-GFP cells to transverse a Matrigel-coated filter for 24 h. Each bar represents the mean ± SE *,#P < 0.05, **,##P < 0.01 and ***P < 0.001 ( n = 6).
A previous study showing reduced random migration by LLC-GFP cells, after 3 days of incubation with 1α,25-D 3 , determined the effect of increasing concentrations of 1α,25-D 3 on tumor cell invasiveness through reconstituted basement membrane-coated filters ( 3 ). The vehicle-treated LLC-GFP cells readily transversed the matrix-coated membranes. In contrast, incubation with 1α,25-D 3 and 22-oxa-1α,25-D 3 caused a dose-dependent decline in the invasive capability of the tumor cells. Invasion was significantly inhibited at 10 −7 M 1α,25-D 3 and 22-oxa-1α, 25-D 3 , with a 70% reduction in the number of cells that could transverse the filter ( Figure 1C ). There was a significant difference between 1α,25-D 3 and 22-oxa-1α,25-D 3 at 10 −8 M.
Effects of continuous treatment with 1α,25-D 3 and 22-oxa-1α,25-D 3 on serum calcium
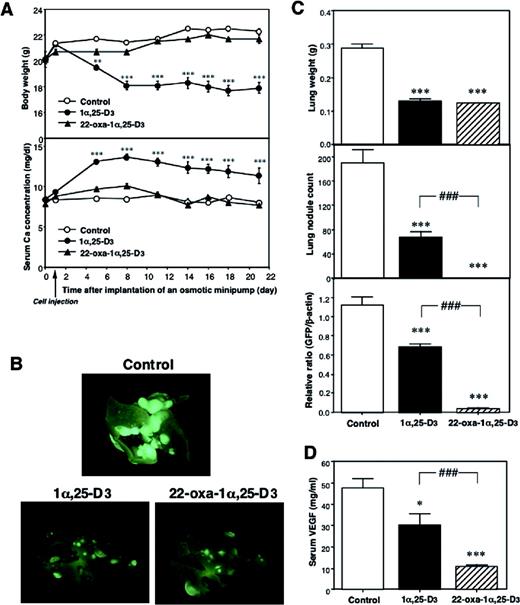
Tumor-bearing animals receiving 1α,25-D 3 showed hypercalcemia and a reduction in weight 4 days after the administration. On the other hand, the 22-oxa-1α,25-D 3 -treated group did not show any significant change in serum calcium when compared with the vehicle-treated control group. In 22-oxa-1α,25-D 3 -treated and vehicle-treated groups, serum calcium concentrations remained normal for the duration of the experiment ( Figure 2A ).
Effects of continuous treatment with 1α,25-D 3 and 22-oxa-1α,25-D 3 on growth, serum calcium and development of lung metastasis in mice injected with LLC-GFP cell suspension. 1α,25-D 3 or 22-oxa-1α,25-D 3 (1 µg/kg/day) or vehicle was infused continuously by an osmotic minipump implanted s.c. on the day before as the LLC-GFP cell injection. Serum and lungs were collected 3 weeks after the cell injection. ( A ) Body weight and serum calcium level. ( B ) External image of the lung of mice under a fluorescence stereo microscope. ( C ) Lung weight, lung nodule counts and GFP mRNA expression in the lung. ( D ) Serum levels of VEGF. Each bar represents the mean ± SE ( n = 10). *P < 0.05 and ***,###P < 0.001, significant difference from vehicle-treated animals.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on the development of lung metastases
The number of mice that developed lung metastases was analyzed by measuring the lung weight, nodule count and GFP mRNA expression in the lung by quantitative RT–PCR. We found values to be significantly lower in the 1α,25-D 3 and 22-oxa-1α,25-D 3 -treated group than in the vehicle-treated group ( Figure 2B ). The lung weight and nodule counts were significantly reduced in animals treated with 1α,25-D 3 and 22-oxa-1α,25-D 3 . GFP mRNA expression, a more quantitative analysis than the measurement of lung weight and nodule count, was markedly decreased in 22-oxa-1α,25-D 3 -treated mice ( Figure 2C ). Moreover, we measured the serum levels of VEGF, an angiogenesis-inducing factor. Although the serum VEGF concentration is usually low, it will increase with the growth of tumor cells highly expressing the VEGF. 1α,25-D 3 and 22-oxa-1α,25-D 3 -treatment groups had significantly lower levels of serum VEGF than the vehicle-treatment group. Serum VEGF levels were correlated with tumor formation levels. The difference in potency between the 1α,25-D 3 -treated and 22-oxa-1α,25-D 3 -treated mice is caused by the calcemic activity of these analogs. The 1α,25-D 3 -treated mice became weak with severe hypercalcemia, but the 22-oxa-1α,25-D 3 -treated mice did not.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on MMP-2 and MMP-9 mRNA expression and MMP activity in LLC-GFP cells
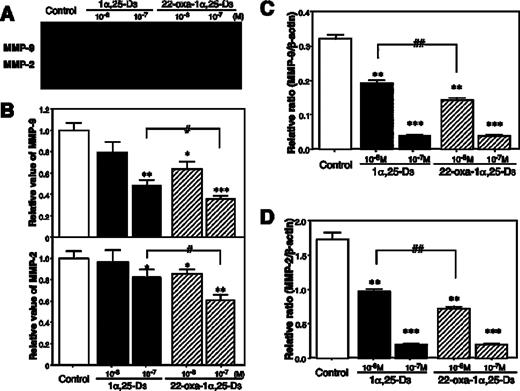
The anti-invasive effect of 1α,25-D 3 has been substantiated by studies showing that these compounds are able to inhibit cancer cell progression by interfering with specific steps in the metastatic cascade, including the proteolytic enzymes. We examined the expression of the metastases-regulating factor secreted from LLC-GFP cells. LLC-GFP cells were found to secrete MMP-2 and MMP-9 into the conditioned medium. MMP-2 and MMP-9 are important factors for tumor invasion and metastasis. Treatment of cells with 1α,25-D 3 and 22-oxa-1α,25-D 3 for 3 days decreased the amount of MMP-2 and MMP-9 secreted to one-third of the control levels ( Figure 3A and B ). 22-Oxa-1α,25-D 3 was able to decrease the secretion of MMP-2 and MMP-9 more than 1α,25-D 3 . Moreover, we analyzed whether 1α,25-D 3 and 22-oxa-1α,25-D 3 could alter MMP-2 and MMP-9 mRNA expression in LLC-GFP cells. After a 24-h treatment of cells with increasing concentrations of 1α,25-D 3 and 22-oxa-1α,25-D 3 , MMP-2 and MMP-9 mRNA expression was decreased ( Figure 3C and D ). Short-term treatment (6, 12 h) with 1α,25-D 3 and 22-oxa-1α,25-D 3 did not significantly alter MMP-2 and MMP-9 mRNA levels, but after a 24-h incubation, a clear concentration-dependent decrease was observed. 1α,25-D 3 and 22-oxa-1α,25-D 3 were clearly more potent in the regulation of MMP-2 and MMP-9 mRNA, as suggested by the data from activity assays. These results suggest that reduced MMP-2 and MMP-9 activity is probably the main mechanism by which 1α,25-D 3 and 22-oxa-1α,25-D 3 inhibit the invasiveness of LLC-GFP cells.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on MMP-2 and MMP-9 mRNA expression and secretion levels in LLC-GFP cells. ( A ) MMP-9 mRNA, ( B ) MMP-2 mRNA, ( C ) zymographic assay with conditioned medium of LLC-GFP cells treated with control, 1α,25-D 3 or 22-oxa-1α,25-D 3 . ( D ) Relative ratio of MMP-2 and MMP-9 activity by gelatin zymography. Each bar represents the mean ± SE. *,#P < 0.05, **,##P < 0.01 and ***P < 0.001 ( n = 6).
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on VEGF mRNA expression and secretion in LLC-GFP cells
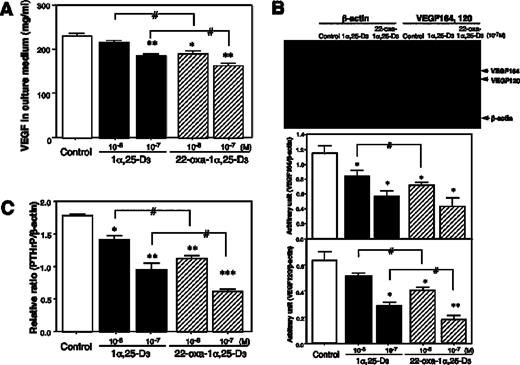
In a multistep process essential for tumor growth and metastasis, it has been established that angiogenesis is necessary for tumor growth beyond 1–2 mm in diameter, and thus tumor growth can be regulated by the inhibition of angiogenesis. VEGF is one of the most selective and potent angiogenic factors secreted from tumor cells. We examined the secretion of VEGF from LLC-GFP cells to determine the effect of 1α,25-D 3 and 22-oxa-1α,25-D 3 on angiogenesis-inducing activity in LLC-GFP cells. Treatment of cells with 1α,25-D 3 and 22-oxa-1α,25-D 3 for 3 days decreased the secreted VEGF in a dose-dependent manner ( Figure 4A ). 22-Oxa-1α,25-D 3 was more potent than 1α,25-D 3 in inhibiting VEGF secretion. Four isoforms of VEGF (VEGF205, VEGF188, VEGF164 and VEGF120) have been reported to be expressed in mouse tissue ( 19 ). The large isoforms, VEGF205 and VEGF188, are cell-associated because of their stronger affinity for cell-surface proteoglycan, whereas the smaller isoforms, VEGF164 and VEGF120, are secretory forms. Several studies have shown VEGF164 and VEGF120 to be most highly expressed in tumors. LLC-GFP cells expressed large amounts of VEGF164 and VEGF120 mRNA, but VEGF205 and VEGF188 mRNA were not detected. A 24-h treatment of cells with increasing concentrations of 1α,25-D 3 and 22-oxa-1α,25-D 3 decreased the VEGF164 and VEGF120 mRNA expression ( Figure 4B ). A clear concentration-dependent decrease in 1α,25-D 3 and 22-oxa-1α,25-D 3 was observed for a 6- to 24-h incubation in a time-dependent manner (data not shown). 22-Oxa-1α,25-D 3 was more effective in downregulating the expression of VEGF164 and VEGF120 mRNA than 1α,25-D 3 . These results indicate that 1α,25-D 3 and 22-oxa-1α,25-D 3 may suppress the angiogenesis-inducing activity of LLC-GFP cells by reducing the expression of VEGF.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on VEGF secretion and VEGF and PTHrP mRNA expression in LLC-GFP cells. ( A ) VEGF concentration in conditioned medium of LLC-GFP cells treated with control, 1α,25-D 3 or 22-oxa-1α,25-D 3 . ( B ) VEGF mRNA expression. ( C ) PTHrP mRNA expression. Each bar represents the mean ± SE. *,#P < 0.05, **P < 0.01 and ***P < 0.001 ( n = 6).
In addition, we measured the PTHrP mRNA expression in LLC-GFP cells. PTHrP was originally isolated from human tumors associated with the hormonal hypercalcemia of malignancy (HHM) syndrome ( 20 ). When high levels of PTHrP are secreted by tumors, PTHrP stimulates osteoclastic bone reabsorption and renal tubular reabsorption of calcium, thus leading to hypercalcemia and osteolytic bone metastases ( 21 ). It was previously reported that LLC causes hypercalcemia ( 22 ). A 24-h treatment with 1α,25-D 3 and 22-oxa-1α,25-D 3 resulted in a significant reduction in the level of PTHrP mRNA. These effects were detected in a concentration- and time-dependent manner (data not shown). In addition, 22-oxa-1α,25-D 3 was more effective in the suppression of VEGF and PTHrP expression than 1α,25-D 3 (10 −7 M).
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on angiogenesis in vivo
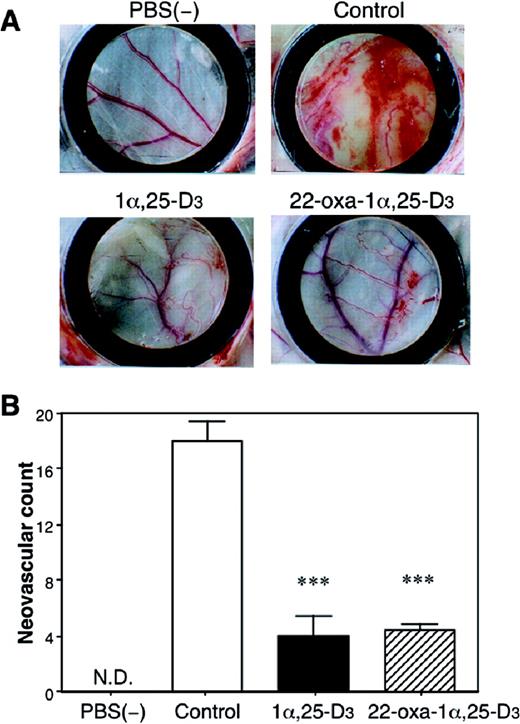
LLC-GFP cells have a high metastatic potential and produce several growth factors. The degree of induced angiogenesis was dependent on the number of LLC-GFP cells in the chamber. The neovascular vessel formation was not induced by the chamber ring including PBS(−) alone. In the vehicle-treated mice, the neovascular vessels were formed under the chamber ring, including LLC-GFP cells. In the 1α,25-D 3 and 22-oxa-1α,25-D 3 -treated mice, the number of neovascular vessels was decreased ( Figure 5A and B ). However, in this assay, it is impossible to discriminate between a direct effect on the tumor cells and an anti-angiogenic effect, since the inhibition of tumor growth limits angiogenesis and vice versa.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on LLC-GFP cell-induced angiogenesis in the mouse dorsal air sac model. ( A ) The chamber containing LLC-GFP cells or PBS(−), which was the negative control, was implanted into a subcutaneous dorsal air sac. At the same time, a osmotic minipump with control (vehicle), 1α,25-D 3 or 22-oxa-1α,25-D 3 , was implanted on the opposite side of the chamber ring in the mice. Ten days after implantation, the mice were killed and the chambers were removed from the fascia. The area that had been in contact with the chamber was photographed. ( B ) Measurement of neovascular counts in the skin of mice bearing Millipore chambers containing LLC-GFP cells. ND, the neovascular vessels were not detected. Each bar represents the mean ± SE. ***P < 0.001 versus vehicle-treated group ( n = 10).
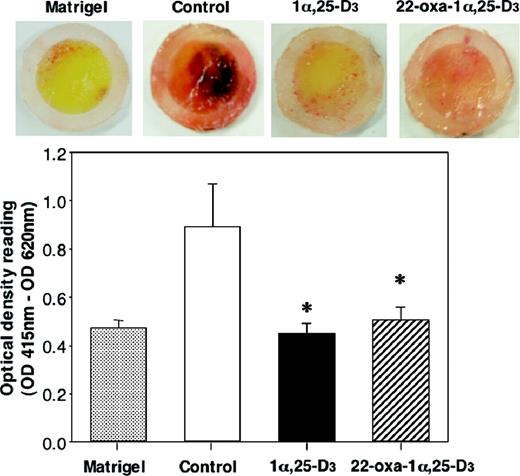
In contrast, the in vivo chamber angiogenesis assay strictly investigates angiogenesis and allows a quantitative analysis of anti-angiogenic activity. This assay was published by Kragh et al . ( 17 ) and reported as a useful tool for drug evaluation as well as research into anti-angiogenesis. Thus, with this assay, we examined the anti-angiogenic effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on the endothelial cells activated by the first angiogenic factor (bFGF) in vivo . Matrigel alone induced angiogenesis on day 10 of post-implantation but Matrigel containing bFGF markedly increased the angiogenesis, measured with OD readings at 415 nm. The continuous administration of 1α,25-D 3 and 22-oxa-1α,25-D 3 completely inhibited the bFGF-induced angiogenesis to a level below that induced by Matrigel alone ( Figure 6 ). The effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 were most pronounced on day 10. We found significant anti-angiogenic effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 in this assay. These results suggest that 1α,25-D 3 and 22-oxa-1α,25-D 3 inhibit the angiogenesis of endothelial cells activated by bFGF without influencing the tumor cells.
Effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on bFGF-induced angiogenesis in the in vitro chamber angiogenesis assay. At the time of the implantation of the chamber ring to store the Matrigel-containing bFGF, a osmotic minipump with 1α,25-D 3 or 22-oxa-1α,25-D 3 , was implanted on the other side of the chamber ring in the mice. Ten days after implantation, the mice were killed and the chambers were removed from the fascia. The angiogenic factor bFGF induced angiogenesis on day 10 post-implantation as determined from OD readings at 415 nm. Each bar represents the mean ± SE. *P < 0.05 versus vehicle-treated group ( n = 10).
Effects of continuous treatment with 22-oxa-1α,25-D 3 on the development of lung metastases in the LLC-GFP cell injected VDR +/+ mice and VDR −/− mice fed a high calcium and vitamin D-deficient diet
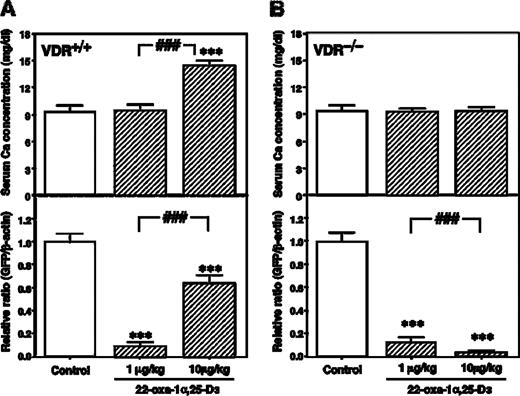
VDR−/− mice exhibit hypocalcemia and extremely high serum levels of 1α,25-D 3 . However, we reported previously that feeding these animals a high calcium and vitamin D-deficient diet resulted in the complete elimination of 1α,25-D 3 and the correction of calcium levels in the serum of both VDR+/+ and VDR−/− mice. Our recent report suggests that VDR−/− mice with corrected hypocalcemia and hypervitaminosis D can be used as an experimental model for screening the anti-cancer effects of new vitamin D analogs in vivo . In this study, using this experimental model, 22-oxa-1α,25-D 3 was administered by an osmotic minipump for 18 days. LLC-GFP cells were injected into VDR+/+ mice receiving a high dose of 22-oxa-1α,25-D 3 (10 µg/kg/day) and these animals subsequently showed hypercalcemia, while VDR−/− mice receiving a high dose of 22-oxa-1α,25-D 3 and similarly injected with tumor cells did not show any significant change in serum calcium levels when compared with the vehicle-treated control group ( Figure 7A and B ).
Effects of continuous treatment with 22-oxa-1α,25-D 3 on the development of lung metastases in LLC-GFP cell-injected VDR+/+ mice and VDR−/− mice fed a high calcium and vitamin D-deficient diet. 22-Oxa-1α,25-D 3 (1 or 10 µg/kg/day) or vehicle was infused continuously by an osmotic minipump implanted s.c. on the same day as the LLC-GFP cell injection. Serum and lungs were collected on day 18 after the cell injection. ( A ) Serum calcium concentration and GFP mRNA expression of the lung in LLC-GFP cell injected VDR+/+ mice. ( B ) Serum calcium concentration and GFP mRNA expression of the lung in LLC-GFP cell injected VDR−/− mice. Each bar represents the mean ± SE ( n = 10). ***P < 0.001, significant difference from vehicle treated animals. ###P < 0.001, significant difference from 22-oxa-1α,25-D 3 (1 µg/kg/day) treated animals.
The number of mice that developed lung metastases was analyzed by measuring the GFP mRNA expression in the lung by quantitative RT–PCR. This parameter was significantly lower in the 22-oxa-1α,25-D 3 -treated group than in the untreated group among VDR+/+ mice and VDR−/− mice fed a high calcium and vitamin D-deficient diet ( Figure 7A and B ). In particular, the anti-tumor effect of 22-oxa-1α,25-D 3 in VDR−/− mice were dose-dependent. This result indicates that 22-oxa-1α,25-D 3 can act directly on cancer cells under conditions without calcemic activity and other actions of 22-oxa-1α,25-D 3 in the host. However, the high dose 22-oxa-1α,25-D 3 -treated VDR+/+ mice increased tumor growth compared with that in the low dose 22-oxa-1α,25-D 3 -treated VDR+/+ mice. These results indicate that hypercalcemia induced by the high dose of 22-oxa-1α,25-D 3 affects the anti-cancer effects.
Discussion
Metastatic cancer of the lung is a particularly challenging problem in the clinical setting. Once tumors have invaded the lung, the response to classical chemotherapeutic agents is weak, and the prognosis for these patients is poor ( 23 ).
In this study, we used an animal model of lung carcinoma to demonstrate that 22-oxa-1α,25-D 3 , a low calcemic analog of 1α,25-D 3 , inhibits the formation of lung tumors. Previous studies had shown that synthetic 1α,25-D 3 analogs with low calcemic activity relative to the native hormone 1α,25-D 3 , are of potential value as anti-cancer agents ( 24 – 28 ). 22-Oxa-1α,25-D 3 has been studied and shown to inhibit the growth of human breast cancer cells and canine osteosarcoma cells in vitro and in vivo ( 29 , 30 ). However, despite the promising effects of 22-oxa-1α,25-D 3 in animal models of cancer, there are no current ongoing clinical trials. Instead, 22-oxa-1α,25-D 3 has been approved and launched for the treatment of secondary hyperparathyroidism ( 31 ). In view of the fact that VDRs are expressed in >90% of human tumors,22-oxa-1α, 25-D 3 may be useful for the treatment of cancer. In the present study, we demonstrated that 22-oxa-1α,25-D 3 reduces not only the development of lung metastases but also the tumor-induced angiogenesis in vitro and in vivo .
The mechanism of action of 22-oxa-1α,25-D 3 in prevention of the development of lung metastasis is unclear. On the basis of both in vitro and in vivo anti-tumor activity of 22-oxa-1α,25-D 3 in several cancer models and the present in vitro data on LLC-GFP cell inhibition, we speculate that our in vivo observation on lung metastases is at least, in part, secondary to a direct effect of 22-oxa-1α,25-D 3 on tumor cell growth within the lung. The mechanisms by which 1α,25-D 3 and 22-oxa-1α,25-D 3 inhibit tumor growth are complex and not fully understood. 1α,25-D 3 induces a growth arrest in G 0 /G 1 and was shown to modulate the expression of cell cycle-associated genes, including p21, p27, cyclin D and cyclin E in breast and prostate cancer and leukemia cells ( 32 – 34 ). In this study, both 1α,25-D 3 and 22-oxa-1α,25-D 3 inhibited cell proliferation and induced growth arrest at G 0 /G 1 in LLC-GFP cells as well as breast and prostate cancer cells. Moreover, 1α,25-D 3 and 22-oxa-1α,25-D 3 inhibited tumor invasive activity. For this reason, we indicate that 1α,25-D 3 and 22-oxa-1α,25-D 3 -treated LLC-GFP cells had decreased mRNA expression and secretion of MMP-2 and MMP-9, a family of proteases capable of degrading extracellular matrix and basement membrane components, including collagens under physiological conditions. In human prostate and breast cancer cells as well as in human phagocytes, 1α,25-D 3 has been reported to reduce MMP-9 activity ( 15 , 35 , 36 ). However, this effect of 1α,25-D 3 was not reported in lung carcinoma cells. LLC cells are susceptible to vitamin D treatment, and their in vitro invasiveness is reduced by 1α,25-D 3 . A more detailed analysis of the MMP-2 and MMP-9 promoter activity is not available. No known vitamin D-inhibitory sequences were found in these promoter regions. Therefore, we do not know whether VDR binds directly to the MMP-2 and MMP-9 promoter. The 5′ end of the MMP-2 and MMP-9 promoter contains Sp-1, cAMP-responsive element-binding protein 1, NFkappaB and activation protein-1 (AP-1) sites, of which one is important for the basal expression of MMP ( 37 , 38 ). Recently, the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10), which is one of the most commonly inactivated genes in human cancer, led to the inhibition of TNFα-induced MMP-9 expression in vascular smooth muscle cells. This downregulation of MMP-9 by PTEN was transcriptionally regulated at NF-kappaB and AP-1 sites in the MMP-9 promoter. In addition, it has been reported that 1α,25-D 3 stimulated the expression of PTEN in leukemic cells ( 39 ). Therefore, the regulation of PTEN gene expression by 1α,25-D 3 may mediate the expression of MMP-2 and MMP-9 genes in LLC-GFP cells.
Moreover, the anti-angiogenic activity of 1α,25-D 3 may contribute to its overall anti-cancer effects. Interestingly, in LLC-GFP cells, 1α,25-D 3 and 22-oxa-1α,25-D 3 reduced the mRNA expression and secretion of VEGF, which selectively induces activation, migration, proliferation and tube formation in endothelial cells. A previous report indicated that the administration of 1α,25-D 3 to mice inoculated with cancer cells of different origin and rats bearing chemically induced colon tumors resulted in a significant decrease in tumor-induced angiogenesis. This finding is based on an immunohistochemical analysis of colon tumors from 1α,25-D 3 -treated rats, which demonstrated a significant decrease in VEGF expression and microvessel counts compared with tumors from untreated animals ( 40 ). However, 1α,25-D 3 induces the expression of VEGF in osteoblast and chondrocyte cells, and has not been examined in cancer cells ( 41 , 42 ). In this study, we examined the effects of 1α,25-D 3 and 22-oxa-1α,25-D 3 on the mRNA expression and secretion of VEGF in LLC-GFP cells. 1α,25-D 3 and 22-oxa-1α,25-D 3 decreased the expression and secretion of VEGF in LLC-GFP cells. The molecular mechanisms of these different effects are not known. Some kind of target cell specificity seems to exist since diverse responses are observed in different cell types. However, our results indicate that 1α,25-D 3 and 22-oxa-1α,25-D 3 could reduce the angiogenesis-inducing activity by decreasing the expression and secretion of VEGF in LLC-GFP cells. Obviously, the mechanisms underlying the anti-cancer effects of 1α,25-D 3 are complex and further investigation within this area is required in order to obtain a better understanding.
Several cancer cells produce a variety of hormones and cytokines that complicate the clinical management of patients with cancer. HHM is one of the most frequent paraneoplastic syndromes and, in most cases, is mediated by the PTHrP ( 20 ). When overproduced and secreted by certain tumors, PTHrP enters the circulation, interacts with the PTH/PTHrP receptor in bone and kidney, and stimulates osteoclastic bone resorption and renal tubular reabsorption of calcium, thus leading to hypercalcemia. A previous report indicated that 22-oxa-1α,25-D 3 suppressed the basal PTHrP gene transcription and secreted peptide levels in a human lung squamous cancer cell line ( 43 ). Here, we have shown that 1α,25-D 3 and 22-oxa-1α,25-D 3 have the potential to suppress the mRNA expression of PTHrP in LLC-GFP cells. Studies of the 5′-flanking sequence of the human and rat PTHrP gene have identified a negative VDRE (nVDRE) ( 44 , 45 ). However, the mouse PTHrP gene has not been identified yet. Other studies have suggested that 1α,25-D 3 can reduce the levels of protein kinase A (PKA) activity in LLC cells. PTHrP gene transcription is activated through the cAMP-response element by PKA ( 46 ). We are currently exploring these possibilities to determine the mechanism by which 1α,25-D 3 and 22-oxa-1α,25-D 3 reduce PKA activity or downregulate promoter activity through nVDRE in the PTHrP gene. Therefore, 22-oxa-1α,25-D 3 may provide a new strategy for treating squamous cell carcinoma of the lung as well as other malignancies, because of its potent antiproliferative effects and ability to prevent PTHrP-induced HHM.
We used two methods to study the anti-angiogenic activity of 1α,25-D 3 and 22-oxa-1α,25-D 3in vivo . In the dorsal air sac assay of LLC-GFP cells, continuous administration of 1α,25-D 3 and 22-oxa-1α,25-D 3 significantly reduced the angiogenesis induced by LLC-GFP cells. However, this method included the direct effect on LLC-GFP cells. On the other hand, the in vivo chamber angiogenesis assay strictly investigates angiogenesis and allows the quantitative analysis of anti-angiogenic activity. Continuous administration of 1α,25-D 3 or 22-oxa-1α,25-D 3 significantly reduced the bFGF-induced angiogenesis. In a previous report, Kragh et al . ( 17 ) reported no significant anti-angiogenic effect of 1α,25-D 3 in this anti-angiogenesis assay. One possible reason for this different result is that we adopted a continuous administration using an osmotic minipump and higher dose of 1α,25-D 3 .
Our recent report suggested that VDR−/− mice with corrected hypocalcemia and hypervitaminosis D could be used as an experimental model to screen the anti-cancer effects of new vitamin D analogs in vivo ( 13 ). In this study, using this experimental model, 22-oxa-1α,25-D 3 was administered for 18 days using an osmotic minipump. The LLC-GFP cells injected VDR−/− mice receiving a high dose of 22-oxa-1α,25-D 3 (10 µg/kg/day) did not show hypercalcemia and significantly decreased the tumor growth. This result clearly indicates that 22-oxa-1α,25-D 3 can act directly on cancer cells under conditions without calcemic activity or other actions of 22-oxa-1α,25-D 3 in the host. However, anti-metastatic effects of the high dose 22-oxa-1α,25-D 3 -treatment were weaker than in the low dose 22-oxa-1α,25-D 3 -treated VDR+/+ mice. These results indicate that hypercalcemia induced by the high dose of 22-oxa-1α,25-D 3 affects the anti-cancer effects. The high dose of 22-oxa-1α,25-D 3 causes hypercalcemia and loss of body weight, resulting in an impairment of the immune system in the host. In the previous reports, it has been suggested that mice treated with 1α,25-D 3 experienced a severe loss of thymocytes, and that thymic atrophy resulted as a consequence of 1α,25-D 3 -induced hypercalcemia ( 47 , 48 ). Therefore, it is considered that the high dose of 22-oxa-1α,25-D 3 -induced hypercalcemia affected the immune system and decreased the host defense in VDR+/+ mice. Thus, our data indicate that hypercalcemia and loss of body weight induced by the high dose of 22-oxa-1α,25-D 3 is a risk factor for tumorigenesis and can occasionally be lethal. However, a low dose of 22-oxa-1α,25-D 3 (1 µg/kg/day) is highly effective in inhibiting the metastatic tumor growth without hypercalcemia. In this experiment, our findings of marked anti-metastatic effects of 22-oxa-1α,25-D 3 , achieved with significantly less toxicity in terms of hypercalcemia and weight loss than 1α,25-D 3 , suggest that 22-oxa-1α,25-D 3 may offer a therapeutic option in advanced lung carcinoma. These results suggest that our experimental model could be useful for studying the pathophysiology of metastatic tumors in the lung microcirculation without the calcemic activity and host's defenses triggered by 1α,25-D 3 analogs. Moreover, this model is more effective in evaluating the influence of the side-effects of 1α,25-D 3 analogs on the in vivo anti-cancer effect.
These results strongly suggest that 22-oxa-1α,25-D 3 has selective effects on target tissues and particularly cancer cells without affecting calcium homeostasis, making it particularly suitable for future clinical trials. The mechanism of action of 22-oxa-1α,25-D 3 in preventing the development of lung metastasis is unclear. However, the present results indicate that 22-oxa-1α,25-D 3 inhibited growth and metastasis and reduced VEGF, MMP-2 and MMP-9 expression and the angiogenesis-inducing activity of LLC-GFP cells. Notably, the anti-angiogenic activity of vitamin D may contribute to its overall anti-cancerous effects. Patients with lung cancer have a very poor prognosis. It is therefore important to develop a therapeutic agent with antiproliferative effects as well as the potential to control angiogenesis-inducing factor and matrix metalloproteinase gene transcription, but which itself has minimal side-effects. The present results indicate that 22-oxa-1α,25-D 3 meets these criteria.
In the present study, we examined the effect of 22-oxa-1α,25-D 3 as a prophylactic for the treatment of lung metastasis. In a clinical setting, this would represent a situation similar to the use of tamoxifen to prevent the recurrence of breast cancer in patients without evidence of tumor spread. Our data clearly indicate the suppression of lung metastasis and tumor-induced angiogenesis on the continuous administration of 22-oxa-1α,25-D 3 . This effect occurs without a significant elevation in the plasma level of calcium, indicating that this analog could be administered safely without undesirable side-effects.
In conclusion, 22-oxa-1α,25-D 3 is highly effective in reducing metastatic lung carcinoma associated with angiogenesis and warrants further study as a therapeutic agent.
We acknowledge the excellent technical assistance provided by Keiko Omori, Saori Kimura, Mima Shimomukai, Yuki Okubo and Kayo Kajioka. This work was supported by the project for the Advanced Research and Technology by Kobe Pharmaceutical University and grants-in-aid received from the Japanese Ministry of Education (12672139, 13218129 and 15590083).
References
Ginsberg,R.J., Vokes,E.E. and Raben,A. (
Young,M.R.I., Ihm,J., Lozano,Y., Wright,M.A. and Prechel,M.M. (
Young,M.R. and Lozano,Y. (
Eisman,J.A., Barkla,D.H. and Tutton,P.J.M. (
Lokeshwar,B.L., Schwartz,G.G., Selzer,M.G., Burnstein,K.L., Zhuang,S.H., Block,N.L. and Binderup,L. (
Cross,H.S., Kallay,E., Farhan,H., Weiland,T. and Manhardt,T. (
Mantell,D.J., Owens,P.E., Bundred,N.J., Mawer,E.B. and Canfield,A.E. (
Abe,J., Morikawa,M., Miyamoto,K., Kaiho,S., Fukushima,M., Miyaura,C., Abe,E., Suda,T. and Nishii,Y. (
Abe,J., Nakano,T., Nishii,Y., Matsumoto,T., Ogata,E. and Ikeda,K. (
Kobayashi,T., Tsugawa,N., Okano,T., Masuda,S., Takeuchi,A., Kubodera,N. and Nishii,Y. (
Oikawa,T., Yoshida,Y., Shimamura,M., Ashino-Fuse,H., Iwaguchi,T. and Tominaga,T. (
Rashidi,B., Yang,M., Jiang,P., Baranov,E., An,Z., Wang,X., Moossa,A.R. and Hoffman,R.M. (
Nakagawa,K., Kawaura,A., Kato,S., Takeda,E. and Okano,T. (
Hovey,R.C., Goldhar,A.S., Baffi,J. and Vonderhaar,B.K. (
Koli,K. and Keski-Oja,J. (
Sakamoto,N., Tanaka,N.G., Tohgo,A. and Ogawa,H. (
Kragh,M., Hjarnaa,P-N.V., Bramm,E., Kristjansen,P.E.G., Rygaard,J. and Binderup,L. (
Yoshizawa,T., Handa,Y., Uematsu,Y., Takeda,S., Sekine,K., Yoshihara,Y., Kawakami,T., Arioka,K., Sato,H., Uchiyama,Y., Masushige,S., Fukamizu,A., Matsumoto,T. and Kato,S. (
Ferrara,N. (
Burtis,W.J., Brady,T.G., Orloff,J.J., Ersbak,J.B., Warrell,R.P., Olson,B.R., Wu,T.L., Mitnick,M.E., Broadus,A.E. and Stewart,A.F. (
Bendre,M., Gaddy,D., Nicholas,R.W. and Suva,L.J. (
Maeda,Y., Yamato,H., Hirai,T., Kobori,N., Fujii,T., Kobayashi,Y., Saitoh,K., Inoguchi,E., Hakozaki,M. and Iijima,H. (
Coven,V. and Kburi,F.R. (
James,S.Y., MacKay,A.G. and Colston,K.W. (
James,S.Y., Mercer,E., Brady,L. and Colston,K. (
Nakagawa,K., Kurobe,M., Konno,K., Fujishima,T., Takayama,H. and Okano,T. (
Nakagawa,K., Sowa,Y., Kurobe,M., Ozono,K., Siu-Caldera,M.L., Reddy,G.S., Uskokovic,M.R. and Okano,T. (
Abe-Hashimoto,J., Kikuchi,T., Matsumoto,T., Nishii,Y., Ogata,E. and Ikeda,K. (
Barroga,E.F., Kadosawa,T., Okumura,M. and Fujinaga,T. (
Hansen,C.M, Binderup,L., Hamberg,K., and Carlberg,C. Vitamin D and cancer: (
Wang,Q.M., Jones,J.B. and Studzinski,G.P. (
Munker,R., Kobayashi,T., Elstner,E., Norman,A.W., Uskokovic,M. and Zhang,W. (
Kumagai,T, O'Kelly,J., Said,J.W. and Koeffler,P. (
Schwartz,G.G., Wang,M.H., Zang,M., Singh,R.K. and Siegal,G.P. (
Lacraz,S., Dayer,J.M., Nicod,L. and Welgus,H.G. (
Bian,J. and Sun,Y. (
Sato,H., Kita,M. and Seiki,M. (
Hisatake,J., O'Kelly,J., Uskokovic,M.R., Tomoyasu,S. and Koeffler,H.P. (
Iseki,K., Tatsuta,M., Uehara,H., Yano,H., Sakai,N. and Ishiguro,S. (
Schlaeppi,J.M., Gutzwiller,S., Finkenzeller,G. and Fournier,B. (
Lin,R., Amizuka,N., Sasaki,T., Aarts,M.M., Ozawa,H., Goltzman,D., Henderson,J.E. and White,J.H. (
Falzon,M. and Zong,J. (
Falzon,M. (
Tovar Sepulveda,V.A. and Falzon,M. (
Ionescu,A.M., Schwarz,E.M., Vinson,C., Puzas,J.E., Rosier,R., Reynolds,P.R. and O'Keefe,R.J. (
Mohamed,M.I., Beckman,M.J., Meehan,J. and DeLuca,H.F. (
Author notes
1Department of Hygienic Sciences, Kobe Pharmaceutical University, Japan, 2Institute of Molecular and Cellular Bioscience, University of Tokyo, Japan and 3Chugai Pharmaceutical Co. Ltd, Japan
- angiogenesis
- vascular endothelial growth factor a
- hypocalcemia
- hypercalcemia
- lung
- cancer
- gelatinase b
- intravenous injections
- neoplasm metastasis
- osmosis
- rna, messenger
- mice
- neoplasms
- vitamin d
- lung cancer
- metastasis to the lung
- tumor cells
- parathyroid hormone-related protein
- poisoning by vitamin d
- tumor cells, malignant