-
PDF
- Split View
-
Views
-
Cite
Cite
Alexis Ceecee Zhang, Manikkuwadura Eranda Harshan De Silva, Richard J MacIsaac, Leslie Roberts, Jordan Kamel, Jennifer P Craig, Ljoudmila Busija, Laura E Downie, Omega-3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta-analysis, Nutrition Reviews, Volume 78, Issue 4, April 2020, Pages 323–341, https://doi.org/10.1093/nutrit/nuz054
Close - Share Icon Share
Abstract
Peripheral nerve damage can occur in a variety of systemic conditions and can have a profound impact on functional and psychological health. Currently, therapeutic interventions for peripheral nerve damage are limited.
The aim of this systematic review, conducted in accordance with the Cochrane Collaboration’s handbook and reported according to the PRISMA checklist, was to evaluate the efficacy and safety of omega-3 oral supplements for improving peripheral nerve structure and function.
PubMed, Embase, and Cochrane databases, along with clinical trial registries, were searched from inception to February 2019. Evidence was identified, critically appraised, and synthesized, and the certainty of evidence was appraised using the Grading of Recommendations Assessment, Development and Evaluation approach.
Randomized controlled trials assessing the effects of omega-3 oral supplementation on outcomes of peripheral nerve structure, peripheral nerve function, or both were eligible for inclusion. Titles and abstracts of identified articles were independently assessed for potential eligibility by 2 review authors. For studies judged as eligible or potentially eligible, full text articles were retrieved and independently assessed by 2 review authors to determine eligibility; disagreements were resolved by consensus.
Fifteen trials were included. Two clinically similar studies that investigated the effect of omega-3 supplementation in individuals receiving chemotherapy were meta-analyzed. Pooled data showed a reduced incidence of peripheral neuropathy (RR = 0.58; 95%CI, 0.43–0.77) and a preservation of sensory nerve action potential amplitudes with omega-3 supplementation compared with placebo (MD = 4.19 µV; 95%CI; 2.19–6.19).
This review finds, with low certainty, that omega-3 supplementation attenuates sensory loss and reduces the incidence of neuropathy secondary to oxaliplatin and paclitaxel treatment relative to placebo. There is currently limited evidence to ascertain whether omega-3 supplementation is beneficial in other systemic conditions characterized by peripheral nerve damage.
PROSPERO registration number CRD 42018086297
INTRODUCTION
The peripheral nervous system mediates sensory, motor, and autonomic functions outside of the brain and spinal cord.1 Function of the peripheral nervous system depends on the anatomical integrity of the system and integrated neural communication across the body.2 Damage to the peripheral nervous system can result in peripheral neuropathy, which adversely affects nerve function, leading to sensory deficits, motor imbalance, or autonomic dysfunction.3 Peripheral neuropathy is a potential complication of multiple systemic conditions, most commonly diabetes mellitus. Other potential causes include infection, inflammation, hereditary conditions, neurotoxicity, and trauma.3
Current management regimens for peripheral neuropathy are typically based on treating the underlying condition(s) and addressing their functional impact. There are some specific conditions, such as chronic inflammatory demyelinating polyneuropathy, Guillain-Barré syndrome, and vasculitic neuropathy, which respond to immunomodulatory treatment.4 However, for most etiologies, including diabetes mellitus, there are currently no established disease-modifying treatments to limit progressive peripheral nerve damage.5 Peripheral neuropathy induced by neurotoxic substances, such as those used in chemotherapy, has long-term negative impacts on the quality of life of cancer survivors.6 Epidemiological studies show that progressive peripheral neuropathy can be associated with certain lifestyle factors, including cigarette smoking and obesity, which affect systemic inflammatory pathways and vascular regulation.7 There is also scientific rationale that an individual’s diet may influence the progression of peripheral neuropathy. A potassium-restrictive diet has been shown to be neuroprotective for peripheral nerve function in individuals at risk of peripheral neuropathy.8 Additionally, omega-3 fatty acid consumption has the potential to affect peripheral nerve health.
Omega-3 fatty acids are one of two major classes of essential polyunsaturated fatty acids (PUFAs) that cannot be synthesized in vivo and thus must be derived from the diet or supplementation. Short-chain omega-3 fatty acids, such as α-linoleic acid, are found in plant sources and are a metabolic precursor to the long-chain omega-3 PUFAs (docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]), which are found mainly in marine sources. Once ingested, omega-3 PUFAs induce a range of biological effects, including the favoring of prostaglandin metabolism toward the production of anti-inflammatory and neuroprotective metabolites; this occurs in competition with the omega-6 pathway, which yields mostly proinflammatory prostaglandins.9
Predisposition to systemic inflammation is affected by the balance of omega-3 to omega-6 PUFA consumption.10 Systematic reviews indicate that diets rich in omega-3 PUFAs are associated with a range of potential health benefits, including lowering of systemic triglycerides and improvement in early cognitive development.11–13 Docosahexaenoic acid is an integral component of neuronal phospholipid membranes and is involved in cortical and visual development.14 Docosahexaenoic acid deficiency has been associated with impaired neuronal function and the development of neurodegenerative disorders.15 Preclinical evidence supports a role for omega-3 fatty acids in promoting neuronal survival and axonal regeneration following peripheral nerve injury.16 Neuroprotectin D-1, a DHA metabolite, reduces oxidative stress–induced cellular apoptosis by suppressing proapoptotic signaling and attenuating proinflammatory responses.17 Both DHA and neuroprotectin D-1 have therapeutic potential in neurodegenerative conditions, such as Alzheimer disease and spinal cord injury,15,18 as well as in corneal injuries, to promote small nerve fiber regeneration.19 Various preclinical studies have associated omega-3 supplementation with neuroprotection, mitigation of functional losses, and promotion of neurite growth.16,20 If similar benefits are evident in clinical populations, omega-3 fatty acids may provide a means to improve peripheral nerve health and, potentially, to have application in the clinical management of peripheral neuropathy.
The aim of this systematic review, conducted according to the guidelines in the Cochrane Collaboration’s handbook and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist,21 was to synthesize the current, best-available evidence on the efficacy and safety of oral omega-3 supplementation for improving peripheral nerve health in order to inform clinical practice and future research in the field.
METHODS
This systematic review was reported in line with the PRISMA statement (see Appendix S1 in the Supporting Information online)21 and conducted according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions.22 The protocol was published23 and prospectively registered on PROSPERO (CRD 42018086297).
Study eligibility criteria
Studies were selected for inclusion on the basis of prespecified PICOS (Population, Intervention, Comparator, Outcomes, Study design) criteria (Table 1). Only randomized controlled trials (RCTs) were included, to minimize potential confounding effects from less robust study designs. Published conference abstracts were eligible for inclusion. Eligible studies included adult participants in whom at least 1 subjective measure of peripheral neuropathy, 1 composite measure of peripheral neuropathy, or 1 objective measure of peripheral nerve structure or function was performed. Studies in which the intervention was administered as a dietary manipulation or in which the intervention was administered in combination with another intervention were excluded, unless the cointervention was administered in the same dose and frequency as in the comparator group.
PICOS criteria for inclusion of studies
| Parameter . | Study selection criteria . |
|---|---|
| Population | Adult participants, aged > 18 years, recruited from within any study setting, in whom the structure or function of peripheral nerves was assessed |
| Intervention | Long- or short-chain omega-3 oral supplements, in any form or dosage |
| Comparator | Placebo or no intervention |
| Outcomes | Primary and secondary outcomes, including both objective and subjective measures of peripheral nerve structure or function (details of prespecified outcomes are described in Table S2 in the Supporting Information online) |
| Study design | Randomized controlled clinical trials, published in any year or language |
| Parameter . | Study selection criteria . |
|---|---|
| Population | Adult participants, aged > 18 years, recruited from within any study setting, in whom the structure or function of peripheral nerves was assessed |
| Intervention | Long- or short-chain omega-3 oral supplements, in any form or dosage |
| Comparator | Placebo or no intervention |
| Outcomes | Primary and secondary outcomes, including both objective and subjective measures of peripheral nerve structure or function (details of prespecified outcomes are described in Table S2 in the Supporting Information online) |
| Study design | Randomized controlled clinical trials, published in any year or language |
PICOS criteria for inclusion of studies
| Parameter . | Study selection criteria . |
|---|---|
| Population | Adult participants, aged > 18 years, recruited from within any study setting, in whom the structure or function of peripheral nerves was assessed |
| Intervention | Long- or short-chain omega-3 oral supplements, in any form or dosage |
| Comparator | Placebo or no intervention |
| Outcomes | Primary and secondary outcomes, including both objective and subjective measures of peripheral nerve structure or function (details of prespecified outcomes are described in Table S2 in the Supporting Information online) |
| Study design | Randomized controlled clinical trials, published in any year or language |
| Parameter . | Study selection criteria . |
|---|---|
| Population | Adult participants, aged > 18 years, recruited from within any study setting, in whom the structure or function of peripheral nerves was assessed |
| Intervention | Long- or short-chain omega-3 oral supplements, in any form or dosage |
| Comparator | Placebo or no intervention |
| Outcomes | Primary and secondary outcomes, including both objective and subjective measures of peripheral nerve structure or function (details of prespecified outcomes are described in Table S2 in the Supporting Information online) |
| Study design | Randomized controlled clinical trials, published in any year or language |
Search methods
Comprehensive searches were performed in the following databases: Ovid MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), the US National Library of Health’s Clinical Trials registry, and the WHO International Clinical Trials Registry Platform. Searches are up to date as of February 21, 2019. The search strategies used are provided in Appendix S2 in the Supporting Information online.23
Data extraction and management
Two review authors independently extracted key study data (see Table S1 in the Supporting Information online) in Covidence24 and resolved any discrepancies by consensus. The following data were collected: study design, methodology, participants, interventions, outcomes, and other relevant information (eg, funding sources). Quantitative data were extracted for prespecified outcomes. Any discrepancies were resolved by discussion and consensus. Study authors were contacted when further information was required. Extracted data were exported into the Cochrane Review Manager software (RevMan)25 by 1 review author (A.C.Z.) and independently verified by a second review author (L.E.D.).
Assessment of risk of bias in included studies
Risk of bias was evaluated using the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.22 Two review authors independently assessed risk of bias in each study as low, unclear, or high in the following domains: (1) selection bias; (2) performance bias; (3) detection bias; (4) attrition bias; (5) reporting bias; and (6) other sources of bias. Disagreements were resolved by consensus.
Measures of treatment effect
Data for primary and secondary outcomes (see Table S2 in the Supporting Information online) were extracted as the mean change from baseline and the standard deviation of the change for the intervention and comparator groups. When change from baseline was not reported, the mean change and standard deviation of the change were extracted at the specified followed-up period in each group. The unit of analysis was the participant. In 1 study in which outcomes were measured in ocular tissues, the unit of analysis was the study eye of the enrolled participant.
Data synthesis
Meta-analyses were performed when the following conditions were met: (1) at least 2 studies reported data in a consistent format (eg, endpoint data); (2) there was an absence of significant statistical heterogeneity (I2< 60% and chi-square test P value > 0.10)23; and (3) the combining of the datasets was considered clinically meaningful (eg, same disease state). Clinical heterogeneity was assessed by considering differences in the characteristics of the intervention (eg, type, dose, and form) and the participants at baseline. A fixed-effect model was used for all meta-analyses that included fewer than 3 studies; otherwise, a random-effects model was used.25 For all adverse events, tabulated results summaries were generated.
The risk of reporting bias (due to selective outcome reporting) was assessed by comparing the outcomes described in trial registries with those in the publication(s). There were 7 studies that had been prospectively registered in a clinical trials registry; for these studies, the outcomes defined in the trial registry entries were compared with those reported in the publications. Subgroup analyses for prognostic factors (eg, type of disease, baseline severity of peripheral neuropathy, and age of participant) and potential intervention modifiers (eg, omega-3 dose, duration of intervention, and type of supplement) could not be performed because there was an insufficient number of trials (fewer than 2 studies per subgroup) to perform these analyses. Originally, the intent was to use funnel plots to detect any potential publication bias by considering relevant factors such as sample size and then interpreting any asymmetries in the funnel plot in association with the trial characteristics. However, as fewer than 10 studies were included in the meta-analyses, a sensitivity analysis could not be performed.
Summary of findings table
A summary of findings table (Table 2) 26–29 was produced for the following outcomes: (1) change in peripheral neuropathy impairment composite score; (2) pain; (3) change in corneal nerve fiber length; (4) sensory nerve action potential (SNAP) amplitudes of the sural nerve; and (5) motor nerve conduction velocity of the peroneal nerve. The GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach was used to assess the certainty of evidence.30
Summary of findings for the prespecified outcomes investigated in the present literature review
| Outcome . | No. of participants (no. of studies) . | Certainty of the evidence (GRADE)a . | Relative effect (95%CI) . | Anticipated absolute effects (95%CI)b
. |
|
|---|---|---|---|---|---|
| Placebo oral supplements . | Omega-3 oral supplements . | ||||
| PN impairments, assessed with a validated, composite neuropathy impairment score(s), with a follow-up range of 16–25 wk | 128 (2 RCTs) | Lowc | RR = 0.58 (0.43–0.77) | Risk: 758 per 1000 | Risk difference: 318 fewer per 1000 (432 fewer to 174 fewer) |
| Symptoms of PN | – | – | – | – | – |
| Pain, assessed using a validated, patient-assessed pain scale(s) at 35 d of follow-up | 41 (1 RCT) | Very lowd | – | Mean pain score: 47.2 | MD relative to placebo: 4.0 units higher (10.18 lower to 18.18 higher) |
| Change in CNFL, defined as the total length of nerves in a given area (mm/mm2), assessed with laser-scanning IVCM at 90 d of follow-up | 12 (1 RCT) | Lowe | – | Mean change in CNFL: −2.7 mm/mm2 | MD relative to placebo: 5.6 mm/mm2 higher (2.31 higher to 8.89 higher) |
| IENFD | – | – | – | – | – |
| SNAP amplitudes (µV) of the sural nerve, with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean SNAP amplitudes of sural nerve: 6.02 µV | MD = 4.19 µV higher (2.19 higher to 6.19 higher) |
| Motor NCV of the peroneal nerve (m/s), with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean motor NCV of peroneal nerve: −43.0 m/s | MD = 1.99 m/s higher (0.51 lower to 4.49 higher) |
| Outcome . | No. of participants (no. of studies) . | Certainty of the evidence (GRADE)a . | Relative effect (95%CI) . | Anticipated absolute effects (95%CI)b
. |
|
|---|---|---|---|---|---|
| Placebo oral supplements . | Omega-3 oral supplements . | ||||
| PN impairments, assessed with a validated, composite neuropathy impairment score(s), with a follow-up range of 16–25 wk | 128 (2 RCTs) | Lowc | RR = 0.58 (0.43–0.77) | Risk: 758 per 1000 | Risk difference: 318 fewer per 1000 (432 fewer to 174 fewer) |
| Symptoms of PN | – | – | – | – | – |
| Pain, assessed using a validated, patient-assessed pain scale(s) at 35 d of follow-up | 41 (1 RCT) | Very lowd | – | Mean pain score: 47.2 | MD relative to placebo: 4.0 units higher (10.18 lower to 18.18 higher) |
| Change in CNFL, defined as the total length of nerves in a given area (mm/mm2), assessed with laser-scanning IVCM at 90 d of follow-up | 12 (1 RCT) | Lowe | – | Mean change in CNFL: −2.7 mm/mm2 | MD relative to placebo: 5.6 mm/mm2 higher (2.31 higher to 8.89 higher) |
| IENFD | – | – | – | – | – |
| SNAP amplitudes (µV) of the sural nerve, with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean SNAP amplitudes of sural nerve: 6.02 µV | MD = 4.19 µV higher (2.19 higher to 6.19 higher) |
| Motor NCV of the peroneal nerve (m/s), with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean motor NCV of peroneal nerve: −43.0 m/s | MD = 1.99 m/s higher (0.51 lower to 4.49 higher) |
Abbreviations: CNFL, corneal nerve fiber length; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; IENFD, intraepidermal nerve fiber density; IVCM, in vivo confocal microscopy; MD, mean difference; NCV, nerve conduction velocity; PN, peripheral neuropathy; RCT, randomized controlled trial; RR, risk ratio; SNAP, sensory nerve action potential. –, indicates no relevant data available.
GRADE Working Group has established 4 grades of evidence. High certainty means the authors are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty means the authors are moderately confident in the effect estimate, ie, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty means the authors’ confidence in the effect estimate is limited, ie, the true effect may be substantially different from the estimate of the effect; and very low certainty means the authors have very little confidence in the effect estimate, ie, the true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95%CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI).
Data derived from 2 studies (Esfahani et al,26 n = 71; Ghoreishi et al,27 n = 57); low number of events does not meet optimal information size (OIS) as defined in the GRADE handbook. Both studies were appraised as having a low risk of bias in most domains, but Esfahani et al26 was funded by industry. This was considered a crucial limitation for 1 criterion in 1 of the 2 included studies, sufficient to lower the confidence in the certainty of the effect.
Data derived from 1 study (Fontani et al,28 n = 46), which was industry funded. Study involved a small number of participants in whom the pain outcome was not measured within the context of peripheral neuropathy.
Data derived from 1 study (Chinnery et al,29 n = 12) that included a very small number of participants.
Summary of findings for the prespecified outcomes investigated in the present literature review
| Outcome . | No. of participants (no. of studies) . | Certainty of the evidence (GRADE)a . | Relative effect (95%CI) . | Anticipated absolute effects (95%CI)b
. |
|
|---|---|---|---|---|---|
| Placebo oral supplements . | Omega-3 oral supplements . | ||||
| PN impairments, assessed with a validated, composite neuropathy impairment score(s), with a follow-up range of 16–25 wk | 128 (2 RCTs) | Lowc | RR = 0.58 (0.43–0.77) | Risk: 758 per 1000 | Risk difference: 318 fewer per 1000 (432 fewer to 174 fewer) |
| Symptoms of PN | – | – | – | – | – |
| Pain, assessed using a validated, patient-assessed pain scale(s) at 35 d of follow-up | 41 (1 RCT) | Very lowd | – | Mean pain score: 47.2 | MD relative to placebo: 4.0 units higher (10.18 lower to 18.18 higher) |
| Change in CNFL, defined as the total length of nerves in a given area (mm/mm2), assessed with laser-scanning IVCM at 90 d of follow-up | 12 (1 RCT) | Lowe | – | Mean change in CNFL: −2.7 mm/mm2 | MD relative to placebo: 5.6 mm/mm2 higher (2.31 higher to 8.89 higher) |
| IENFD | – | – | – | – | – |
| SNAP amplitudes (µV) of the sural nerve, with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean SNAP amplitudes of sural nerve: 6.02 µV | MD = 4.19 µV higher (2.19 higher to 6.19 higher) |
| Motor NCV of the peroneal nerve (m/s), with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean motor NCV of peroneal nerve: −43.0 m/s | MD = 1.99 m/s higher (0.51 lower to 4.49 higher) |
| Outcome . | No. of participants (no. of studies) . | Certainty of the evidence (GRADE)a . | Relative effect (95%CI) . | Anticipated absolute effects (95%CI)b
. |
|
|---|---|---|---|---|---|
| Placebo oral supplements . | Omega-3 oral supplements . | ||||
| PN impairments, assessed with a validated, composite neuropathy impairment score(s), with a follow-up range of 16–25 wk | 128 (2 RCTs) | Lowc | RR = 0.58 (0.43–0.77) | Risk: 758 per 1000 | Risk difference: 318 fewer per 1000 (432 fewer to 174 fewer) |
| Symptoms of PN | – | – | – | – | – |
| Pain, assessed using a validated, patient-assessed pain scale(s) at 35 d of follow-up | 41 (1 RCT) | Very lowd | – | Mean pain score: 47.2 | MD relative to placebo: 4.0 units higher (10.18 lower to 18.18 higher) |
| Change in CNFL, defined as the total length of nerves in a given area (mm/mm2), assessed with laser-scanning IVCM at 90 d of follow-up | 12 (1 RCT) | Lowe | – | Mean change in CNFL: −2.7 mm/mm2 | MD relative to placebo: 5.6 mm/mm2 higher (2.31 higher to 8.89 higher) |
| IENFD | – | – | – | – | – |
| SNAP amplitudes (µV) of the sural nerve, with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean SNAP amplitudes of sural nerve: 6.02 µV | MD = 4.19 µV higher (2.19 higher to 6.19 higher) |
| Motor NCV of the peroneal nerve (m/s), with a follow-up range of 16–25 wk | 116 (2 RCTs) | Lowc | – | Mean motor NCV of peroneal nerve: −43.0 m/s | MD = 1.99 m/s higher (0.51 lower to 4.49 higher) |
Abbreviations: CNFL, corneal nerve fiber length; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; IENFD, intraepidermal nerve fiber density; IVCM, in vivo confocal microscopy; MD, mean difference; NCV, nerve conduction velocity; PN, peripheral neuropathy; RCT, randomized controlled trial; RR, risk ratio; SNAP, sensory nerve action potential. –, indicates no relevant data available.
GRADE Working Group has established 4 grades of evidence. High certainty means the authors are very confident that the true effect lies close to that of the estimate of the effect; moderate certainty means the authors are moderately confident in the effect estimate, ie, the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty means the authors’ confidence in the effect estimate is limited, ie, the true effect may be substantially different from the estimate of the effect; and very low certainty means the authors have very little confidence in the effect estimate, ie, the true effect is likely to be substantially different from the estimate of effect.
The risk in the intervention group (and its 95%CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95%CI).
Data derived from 2 studies (Esfahani et al,26 n = 71; Ghoreishi et al,27 n = 57); low number of events does not meet optimal information size (OIS) as defined in the GRADE handbook. Both studies were appraised as having a low risk of bias in most domains, but Esfahani et al26 was funded by industry. This was considered a crucial limitation for 1 criterion in 1 of the 2 included studies, sufficient to lower the confidence in the certainty of the effect.
Data derived from 1 study (Fontani et al,28 n = 46), which was industry funded. Study involved a small number of participants in whom the pain outcome was not measured within the context of peripheral neuropathy.
Data derived from 1 study (Chinnery et al,29 n = 12) that included a very small number of participants.
RESULTS
Characteristics of included studies
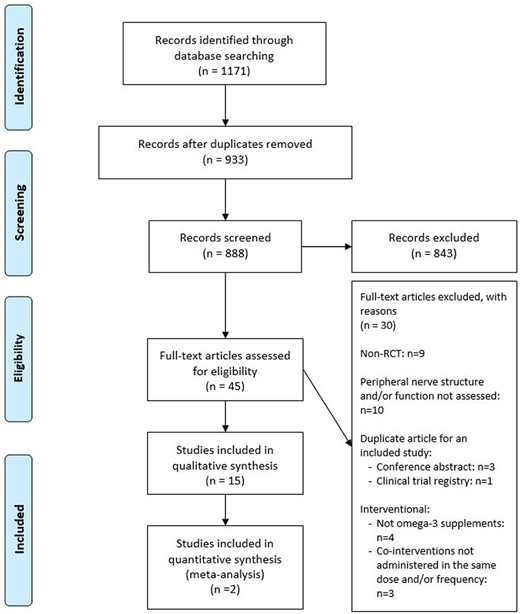
The electronic searches yielded 933 nonduplicate citations (Figure 1). Full texts judged to meet or potentially meet the eligibility criteria were obtained for 45 studies, including 3 articles that were translated into English (2 in Russian,31,32 1 in Chinese33). In total, 15 RCTs met the eligibility criteria and were included. Table S3 in the Supporting Information online provides a list of studies excluded after full-text review, along with the primary reason for exclusion.
Flow diagram of the literature search process. Abbreviation: RCT, randomized controlled trial.
Table 326–29,34–44 summarizes the main characteristics of the included studies. Detailed study information is provided in Table S1 in the Supporting Information online. Of the 15 RCTs, 12 were full-text articles,26–29,34–37,39,40,42,44 2 were conference abstracts,38,43 and 1 was a trial registry entry.41 Four of the studies were considered to be from the same trial (Wessex Evaluation of fatty Liver and Cardiovascular markers in non-alcoholic fatty liver disease with OMacor thErapy trial [WELCOME] trial), including 2 full-text articles37,39 and 2 conference abstracts.38,43 The trials were conducted in 7 countries: 1 in Australia,29 3 in Iran,26,27,34 1 in Italy,28 1 in Japan,42 1 in Spain,44 5 in the United Kingdom,37–40,43 and 3 in the United States.35,36,41
Main characteristics of the included studies
| Reference . | Article type . | Type of participants . | Intervention (dosage/day) . | Comparator (dosage/day) . | Follow-up period . |
|---|---|---|---|---|---|
| Anoushirvani et al (2018)34 | Full text | 63 adults undergoing Taxol treatment (omega-3 group, n=21; vitamin E group, n=21; control group, n=21) | Omega-3 capsules: 640 mg, 3 × /d (total dosage, 1920 mg/d); form NR | Placebo (type and dosage NR) | 3 mo |
| Carter et al (2012)35 | Full text | 67 adults with normotension (treatment group, n=19; control group, n=19) or prehypertension (treatment group, n=15; control group, n=14) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Carter et al (2013)36 | Full text | 67 adults with normotension who were placed under mental stress (treatment group, n=34; control group, n=33) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Chinnery et al (2017)29 | Full text | 12 adults with moderate dry eye disease (treatment group, n=8; control group, n=4) | Omega-3 capsules containing DHA (≈ 500 mg) + EPA (≈ 1000 mg) in either re-esterified triacylglyceride or phospholipid form | Olive oil (1500 mg) | 90 d |
| Clough et al (2016)37,a | Full text | 90 adults with NAFLD, with or without T2DM, without diabetic neuropathy or retinopathy (treatment group, n=44; control group, n=46) | Omacor omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | Olive oil (4000 mg), reported in clinical trials registry | 15–18 mo |
| Esfahani et al (2016)26 | Full text | 71 adults with metastatic colon cancer (stage III), undergoing treatment with IV oxaliplatin (130 mg/m2) and oral capecitabine (1000 mg/m2, twice daily) for 8 cycles (treatment group, n=36; control group, n=35) | Omega-3 pearls (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 25 wk |
| Fontani et al (2010)28 | Full text | 46 female adults with fibromyalgia or widespread musculoskeletal pain (treatment group, n=23; control group, n=23) | Omega-3 capsules (4000 mg) containing DHA (800 mg), EPA (1600 mg), and other types of omega-3 PUFAs: α-linolenic, stearidonic, eicosatetraenoic, and docosapentaenoic (400 mg); form NR | Sunflower oil containing oleic acid (4000 mg) | 35 d |
| Ghoreishi et al (2012)27 | Full text | 69b female adults with breast cancer, undergoing treatment with 4 cycles of paclitaxel, 175 mg/m2 (treatment group, n=30; control group, n=27) | Omega-3 capsules (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 16 wk |
| McCormick et al (2015a)38,a | Conference abstract | 101 adults with NAFLD, with and without T2DM, without diabetic neuropathy (assignment NR) | Omega-3 (4000 mg) in ethyl ester form | Placebo, type NR | 15–18 mo |
| McCormick et al (2015b)39,a | Full text | 100 adults with NAFLD, with or without T2DM, without overt neuropathy or retinopathy (treatment group, n=51; control group, n=49) | Omacor omega-3 capsules (4000 mg) containing DHA (1520 mg) + EPA (1840 mg) in ethyl ester form | Olive oil (4000 mg) containing ≈ 67% oleic acid, ≈ 15% linoleic acid, ≈ 15% palmitic acid, ≈ 2% stearic acid, ≈ 1% α-linolenic acid | 15–18 mo |
| Monahan et al (2004)40 | Full text | 18 healthy adults being put under physiological stress (treatment group, n=9; control group, n=9) | Omega-3 capsules (10 000 mg) containing DHA (2000 mg) + EPA (3000 mg); form NR | Olive oil (10 000 mg) | 1 mo |
| Clinical trial, ID NCT00931879 (2017)41 | Clinical trials registry | 38 adults with T2DM (treatment group, n=19; control group, n=19) | Lovaza omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | NR | 12 mo |
| Ochi et al (2017)42 | Full text | 21 healthy adults undergoing eccentric contraction exercises (treatment group, n=10; control group, n=11) | Fish oil capsules (2400 mg) containing DHA (260 mg) + EPA (600 mg); form NR | Corn oil (2400 mg) | 62 d |
| Palmer et al (2014)43,a | Conference abstract | 86 adults with nonalcoholic fatty liver disease (assignment NR) | High-dose purified omega-3 fatty acids (4000 mg) | Placebo, type NR | 15–18 mo |
| Stiefel et al (1999)44 | Full text | 18 adults with T1DM (treatment group, n=8; control group, n=10) | Omega-3 capsules containing DHA (330 mg) + EPA (630 mg); form NR | Usual diet, no supplements | 90 d |
| Reference . | Article type . | Type of participants . | Intervention (dosage/day) . | Comparator (dosage/day) . | Follow-up period . |
|---|---|---|---|---|---|
| Anoushirvani et al (2018)34 | Full text | 63 adults undergoing Taxol treatment (omega-3 group, n=21; vitamin E group, n=21; control group, n=21) | Omega-3 capsules: 640 mg, 3 × /d (total dosage, 1920 mg/d); form NR | Placebo (type and dosage NR) | 3 mo |
| Carter et al (2012)35 | Full text | 67 adults with normotension (treatment group, n=19; control group, n=19) or prehypertension (treatment group, n=15; control group, n=14) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Carter et al (2013)36 | Full text | 67 adults with normotension who were placed under mental stress (treatment group, n=34; control group, n=33) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Chinnery et al (2017)29 | Full text | 12 adults with moderate dry eye disease (treatment group, n=8; control group, n=4) | Omega-3 capsules containing DHA (≈ 500 mg) + EPA (≈ 1000 mg) in either re-esterified triacylglyceride or phospholipid form | Olive oil (1500 mg) | 90 d |
| Clough et al (2016)37,a | Full text | 90 adults with NAFLD, with or without T2DM, without diabetic neuropathy or retinopathy (treatment group, n=44; control group, n=46) | Omacor omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | Olive oil (4000 mg), reported in clinical trials registry | 15–18 mo |
| Esfahani et al (2016)26 | Full text | 71 adults with metastatic colon cancer (stage III), undergoing treatment with IV oxaliplatin (130 mg/m2) and oral capecitabine (1000 mg/m2, twice daily) for 8 cycles (treatment group, n=36; control group, n=35) | Omega-3 pearls (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 25 wk |
| Fontani et al (2010)28 | Full text | 46 female adults with fibromyalgia or widespread musculoskeletal pain (treatment group, n=23; control group, n=23) | Omega-3 capsules (4000 mg) containing DHA (800 mg), EPA (1600 mg), and other types of omega-3 PUFAs: α-linolenic, stearidonic, eicosatetraenoic, and docosapentaenoic (400 mg); form NR | Sunflower oil containing oleic acid (4000 mg) | 35 d |
| Ghoreishi et al (2012)27 | Full text | 69b female adults with breast cancer, undergoing treatment with 4 cycles of paclitaxel, 175 mg/m2 (treatment group, n=30; control group, n=27) | Omega-3 capsules (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 16 wk |
| McCormick et al (2015a)38,a | Conference abstract | 101 adults with NAFLD, with and without T2DM, without diabetic neuropathy (assignment NR) | Omega-3 (4000 mg) in ethyl ester form | Placebo, type NR | 15–18 mo |
| McCormick et al (2015b)39,a | Full text | 100 adults with NAFLD, with or without T2DM, without overt neuropathy or retinopathy (treatment group, n=51; control group, n=49) | Omacor omega-3 capsules (4000 mg) containing DHA (1520 mg) + EPA (1840 mg) in ethyl ester form | Olive oil (4000 mg) containing ≈ 67% oleic acid, ≈ 15% linoleic acid, ≈ 15% palmitic acid, ≈ 2% stearic acid, ≈ 1% α-linolenic acid | 15–18 mo |
| Monahan et al (2004)40 | Full text | 18 healthy adults being put under physiological stress (treatment group, n=9; control group, n=9) | Omega-3 capsules (10 000 mg) containing DHA (2000 mg) + EPA (3000 mg); form NR | Olive oil (10 000 mg) | 1 mo |
| Clinical trial, ID NCT00931879 (2017)41 | Clinical trials registry | 38 adults with T2DM (treatment group, n=19; control group, n=19) | Lovaza omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | NR | 12 mo |
| Ochi et al (2017)42 | Full text | 21 healthy adults undergoing eccentric contraction exercises (treatment group, n=10; control group, n=11) | Fish oil capsules (2400 mg) containing DHA (260 mg) + EPA (600 mg); form NR | Corn oil (2400 mg) | 62 d |
| Palmer et al (2014)43,a | Conference abstract | 86 adults with nonalcoholic fatty liver disease (assignment NR) | High-dose purified omega-3 fatty acids (4000 mg) | Placebo, type NR | 15–18 mo |
| Stiefel et al (1999)44 | Full text | 18 adults with T1DM (treatment group, n=8; control group, n=10) | Omega-3 capsules containing DHA (330 mg) + EPA (630 mg); form NR | Usual diet, no supplements | 90 d |
Abbreviations: CNFL, corneal nerve fiber length; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IENFD, intraepidermal nerve fiber density; IVCM, in vivo confocal microscope; IV, intravenous; MD, mean difference; NAFLD, nonalcoholic fatty liver disease; NCV, nerve conduction velocity; NR, not reported; PN, peripheral neuropathy; PUFA, polyunsaturated fatty acid; RR, risk ratio; SNAP, sensory nerve action potential; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
As these studies are derived from the same trial (the WELCOME study), data may derive from the same cohort of participants.
Twelve participants (5 in treatment group, 7 in placebo group) were lost to follow-up.
Main characteristics of the included studies
| Reference . | Article type . | Type of participants . | Intervention (dosage/day) . | Comparator (dosage/day) . | Follow-up period . |
|---|---|---|---|---|---|
| Anoushirvani et al (2018)34 | Full text | 63 adults undergoing Taxol treatment (omega-3 group, n=21; vitamin E group, n=21; control group, n=21) | Omega-3 capsules: 640 mg, 3 × /d (total dosage, 1920 mg/d); form NR | Placebo (type and dosage NR) | 3 mo |
| Carter et al (2012)35 | Full text | 67 adults with normotension (treatment group, n=19; control group, n=19) or prehypertension (treatment group, n=15; control group, n=14) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Carter et al (2013)36 | Full text | 67 adults with normotension who were placed under mental stress (treatment group, n=34; control group, n=33) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Chinnery et al (2017)29 | Full text | 12 adults with moderate dry eye disease (treatment group, n=8; control group, n=4) | Omega-3 capsules containing DHA (≈ 500 mg) + EPA (≈ 1000 mg) in either re-esterified triacylglyceride or phospholipid form | Olive oil (1500 mg) | 90 d |
| Clough et al (2016)37,a | Full text | 90 adults with NAFLD, with or without T2DM, without diabetic neuropathy or retinopathy (treatment group, n=44; control group, n=46) | Omacor omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | Olive oil (4000 mg), reported in clinical trials registry | 15–18 mo |
| Esfahani et al (2016)26 | Full text | 71 adults with metastatic colon cancer (stage III), undergoing treatment with IV oxaliplatin (130 mg/m2) and oral capecitabine (1000 mg/m2, twice daily) for 8 cycles (treatment group, n=36; control group, n=35) | Omega-3 pearls (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 25 wk |
| Fontani et al (2010)28 | Full text | 46 female adults with fibromyalgia or widespread musculoskeletal pain (treatment group, n=23; control group, n=23) | Omega-3 capsules (4000 mg) containing DHA (800 mg), EPA (1600 mg), and other types of omega-3 PUFAs: α-linolenic, stearidonic, eicosatetraenoic, and docosapentaenoic (400 mg); form NR | Sunflower oil containing oleic acid (4000 mg) | 35 d |
| Ghoreishi et al (2012)27 | Full text | 69b female adults with breast cancer, undergoing treatment with 4 cycles of paclitaxel, 175 mg/m2 (treatment group, n=30; control group, n=27) | Omega-3 capsules (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 16 wk |
| McCormick et al (2015a)38,a | Conference abstract | 101 adults with NAFLD, with and without T2DM, without diabetic neuropathy (assignment NR) | Omega-3 (4000 mg) in ethyl ester form | Placebo, type NR | 15–18 mo |
| McCormick et al (2015b)39,a | Full text | 100 adults with NAFLD, with or without T2DM, without overt neuropathy or retinopathy (treatment group, n=51; control group, n=49) | Omacor omega-3 capsules (4000 mg) containing DHA (1520 mg) + EPA (1840 mg) in ethyl ester form | Olive oil (4000 mg) containing ≈ 67% oleic acid, ≈ 15% linoleic acid, ≈ 15% palmitic acid, ≈ 2% stearic acid, ≈ 1% α-linolenic acid | 15–18 mo |
| Monahan et al (2004)40 | Full text | 18 healthy adults being put under physiological stress (treatment group, n=9; control group, n=9) | Omega-3 capsules (10 000 mg) containing DHA (2000 mg) + EPA (3000 mg); form NR | Olive oil (10 000 mg) | 1 mo |
| Clinical trial, ID NCT00931879 (2017)41 | Clinical trials registry | 38 adults with T2DM (treatment group, n=19; control group, n=19) | Lovaza omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | NR | 12 mo |
| Ochi et al (2017)42 | Full text | 21 healthy adults undergoing eccentric contraction exercises (treatment group, n=10; control group, n=11) | Fish oil capsules (2400 mg) containing DHA (260 mg) + EPA (600 mg); form NR | Corn oil (2400 mg) | 62 d |
| Palmer et al (2014)43,a | Conference abstract | 86 adults with nonalcoholic fatty liver disease (assignment NR) | High-dose purified omega-3 fatty acids (4000 mg) | Placebo, type NR | 15–18 mo |
| Stiefel et al (1999)44 | Full text | 18 adults with T1DM (treatment group, n=8; control group, n=10) | Omega-3 capsules containing DHA (330 mg) + EPA (630 mg); form NR | Usual diet, no supplements | 90 d |
| Reference . | Article type . | Type of participants . | Intervention (dosage/day) . | Comparator (dosage/day) . | Follow-up period . |
|---|---|---|---|---|---|
| Anoushirvani et al (2018)34 | Full text | 63 adults undergoing Taxol treatment (omega-3 group, n=21; vitamin E group, n=21; control group, n=21) | Omega-3 capsules: 640 mg, 3 × /d (total dosage, 1920 mg/d); form NR | Placebo (type and dosage NR) | 3 mo |
| Carter et al (2012)35 | Full text | 67 adults with normotension (treatment group, n=19; control group, n=19) or prehypertension (treatment group, n=15; control group, n=14) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Carter et al (2013)36 | Full text | 67 adults with normotension who were placed under mental stress (treatment group, n=34; control group, n=33) | Fish oil pills (9000 mg) containing DHA (1100 mg) + EPA (1600 mg); form NR | Olive oil (9000 mg) | 8 wk |
| Chinnery et al (2017)29 | Full text | 12 adults with moderate dry eye disease (treatment group, n=8; control group, n=4) | Omega-3 capsules containing DHA (≈ 500 mg) + EPA (≈ 1000 mg) in either re-esterified triacylglyceride or phospholipid form | Olive oil (1500 mg) | 90 d |
| Clough et al (2016)37,a | Full text | 90 adults with NAFLD, with or without T2DM, without diabetic neuropathy or retinopathy (treatment group, n=44; control group, n=46) | Omacor omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | Olive oil (4000 mg), reported in clinical trials registry | 15–18 mo |
| Esfahani et al (2016)26 | Full text | 71 adults with metastatic colon cancer (stage III), undergoing treatment with IV oxaliplatin (130 mg/m2) and oral capecitabine (1000 mg/m2, twice daily) for 8 cycles (treatment group, n=36; control group, n=35) | Omega-3 pearls (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 25 wk |
| Fontani et al (2010)28 | Full text | 46 female adults with fibromyalgia or widespread musculoskeletal pain (treatment group, n=23; control group, n=23) | Omega-3 capsules (4000 mg) containing DHA (800 mg), EPA (1600 mg), and other types of omega-3 PUFAs: α-linolenic, stearidonic, eicosatetraenoic, and docosapentaenoic (400 mg); form NR | Sunflower oil containing oleic acid (4000 mg) | 35 d |
| Ghoreishi et al (2012)27 | Full text | 69b female adults with breast cancer, undergoing treatment with 4 cycles of paclitaxel, 175 mg/m2 (treatment group, n=30; control group, n=27) | Omega-3 capsules (1920 mg) containing DHA (1037 mg) + EPA (192 mg); form NR | Sunflower oil (dosage NR) | 16 wk |
| McCormick et al (2015a)38,a | Conference abstract | 101 adults with NAFLD, with and without T2DM, without diabetic neuropathy (assignment NR) | Omega-3 (4000 mg) in ethyl ester form | Placebo, type NR | 15–18 mo |
| McCormick et al (2015b)39,a | Full text | 100 adults with NAFLD, with or without T2DM, without overt neuropathy or retinopathy (treatment group, n=51; control group, n=49) | Omacor omega-3 capsules (4000 mg) containing DHA (1520 mg) + EPA (1840 mg) in ethyl ester form | Olive oil (4000 mg) containing ≈ 67% oleic acid, ≈ 15% linoleic acid, ≈ 15% palmitic acid, ≈ 2% stearic acid, ≈ 1% α-linolenic acid | 15–18 mo |
| Monahan et al (2004)40 | Full text | 18 healthy adults being put under physiological stress (treatment group, n=9; control group, n=9) | Omega-3 capsules (10 000 mg) containing DHA (2000 mg) + EPA (3000 mg); form NR | Olive oil (10 000 mg) | 1 mo |
| Clinical trial, ID NCT00931879 (2017)41 | Clinical trials registry | 38 adults with T2DM (treatment group, n=19; control group, n=19) | Lovaza omega-3 capsules containing DHA + EPA (combined 4000 mg/d) in ethyl ester form | NR | 12 mo |
| Ochi et al (2017)42 | Full text | 21 healthy adults undergoing eccentric contraction exercises (treatment group, n=10; control group, n=11) | Fish oil capsules (2400 mg) containing DHA (260 mg) + EPA (600 mg); form NR | Corn oil (2400 mg) | 62 d |
| Palmer et al (2014)43,a | Conference abstract | 86 adults with nonalcoholic fatty liver disease (assignment NR) | High-dose purified omega-3 fatty acids (4000 mg) | Placebo, type NR | 15–18 mo |
| Stiefel et al (1999)44 | Full text | 18 adults with T1DM (treatment group, n=8; control group, n=10) | Omega-3 capsules containing DHA (330 mg) + EPA (630 mg); form NR | Usual diet, no supplements | 90 d |
Abbreviations: CNFL, corneal nerve fiber length; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IENFD, intraepidermal nerve fiber density; IVCM, in vivo confocal microscope; IV, intravenous; MD, mean difference; NAFLD, nonalcoholic fatty liver disease; NCV, nerve conduction velocity; NR, not reported; PN, peripheral neuropathy; PUFA, polyunsaturated fatty acid; RR, risk ratio; SNAP, sensory nerve action potential; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
As these studies are derived from the same trial (the WELCOME study), data may derive from the same cohort of participants.
Twelve participants (5 in treatment group, 7 in placebo group) were lost to follow-up.
Studies had sample sizes ranging from 12 to 101 participants and examined the effects of omega-3 PUFA oral supplementation in clinical populations that were undergoing chemotherapy26,27,34 or had non-alcoholic fatty liver disease,37–39,43 mental or physiological stress,36,40,42 diabetes mellitus,41,44 dry eye disease,29 chronic inflammatory pain,28 or prehypertension.35 The follow-up periods of included studies ranged from 1 to 18 months. Thirteen of the included studies assessed peripheral nerve structure or function in the upper or lower extremities (ie, arm or leg).26–28,35–42,44 One study assessed peripheral nerve structure in the cornea,29 and 1 conference abstract did not report the location of the peripheral nerve assessment.43 The unit of analysis for all studies was the participant, except for the 1 study in which peripheral nerve assessment was conducted in the eye.29 In that case, the unit of analysis was the enrolled eye of the participant.
The daily dose of omega-3 supplements ranged from 640 mg/d to 5000 mg/d (DHA and EPA combined). The DHA component ranged from 260 mg/d to 2000 mg/d, and the EPA component ranged from 192 mg/d to 3000 mg/d. None of the omega-3 interventions were administered with any other cointerventions. One study included an additional intervention arm involving a vitamin E treatment.34 For the comparator (control) intervention, 6 studies used olive oil supplements,29,35–37,39,40 3 used sunflower oil supplements,26–28 1 used corn oil supplements,42 1 used usual diet (ie, no supplement intervention),44 and 4 did not specify the form or concentration of the placebo.34,38,41,43
Risk of bias in included studies
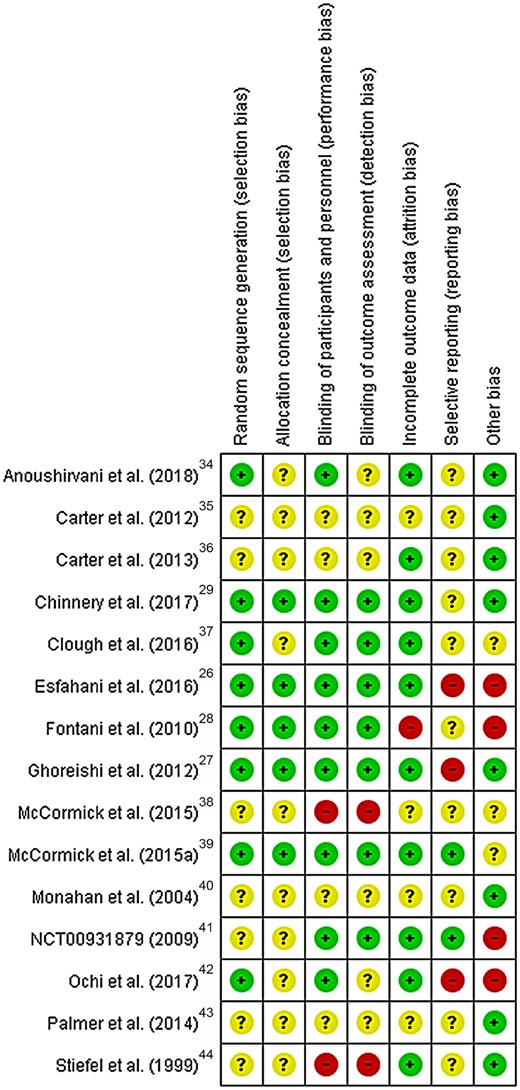
Figure 226–29,34–44 summarizes the risk of bias assessments. No study was judged as having a low risk of bias in all 7 domains (see Figure S1 in the Supporting Information online). The domains with the most number of studies judged to have a low risk of bias were attrition bias (10 of 15 studies judged to have low risk) and blinding of participants and personnel (9 of 15 studies judged to have low risk). None of the studies were considered to have a high risk of selection bias. The domains with the most number of studies judged to have a high risk of bias were reporting bias related to incomplete outcome reporting (3 of 15 studies judged to have high risk) and other risk of bias (4 of 15 studies judged to have high risk because of industry funding).
Risk of bias summary. Review authors' judgments about each risk of bias item for each included study. Abbreviation and symbols: RCT, randomized controlled trial; +, high risk of bias; −, low risk of bias; ?, unclear risk of bias.
Effects of interventions
Table 2 summarizes findings for the prespecified outcomes. There were no extractable data for the following outcomes: peripheral neuropathy symptoms; patient-reported disability; intraepidermal nerve fiber density; minimum F-wave latency of the peroneal, tibial, median, or ulnar nerve; corneal sensory function; or warm detection thresholds in the skin.
Primary outcome
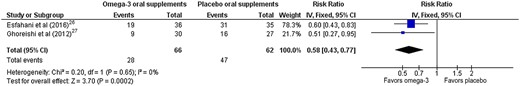
Two studies reported data on the incidence of peripheral neuropathy, quantified using a validated composite score.26,27 Both studies considered populations at risk of chemotherapy-induced peripheral neuropathy, where none of the participants had peripheral neuropathy at baseline, and the incidence of neuropathy was quantified at the study endpoint using the reduced Total Neuropathy Score.45 Pooled data (Figure 3)26,27 assessed at the end of the follow-up period (ranging from 16 to 25 weeks) showed a significant reduction in the relative risk (RR) of developing peripheral neuropathy with omega-3 supplementation (RR = 0.58; 95%CI, 0.43–0.77; P = 0.0002; n = 128 participants) relative to placebo. The level of heterogeneity was negligible (I2 = 0%). One additional study reported the incidence of peripheral neuropathy in a population at risk of chemotherapy-induced peripheral neuropathy.34 Results from this study could not be pooled because the type of scoring system was not reported by the authors, and a response was not received from the authors 4 weeks after requesting this information via email. In this study, the incidence of chemotherapy-induced peripheral neuropathy in the omega-3 group was reported to be 28.6% (6 of 21 participants), compared with 71.4% (15 of 21 participants) in the placebo group.34 The certainty of evidence, judged using GRADE, was low (downgraded because of imprecision and risk of bias).
Forest plot of comparison for incidence of peripheral neuropathy in chemotherapy-induced peripheral neuropathy. Omega-3 vs placebo oral supplements for peripheral neuropathy impairments (quantified by reduced Total Neuropathy Score). Data are reported as the incidence of peripheral neuropathy at the end of the follow-up period, which ranged from 16 weeks (Ghoreishi et al27) to 25 weeks (Esfahani et al26). Abbreviation: IV, inverse variance.
Secondary outcomes
Pain
One study reported data related to pain intensity, measured using visual analog scores ranging from 0 to 100, in individuals with chronic inflammatory disorders.28 No significant difference was observed between the omega-3 and placebo supplement groups after 35 days (mean ± standard error of the mean [SEM]: omega-3, 47.2 ± 4.7, n = 23 participants, vs placebo, 43.2 ± 5.5, n = 23 participants; mean difference [MD] calculated using values reported in the article: 4.0 units, 95%CI, −10.18 to 18.18; P = 0.58). The certainty of evidence, judged using GRADE, was very low (downgraded for risk of bias, indirectness, and imprecision, as data were from 1 small study [n = 46 participants] that was industry funded, and pain outcomes were not measured within the context of peripheral neuropathy).
Anatomical markers
Data related to the change from baseline in central corneal nerve fiber length, defined as the total length of all nerve fibers in the image capture frame (mm/mm2) obtained with in vivo confocal microscopy, were collected from 1 study.29 In this RCT, a significant increase in corneal nerve fiber length in people with dry eye disease was reported after 90 days of omega-3 PUFA oral supplementation compared with placebo (mean ± SEM: omega-3, 2.90 ± 1.6 mm/mm2, n = 8 participants, vs placebo, −2.7 ± 1.6 mm/mm2, n = 4 participants; P = 0.01). The certainty of evidence for this outcome, judged using GRADE, was low (downgraded for imprecision, as data were from a single study involving only 12 participants).
Nerve conduction studies
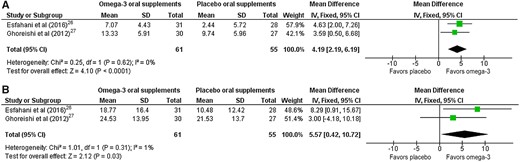
For nerve conduction studies, a quantitative data synthesis was performed to combine 2 studies26,27 in which the clinical questions were similar and the study outcomes were reported as means (± standard deviations) or medians (interquartile ranges) at the end of follow-up (summarized in Table 426,27,41,44 and Table 526,27,41,44). Both of these trials investigated the effects of omega-3 supplementation on the development of chemotherapy-induced peripheral neuropathy. One additional study reported on the effects of omega-3 supplementation on the development of chemotherapy-induced peripheral neuropathy34; however, it could not be included in the meta-analyses as the results reported in the article could not be interpreted, and no response from the authors to a request for missing outcome data was received. Results as reported in the publication are supplied in Table S1 in the Supporting Information online.
Results of the sensory nerve conduction studies included in the present review
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| SNAP amplitudes (µV) | Sural nerve | MD=4.19; 95%CI, 2.19–6.19; P < 0.0001, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD=0.32; 95%CI, −4.38 to 5.02; P = 0.89) | No significant intergroup difference in change from baseline (MD = −4.20; 95%CI, −8.72 to 0.32; P = 0.07) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=3.6; 95%CI, −8.02 to 15.22; P = 0.54) | No significant intergroup difference in change from baseline between groups (MD = −3.6; 95%CI, −11.54 to 4.34; P = 0.37) | |
| Ulnar nerve | MD=5.57; 95%CI, 0.42–10.72; P = 0.03, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD = −5.28; 95%CI, −17.33 to 6.77; P = 0.37) | No data available | |
| SNAP latencies (ms) | Sural nerve | No data available | No significant intergroup difference in endpoint values (MD=0.02; 95%CI, −0.38 to 0.42; P = 0.92) | No data available |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD = −0.47; 95%CI, −1.19 to 0.25; P = 0.20) | No data available | |
| Ulnar nerve | No data available | No significant intergroup difference in endpoint values (MD=0.11; 95%CI, −0.22 to 0.44; P = 0.52) | No data available | |
| Sensory NCV (m/s) | Sural nerve | Because of considerable statistical heterogeneity (I2 = 87%), a pooled estimate is not presented. Individual study findings: Esfahani et al (2016)26: significant difference in endpoint values favoring the omega-3 group: omega-3 (42.29 ± 15.51 m/s) vs placebo (29.67 ± 13.01 m/s); P = 0.018 Ghoreishi et al (2012)22: no significant intergroup difference in endpoint values: omega-3 (54.79 ± 8.35 m/s) vs placebo (52.52 ± 8.05 m/s); P = 0.514 | No significant intergroup difference in endpoint values (MD = −0.42; 95%CI, −4.84 to 3.98; P = 0.85) | No significant intergroup difference in change from baseline (MD = −2.87; 95%CI, −6.78 to 1.04; P = 0.15) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=2.83; 95%CI, −4.23 to 9.89; P = 0.43) | No significant intergroup difference in change from baseline (MD = −0.48; 95%CI, −2.09 to 1.13; P = 0.56) | |
| Ulnar nerve | No significant effect: MD=2.21; 95%CI, −0.64 to 5.06; P = 0.13 | No significant intergroup difference in endpoint values (MD = −1.72; 95%CI, −6.54 to 3.10; P = 0.48) | No data available |
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| SNAP amplitudes (µV) | Sural nerve | MD=4.19; 95%CI, 2.19–6.19; P < 0.0001, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD=0.32; 95%CI, −4.38 to 5.02; P = 0.89) | No significant intergroup difference in change from baseline (MD = −4.20; 95%CI, −8.72 to 0.32; P = 0.07) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=3.6; 95%CI, −8.02 to 15.22; P = 0.54) | No significant intergroup difference in change from baseline between groups (MD = −3.6; 95%CI, −11.54 to 4.34; P = 0.37) | |
| Ulnar nerve | MD=5.57; 95%CI, 0.42–10.72; P = 0.03, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD = −5.28; 95%CI, −17.33 to 6.77; P = 0.37) | No data available | |
| SNAP latencies (ms) | Sural nerve | No data available | No significant intergroup difference in endpoint values (MD=0.02; 95%CI, −0.38 to 0.42; P = 0.92) | No data available |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD = −0.47; 95%CI, −1.19 to 0.25; P = 0.20) | No data available | |
| Ulnar nerve | No data available | No significant intergroup difference in endpoint values (MD=0.11; 95%CI, −0.22 to 0.44; P = 0.52) | No data available | |
| Sensory NCV (m/s) | Sural nerve | Because of considerable statistical heterogeneity (I2 = 87%), a pooled estimate is not presented. Individual study findings: Esfahani et al (2016)26: significant difference in endpoint values favoring the omega-3 group: omega-3 (42.29 ± 15.51 m/s) vs placebo (29.67 ± 13.01 m/s); P = 0.018 Ghoreishi et al (2012)22: no significant intergroup difference in endpoint values: omega-3 (54.79 ± 8.35 m/s) vs placebo (52.52 ± 8.05 m/s); P = 0.514 | No significant intergroup difference in endpoint values (MD = −0.42; 95%CI, −4.84 to 3.98; P = 0.85) | No significant intergroup difference in change from baseline (MD = −2.87; 95%CI, −6.78 to 1.04; P = 0.15) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=2.83; 95%CI, −4.23 to 9.89; P = 0.43) | No significant intergroup difference in change from baseline (MD = −0.48; 95%CI, −2.09 to 1.13; P = 0.56) | |
| Ulnar nerve | No significant effect: MD=2.21; 95%CI, −0.64 to 5.06; P = 0.13 | No significant intergroup difference in endpoint values (MD = −1.72; 95%CI, −6.54 to 3.10; P = 0.48) | No data available |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; MD, mean difference; NCV, nerve conduction velocity; SNAP, sensory nerve action potential; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Data are reported as the mean difference in endpoint values between omega-3 supplementation group and placebo group at the end of follow-up (at 6 months of follow-up, with acceptable follow-up periods ranging between 3 and 9 months from baseline).
Mean differences and P values are calculated on the basis of endpoint data provided (mean ± standard error of the mean) in the clinical trials registry for the omega-3 supplementation group compared with the placebo group, at 12 months follow-up.
Mean differences and P values are calculated on the basis of values provided (as change from baseline) for the omega-3 supplementation group compared with the placebo group, at 12 months of follow-up.
Results of the sensory nerve conduction studies included in the present review
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| SNAP amplitudes (µV) | Sural nerve | MD=4.19; 95%CI, 2.19–6.19; P < 0.0001, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD=0.32; 95%CI, −4.38 to 5.02; P = 0.89) | No significant intergroup difference in change from baseline (MD = −4.20; 95%CI, −8.72 to 0.32; P = 0.07) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=3.6; 95%CI, −8.02 to 15.22; P = 0.54) | No significant intergroup difference in change from baseline between groups (MD = −3.6; 95%CI, −11.54 to 4.34; P = 0.37) | |
| Ulnar nerve | MD=5.57; 95%CI, 0.42–10.72; P = 0.03, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD = −5.28; 95%CI, −17.33 to 6.77; P = 0.37) | No data available | |
| SNAP latencies (ms) | Sural nerve | No data available | No significant intergroup difference in endpoint values (MD=0.02; 95%CI, −0.38 to 0.42; P = 0.92) | No data available |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD = −0.47; 95%CI, −1.19 to 0.25; P = 0.20) | No data available | |
| Ulnar nerve | No data available | No significant intergroup difference in endpoint values (MD=0.11; 95%CI, −0.22 to 0.44; P = 0.52) | No data available | |
| Sensory NCV (m/s) | Sural nerve | Because of considerable statistical heterogeneity (I2 = 87%), a pooled estimate is not presented. Individual study findings: Esfahani et al (2016)26: significant difference in endpoint values favoring the omega-3 group: omega-3 (42.29 ± 15.51 m/s) vs placebo (29.67 ± 13.01 m/s); P = 0.018 Ghoreishi et al (2012)22: no significant intergroup difference in endpoint values: omega-3 (54.79 ± 8.35 m/s) vs placebo (52.52 ± 8.05 m/s); P = 0.514 | No significant intergroup difference in endpoint values (MD = −0.42; 95%CI, −4.84 to 3.98; P = 0.85) | No significant intergroup difference in change from baseline (MD = −2.87; 95%CI, −6.78 to 1.04; P = 0.15) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=2.83; 95%CI, −4.23 to 9.89; P = 0.43) | No significant intergroup difference in change from baseline (MD = −0.48; 95%CI, −2.09 to 1.13; P = 0.56) | |
| Ulnar nerve | No significant effect: MD=2.21; 95%CI, −0.64 to 5.06; P = 0.13 | No significant intergroup difference in endpoint values (MD = −1.72; 95%CI, −6.54 to 3.10; P = 0.48) | No data available |
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| SNAP amplitudes (µV) | Sural nerve | MD=4.19; 95%CI, 2.19–6.19; P < 0.0001, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD=0.32; 95%CI, −4.38 to 5.02; P = 0.89) | No significant intergroup difference in change from baseline (MD = −4.20; 95%CI, −8.72 to 0.32; P = 0.07) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=3.6; 95%CI, −8.02 to 15.22; P = 0.54) | No significant intergroup difference in change from baseline between groups (MD = −3.6; 95%CI, −11.54 to 4.34; P = 0.37) | |
| Ulnar nerve | MD=5.57; 95%CI, 0.42–10.72; P = 0.03, favoring omega-3 supplements | No significant intergroup difference in endpoint values (MD = −5.28; 95%CI, −17.33 to 6.77; P = 0.37) | No data available | |
| SNAP latencies (ms) | Sural nerve | No data available | No significant intergroup difference in endpoint values (MD=0.02; 95%CI, −0.38 to 0.42; P = 0.92) | No data available |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD = −0.47; 95%CI, −1.19 to 0.25; P = 0.20) | No data available | |
| Ulnar nerve | No data available | No significant intergroup difference in endpoint values (MD=0.11; 95%CI, −0.22 to 0.44; P = 0.52) | No data available | |
| Sensory NCV (m/s) | Sural nerve | Because of considerable statistical heterogeneity (I2 = 87%), a pooled estimate is not presented. Individual study findings: Esfahani et al (2016)26: significant difference in endpoint values favoring the omega-3 group: omega-3 (42.29 ± 15.51 m/s) vs placebo (29.67 ± 13.01 m/s); P = 0.018 Ghoreishi et al (2012)22: no significant intergroup difference in endpoint values: omega-3 (54.79 ± 8.35 m/s) vs placebo (52.52 ± 8.05 m/s); P = 0.514 | No significant intergroup difference in endpoint values (MD = −0.42; 95%CI, −4.84 to 3.98; P = 0.85) | No significant intergroup difference in change from baseline (MD = −2.87; 95%CI, −6.78 to 1.04; P = 0.15) |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=2.83; 95%CI, −4.23 to 9.89; P = 0.43) | No significant intergroup difference in change from baseline (MD = −0.48; 95%CI, −2.09 to 1.13; P = 0.56) | |
| Ulnar nerve | No significant effect: MD=2.21; 95%CI, −0.64 to 5.06; P = 0.13 | No significant intergroup difference in endpoint values (MD = −1.72; 95%CI, −6.54 to 3.10; P = 0.48) | No data available |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; MD, mean difference; NCV, nerve conduction velocity; SNAP, sensory nerve action potential; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Data are reported as the mean difference in endpoint values between omega-3 supplementation group and placebo group at the end of follow-up (at 6 months of follow-up, with acceptable follow-up periods ranging between 3 and 9 months from baseline).
Mean differences and P values are calculated on the basis of endpoint data provided (mean ± standard error of the mean) in the clinical trials registry for the omega-3 supplementation group compared with the placebo group, at 12 months follow-up.
Mean differences and P values are calculated on the basis of values provided (as change from baseline) for the omega-3 supplementation group compared with the placebo group, at 12 months of follow-up.
Results of the motor nerve conduction studies included in the present review
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
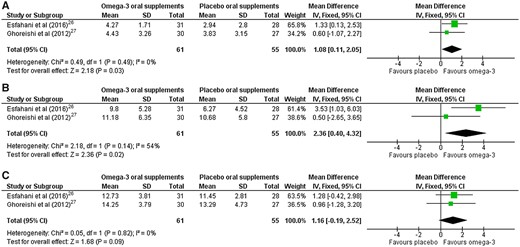
| Distal CMAP amplitudes (mV) | Peroneal nerve | MD=1.08; 95%CI, 0.11–2.05; P = 0.03, favoring omega-3 group | MD = −0.53; 95%CI, −0.78 to −0.28; P < 0.00001, favoring control group | No significant intergroup difference in change from baseline (MD = −4.03; 95%CI, −11.92 to 3.86; P=0.32) |
| Tibial nerve | MD=2.36; 95%CI, 0.40–4.32; P = 0.02, favoring omega-3 group | No significant intergroup difference in endpoint values (MD = −0.28; 95%CI, −0.70 to 0.14; P = 0.19) | No data available | |
| Median nerve | No data available | MD=0.85; 95%CI, 0.49–1.21; P < 0.00001, favoring omega-3 group | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −2.66 to 4.36; P=0.64) | |
| Ulnar nerve | No significant intergroup difference in change from baseline (MD=1.16; 95%CI, −0.19 to 2.52; P = 0.09) | No data available | No data available | |
| Distal CMAP latencies (ms) | Peroneal nerve | No significant intergroup difference in change from baseline (MD = −0.59; 95%CI, −1.28 to 0.09; P = 0.09) | No significant inter-group difference in endpoint values (MD = −0.38; 95%CI, −0.77 to 0.01; P = 0.06) | No data available |
| Tibial nerve | MD = –1.02; 95%CI, −1.45 to −0.59; P < 0.00001, favoring omega-3 group | MD=1.76; 95%CI, 1.38–2.14; P < 0.00001, favoring control group | No data available | |
| Median nerve | No data available | MD = −0.37; 95%CI, −0.62 to −0.12; P = 0.004, favoring omega-3 group | No data available | |
| Ulnar nerve | MD = –0.27; 95%CI, −0.53 to −0.01; P = 0.04, favoring omega-3 group | No data available | No data available | |
| Motor NCV (m/s) | Peroneal nerve | No significant intergroup difference (MD=1.99; 95%CI, −0.51 to 4.49; P = 0.12) | No significant intergroup difference in endpoint values (MD = −0.40; 95%CI, −1.25 to 0.45); P = 0.35) | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −1.63 to 3.33; P=0.50) |
| Tibial nerve | Because of considerable statistical heterogeneity (I2 = 74%), a pooled estimate is not presented. Both Esfahani et al26 and Ghoreishi et al27 reported no significant intergroup difference in endpoint values (Esfahani, mean ± SD, omega-3: 45.67 ± 6.1 m/s, vs placebo, 42.34 ± 4.68 m/s; P = 0.123. Ghoreishi, mean ± SD, omega-3: 45.16 ± 4.24 m/s, vs placebo, 46.03 ± 6.65 m/s; P = 0.359) | MD = −3.55; 95%CI, −6.59 to −0.51; P = 0.02, favoring control group | No data available | |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=0.85; 95%CI, −0.12 to 1.82; P = 0.08) | Significant change relative to baseline in the omega-3 group (+ 2.12 ± 1.35) vs placebo group (−0.80 ± 2.34; P < 0.01). Calculated MD=2.92; 95%CI, 1.19–4.65; P = 0.0009 |
|
| Ulnar nerve | No significant intergroup difference (MD=1.92; 95%CI, −1.19 to 5.02; P = 0.23) | No data available | No data available | |
| F-wave latencies(s) | Peroneal nerve | No data available | No data available | No data available |
| Tibial nerve | No data available | No data available | No data available | |
| Median nerve | No data available | No data available | No data available | |
| Ulnar nerve | No data available | No data available | No data available |
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| Distal CMAP amplitudes (mV) | Peroneal nerve | MD=1.08; 95%CI, 0.11–2.05; P = 0.03, favoring omega-3 group | MD = −0.53; 95%CI, −0.78 to −0.28; P < 0.00001, favoring control group | No significant intergroup difference in change from baseline (MD = −4.03; 95%CI, −11.92 to 3.86; P=0.32) |
| Tibial nerve | MD=2.36; 95%CI, 0.40–4.32; P = 0.02, favoring omega-3 group | No significant intergroup difference in endpoint values (MD = −0.28; 95%CI, −0.70 to 0.14; P = 0.19) | No data available | |
| Median nerve | No data available | MD=0.85; 95%CI, 0.49–1.21; P < 0.00001, favoring omega-3 group | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −2.66 to 4.36; P=0.64) | |
| Ulnar nerve | No significant intergroup difference in change from baseline (MD=1.16; 95%CI, −0.19 to 2.52; P = 0.09) | No data available | No data available | |
| Distal CMAP latencies (ms) | Peroneal nerve | No significant intergroup difference in change from baseline (MD = −0.59; 95%CI, −1.28 to 0.09; P = 0.09) | No significant inter-group difference in endpoint values (MD = −0.38; 95%CI, −0.77 to 0.01; P = 0.06) | No data available |
| Tibial nerve | MD = –1.02; 95%CI, −1.45 to −0.59; P < 0.00001, favoring omega-3 group | MD=1.76; 95%CI, 1.38–2.14; P < 0.00001, favoring control group | No data available | |
| Median nerve | No data available | MD = −0.37; 95%CI, −0.62 to −0.12; P = 0.004, favoring omega-3 group | No data available | |
| Ulnar nerve | MD = –0.27; 95%CI, −0.53 to −0.01; P = 0.04, favoring omega-3 group | No data available | No data available | |
| Motor NCV (m/s) | Peroneal nerve | No significant intergroup difference (MD=1.99; 95%CI, −0.51 to 4.49; P = 0.12) | No significant intergroup difference in endpoint values (MD = −0.40; 95%CI, −1.25 to 0.45); P = 0.35) | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −1.63 to 3.33; P=0.50) |
| Tibial nerve | Because of considerable statistical heterogeneity (I2 = 74%), a pooled estimate is not presented. Both Esfahani et al26 and Ghoreishi et al27 reported no significant intergroup difference in endpoint values (Esfahani, mean ± SD, omega-3: 45.67 ± 6.1 m/s, vs placebo, 42.34 ± 4.68 m/s; P = 0.123. Ghoreishi, mean ± SD, omega-3: 45.16 ± 4.24 m/s, vs placebo, 46.03 ± 6.65 m/s; P = 0.359) | MD = −3.55; 95%CI, −6.59 to −0.51; P = 0.02, favoring control group | No data available | |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=0.85; 95%CI, −0.12 to 1.82; P = 0.08) | Significant change relative to baseline in the omega-3 group (+ 2.12 ± 1.35) vs placebo group (−0.80 ± 2.34; P < 0.01). Calculated MD=2.92; 95%CI, 1.19–4.65; P = 0.0009 |
|
| Ulnar nerve | No significant intergroup difference (MD=1.92; 95%CI, −1.19 to 5.02; P = 0.23) | No data available | No data available | |
| F-wave latencies(s) | Peroneal nerve | No data available | No data available | No data available |
| Tibial nerve | No data available | No data available | No data available | |
| Median nerve | No data available | No data available | No data available | |
| Ulnar nerve | No data available | No data available | No data available |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; CMAP, compound motor action potential; MD, mean difference; NCV, nerve conduction velocity.
Data are reported as the mean difference in endpoint values between the omega-3 and placebo supplement groups at the end of the follow-up period (at 6 months of follow-up, with acceptable follow-up periods ranging between 3 and 9 months from baseline).
Mean differences and P values are calculated on the basis of the endpoint data provided (mean ± SEM) in the clinical trial registry for omega-3 supplementation and placebo groups at 12 months of follow-up.
Mean differences and P values are calculated on the basis of the values provided, as change from baseline, for the omega-3 supplementation group compared with the placebo group at 12 months of follow-up.
Results of the motor nerve conduction studies included in the present review
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| Distal CMAP amplitudes (mV) | Peroneal nerve | MD=1.08; 95%CI, 0.11–2.05; P = 0.03, favoring omega-3 group | MD = −0.53; 95%CI, −0.78 to −0.28; P < 0.00001, favoring control group | No significant intergroup difference in change from baseline (MD = −4.03; 95%CI, −11.92 to 3.86; P=0.32) |
| Tibial nerve | MD=2.36; 95%CI, 0.40–4.32; P = 0.02, favoring omega-3 group | No significant intergroup difference in endpoint values (MD = −0.28; 95%CI, −0.70 to 0.14; P = 0.19) | No data available | |
| Median nerve | No data available | MD=0.85; 95%CI, 0.49–1.21; P < 0.00001, favoring omega-3 group | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −2.66 to 4.36; P=0.64) | |
| Ulnar nerve | No significant intergroup difference in change from baseline (MD=1.16; 95%CI, −0.19 to 2.52; P = 0.09) | No data available | No data available | |
| Distal CMAP latencies (ms) | Peroneal nerve | No significant intergroup difference in change from baseline (MD = −0.59; 95%CI, −1.28 to 0.09; P = 0.09) | No significant inter-group difference in endpoint values (MD = −0.38; 95%CI, −0.77 to 0.01; P = 0.06) | No data available |
| Tibial nerve | MD = –1.02; 95%CI, −1.45 to −0.59; P < 0.00001, favoring omega-3 group | MD=1.76; 95%CI, 1.38–2.14; P < 0.00001, favoring control group | No data available | |
| Median nerve | No data available | MD = −0.37; 95%CI, −0.62 to −0.12; P = 0.004, favoring omega-3 group | No data available | |
| Ulnar nerve | MD = –0.27; 95%CI, −0.53 to −0.01; P = 0.04, favoring omega-3 group | No data available | No data available | |
| Motor NCV (m/s) | Peroneal nerve | No significant intergroup difference (MD=1.99; 95%CI, −0.51 to 4.49; P = 0.12) | No significant intergroup difference in endpoint values (MD = −0.40; 95%CI, −1.25 to 0.45); P = 0.35) | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −1.63 to 3.33; P=0.50) |
| Tibial nerve | Because of considerable statistical heterogeneity (I2 = 74%), a pooled estimate is not presented. Both Esfahani et al26 and Ghoreishi et al27 reported no significant intergroup difference in endpoint values (Esfahani, mean ± SD, omega-3: 45.67 ± 6.1 m/s, vs placebo, 42.34 ± 4.68 m/s; P = 0.123. Ghoreishi, mean ± SD, omega-3: 45.16 ± 4.24 m/s, vs placebo, 46.03 ± 6.65 m/s; P = 0.359) | MD = −3.55; 95%CI, −6.59 to −0.51; P = 0.02, favoring control group | No data available | |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=0.85; 95%CI, −0.12 to 1.82; P = 0.08) | Significant change relative to baseline in the omega-3 group (+ 2.12 ± 1.35) vs placebo group (−0.80 ± 2.34; P < 0.01). Calculated MD=2.92; 95%CI, 1.19–4.65; P = 0.0009 |
|
| Ulnar nerve | No significant intergroup difference (MD=1.92; 95%CI, −1.19 to 5.02; P = 0.23) | No data available | No data available | |
| F-wave latencies(s) | Peroneal nerve | No data available | No data available | No data available |
| Tibial nerve | No data available | No data available | No data available | |
| Median nerve | No data available | No data available | No data available | |
| Ulnar nerve | No data available | No data available | No data available |
| Study type . | Nerve . | Pooled data from 2 studies in individuals at risk of CIPN [Esfahani et al (2016)26 & Ghoreishi et al (2012)27]a . | Unpublished data extracted from 1 study in individuals with T2DM [clinical trial, ID NCT00931879 (2009)41]b . | Data extracted from 1 study in individuals with T1DM [Stiefel et al (1999)44]c . |
|---|---|---|---|---|
| Distal CMAP amplitudes (mV) | Peroneal nerve | MD=1.08; 95%CI, 0.11–2.05; P = 0.03, favoring omega-3 group | MD = −0.53; 95%CI, −0.78 to −0.28; P < 0.00001, favoring control group | No significant intergroup difference in change from baseline (MD = −4.03; 95%CI, −11.92 to 3.86; P=0.32) |
| Tibial nerve | MD=2.36; 95%CI, 0.40–4.32; P = 0.02, favoring omega-3 group | No significant intergroup difference in endpoint values (MD = −0.28; 95%CI, −0.70 to 0.14; P = 0.19) | No data available | |
| Median nerve | No data available | MD=0.85; 95%CI, 0.49–1.21; P < 0.00001, favoring omega-3 group | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −2.66 to 4.36; P=0.64) | |
| Ulnar nerve | No significant intergroup difference in change from baseline (MD=1.16; 95%CI, −0.19 to 2.52; P = 0.09) | No data available | No data available | |
| Distal CMAP latencies (ms) | Peroneal nerve | No significant intergroup difference in change from baseline (MD = −0.59; 95%CI, −1.28 to 0.09; P = 0.09) | No significant inter-group difference in endpoint values (MD = −0.38; 95%CI, −0.77 to 0.01; P = 0.06) | No data available |
| Tibial nerve | MD = –1.02; 95%CI, −1.45 to −0.59; P < 0.00001, favoring omega-3 group | MD=1.76; 95%CI, 1.38–2.14; P < 0.00001, favoring control group | No data available | |
| Median nerve | No data available | MD = −0.37; 95%CI, −0.62 to −0.12; P = 0.004, favoring omega-3 group | No data available | |
| Ulnar nerve | MD = –0.27; 95%CI, −0.53 to −0.01; P = 0.04, favoring omega-3 group | No data available | No data available | |
| Motor NCV (m/s) | Peroneal nerve | No significant intergroup difference (MD=1.99; 95%CI, −0.51 to 4.49; P = 0.12) | No significant intergroup difference in endpoint values (MD = −0.40; 95%CI, −1.25 to 0.45); P = 0.35) | No significant intergroup difference in change from baseline (MD=0.85; 95%CI, −1.63 to 3.33; P=0.50) |
| Tibial nerve | Because of considerable statistical heterogeneity (I2 = 74%), a pooled estimate is not presented. Both Esfahani et al26 and Ghoreishi et al27 reported no significant intergroup difference in endpoint values (Esfahani, mean ± SD, omega-3: 45.67 ± 6.1 m/s, vs placebo, 42.34 ± 4.68 m/s; P = 0.123. Ghoreishi, mean ± SD, omega-3: 45.16 ± 4.24 m/s, vs placebo, 46.03 ± 6.65 m/s; P = 0.359) | MD = −3.55; 95%CI, −6.59 to −0.51; P = 0.02, favoring control group | No data available | |
| Median nerve | No data available | No significant intergroup difference in endpoint values (MD=0.85; 95%CI, −0.12 to 1.82; P = 0.08) | Significant change relative to baseline in the omega-3 group (+ 2.12 ± 1.35) vs placebo group (−0.80 ± 2.34; P < 0.01). Calculated MD=2.92; 95%CI, 1.19–4.65; P = 0.0009 |
|
| Ulnar nerve | No significant intergroup difference (MD=1.92; 95%CI, −1.19 to 5.02; P = 0.23) | No data available | No data available | |
| F-wave latencies(s) | Peroneal nerve | No data available | No data available | No data available |
| Tibial nerve | No data available | No data available | No data available | |
| Median nerve | No data available | No data available | No data available | |
| Ulnar nerve | No data available | No data available | No data available |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; CMAP, compound motor action potential; MD, mean difference; NCV, nerve conduction velocity.
Data are reported as the mean difference in endpoint values between the omega-3 and placebo supplement groups at the end of the follow-up period (at 6 months of follow-up, with acceptable follow-up periods ranging between 3 and 9 months from baseline).
Mean differences and P values are calculated on the basis of the endpoint data provided (mean ± SEM) in the clinical trial registry for omega-3 supplementation and placebo groups at 12 months of follow-up.
Mean differences and P values are calculated on the basis of the values provided, as change from baseline, for the omega-3 supplementation group compared with the placebo group at 12 months of follow-up.
As for the other 2 studies that reported on this outcome, 1 RCT reported the change from baseline of sensory nerve conduction studies in the sural and median nerves and of motor nerve conduction studies in the median and peroneal nerves at 90 days of follow-up in individuals with type 1 diabetes.44 One unpublished study provided endpoint data from sensory nerve conduction studies in the sural, median, and ulnar nerves and from motor nerve conduction studies in the median, tibial, and peroneal nerves in a population with type 2 diabetes mellitus at 12 months of follow-up.41 Data from these 2 studies could not be pooled, owing to the reporting of data in different formats.
Sensory nerve conduction studies
As summarized in Table 4, pooled data for SNAP amplitudes from the 2 trials of chemotherapy-induced peripheral neuropathy showed a significant difference in mean values between the intervention and control groups, favoring omega-3 PUFA supplementation in both the sural nerve (MD=4.19 µV; 95%CI, 2.19–6.19; P < 0.0001; n = 116 participants) (Figure 4A)26,27 and the ulnar nerve (MD=5.57 µV; 95%CI, 0.42–10.72; P = 0.03; n = 116 participants) (Figure 4B)26,27 at the study endpoint (ranging from 16 to 25 weeks). For both these analyses, the level of heterogeneity was negligible (I2 < 2%).
Forest plots of comparison for sensory nerve action potential (SNAP) amplitudes. Omega-3 vs placebo oral supplements for the effect on SNAP amplitudes of the (A) sural nerve and (B) ulnar nerve (µV) in chemotherapy-induced peripheral neuropathy. Data are those reported at the end of the follow-up period, which ranged from 16 weeks (Ghoreishi et al27) to 25 weeks (Esfahani et al,26 data calculated from median and interquartile range). Abbreviation: IV, inverse variance.
One of these studies26 had significant intergroup differences at baseline, with the omega-3 PUFA supplement group having higher baseline SNAP amplitudes (sural nerve, median [interquartile range]: omega-3: 9.95 µV [5.52–16.40] vs placebo: 5.50 µV [3.40–14.10]; P = 0.033; ulnar nerve, median [interquartile range]: omega-3: 27.90 µV [16.82–42.77] vs placebo: 17.50 µV [12.40–35.50]; P = 0.144). In this study, the authors also reported the percentage change from baseline for SNAP amplitudes in both nerves, with a greater relative reduction in the placebo group (sural nerve, omega-3: −36.68%, vs placebo: −100.00%; P value not reported; ulnar nerve, omega-3: −18.28%, vs placebo: −42.06%; P value not reported). The certainty of evidence for the effect of omega-3 PUFA supplementation on sural SNAP amplitudes was considered low, on the basis of imprecision and risk of bias.
When data from the 2 trials26,27 were pooled, nerve conduction velocity in the ulnar nerve was not significantly different between groups (MD=2.21 m/s; 95%CI, −0.64 to 5.06; P = 0.13; n = 116 participants) (see Figure S2 in the Supporting Information online) at the end of the follow-up period (ranging from 16 to 25 weeks). For sural nerve conduction velocity, a pooled estimate is not presented because considerable statistical heterogeneity was evident (I2 = 87%). In the study by Esfahani et al,26 a significant difference was found, favoring the omega-3 intervention compared with placebo for sural nerve conduction velocity at the end of 25 weeks of follow-up (mean ± SD: omega-3, 42.29 ± 15.51 m/s, vs placebo, 29.67 ± 13.01 m/s; P = 0.018). Ghoreishi et al27 reported no significant intergroup difference at 16 weeks (mean ± SD: omega-3, 54.79 ± 8.35 m/s, vs placebo, 52.52 ± 8.05 m/s; P = 0.514).
Motor nerve conduction studies
Table 5 summarizes data related to motor nerve conduction studies. When data from the same 2 trials investigating chemotherapy-induced peripheral neuropathy26,27 were pooled, there was a significant intergroup difference in distal compound motor action potential amplitudes, favoring the omega-3 PUFA intervention, in both the peroneal nerve (MD=1.08 mV; 95%CI, 0.11–2.05; P = 0.03; n = 116 participants) (Figure 5A)26,27 and the tibial nerve (MD=2.36 mV; 95%CI, 0.40–4.32; P = 0.02; n = 116 participants) (Figure 5B)26,27 at the end of the follow-up period (ranging from 16 to 25 weeks). For both of these analyses, statistical heterogeneity was below the predefined threshold of significance (I2 < 60%).
Forest plots of comparison for compound motor action potential (CMAP) amplitudes. Omega-3 vs placebo oral supplements for distal CMAP amplitude of (A) the peroneal nerve, (B) the tibial nerve, and (C) the ulnar nerve (mV) in chemotherapy-induced peripheral neuropathy. Data are those reported at the end of the follow-up period, which ranged from 16 weeks (Ghoreishi et al.27) to 25 weeks (Esfahani et al,26 data calculated from median values and interquartile ranges). Abbreviation: IV, inverse variance.
No significant intergroup difference was evident for distal compound motor action potential amplitude of the ulnar nerve (MD=1.16 mV; 95%CI, −0.19 to 2.52; P = 0.09; n = 116 participants) (Figure 5C)26,27. There was a significant difference in distal compound motor action potential latency at the study endpoint, favoring the omega-3 intervention, in both the tibial nerve (MD = −1.02 ms; 95%CI, −1.45 to −0.59; P < 0.00001; n = 116 participants) (see Figure S3A in the Supporting Information online) and the ulnar nerve (MD = −0.27 ms; 95%CI, −0.53 to −0.01; P = 0.04; n = 116 participants) (see Figure S3B in the Supporting Information online). No significant intergroup differences were found in the distal compound motor action potential latency of the peroneal nerve (MD = −0.59 ms; 95%CI, −1.28 to 0.09; P = 0.09; n = 116 participants) (see Figure S3C in the Supporting Information online). For motor nerve conduction velocity, there were no significant differences between treatment groups in the peroneal (MD = 1.99 m/s; 95%CI, −0.51 to 4.49; P = 0.12; n = 116 participants) see (Figure S4A in the Supporting Information online) or ulnar nerves (MD = 1.92 m/s; 95%CI, −1.19 to 5.02; P = 0.23; n = 116 participants) (see Figure S4B in the Supporting Information online) at the end of the follow-up period. For each of these analyses, statistical heterogeneity was below the predefined threshold of significance. For motor nerve conduction velocity in the tibial nerve, a pooled estimate is not presented because considerable statistical heterogeneity was evident (I2= 74%). Neither study reported a significant intergroup difference in tibial nerve conduction velocity at the study endpoint (Table 5). The certainty of evidence for the effect of omega-3 PUFA supplementation on peroneal motor nerve conduction velocity, downgraded on the basis of imprecision and risk of bias, was considered low.
Small nerve fiber function
One study reported data related to small nerve fiber function, measured using Quantitative Sensory Testing, in a population with musculoskeletal pain and fibromyalgia.28 Although there was a reduction in the number of positive tender points clinically, the authors reported no significant difference between the omega-3 (n = 23 participants) and placebo (n = 22 participants) groups for mechanical thresholds (measured using von Frey hair esthesiometry, mean±SEM: omega-3, 432.0±47.0 g, vs placebo, 422±55.0 g; P values not reported), heat-pain thresholds (mean±SEM: omega-3, 41.7±0.5°C, vs placebo, 43.2±0.6°C; P values not reported), cold-detection thresholds (mean±SEM: omega-3, 7.7±1.1 s vs placebo, 6.9±1.3 s; P values not reported), or cold-pain thresholds (mean±SEM: omega-3, 0.2±1.8°C, vs placebo, −0.3±2.1°C; P values not reported) at 35 days of follow-up.
Another unpublished study reported the change from baseline in cold-detection thresholds and cold-pain thresholds (measured from the big toe) in people with type 2 diabetes at 12 months of follow-up.41 Intergroup differences were calculated using values provided in the clinical trial registry. Cold-detection thresholds were not significantly different between the omega-3 (n = 19 participants) and placebo (n = 19 participants) groups (mean±SEM: omega-3, −3.76±1.54°C, vs placebo, −1.17±1.18°C; calculated MD = −2.59°C; 95%CI, −6.57 to 1.38; P = 0.20). Omega-3 supplementation was calculated to preserve cold-pain thresholds as compared with placebo (omega-3, −1.41±2.16°C, vs placebo, 5.66±1.91°C; 95%CI, −12.71 to −1.42; P = 0.01) at 12 months of follow-up.
Adverse events. As summarized in Table S4 in the Supporting Information online, 5 trials reported data related to adverse events.27,28,37,39,41 Together, these studies involved 343 participants (omega-3, n = 172; placebo, n = 171). One study involving 46 participants reported no adverse events over a 35-day period.28 In 2 trials (Clough et al,37 n = 90 participants; McCormick et al,39 n = 100 participants), it was reported that there were no important adverse events over the course of each study (ranging from 450 to 540 days). In Ghoreishi et al,27 4 of 69 participants discontinued the study because of “critical conditions” (omega-3, n = 3, vs placebo, n = 1; calculated RR = 2.91; 95%CI; 0.32–26.66). Although there was no significant intergroup difference in the risk of developing a critical condition, this finding should be viewed with caution because of the low number of events and the wide confidence intervals. In the clinical trial NCT00931879,41 which involved 69 participants over a 180-day intervention period, a total of 10 adverse events (with 3 judged to be serious) occurred in individuals supplemented with omega-3 PUFAs, while 11 adverse events (with 4 judged to be serious) were reported in the placebo group.
Data from 3 studies were pooled to assess the risk of developing an adverse event.27,28,41 There was no significant difference in the relative risk of developing an adverse event (see Figure S5A in the Supporting Information online) with an omega-3 or placebo oral supplement (RR = 0.98; 95%CI, 0.56–1.70; P = 0.94; n = 148 participants). To assess the specific risk of a serious adverse event (defined as an event requiring hospitalization or prolonged admission, a life-threatening event, or death), data from 4 trials were combined,28,37,39,41 with no significant difference detected between interventions (RR = 0.50; 95%CI, 0.10–2.41; P = 0.39; n = 439 participants) (see Figure S5B in the Supporting Information online).
DISCUSSION
This is the first systematic review to consider the effects of omega-3 PUFA oral supplementation on peripheral nerve health. Fifteen relevant RCTs, involving a total of more than 590 adult participants, were identified. These RCTs reported data related to peripheral nerve structure or function in a variety of acute and chronic conditions, including chemotherapy-induced peripheral neuropathy and diabetes. On the basis of data from 2 trials, this review finds, with low certainty (as judged using the GRADE approach), that omega-3 fatty acid supplementation is beneficial for reducing the incidence of peripheral neuropathy secondary to neurotoxicity caused by the chemotherapeutic agents oxaliplatin and paclitaxel. Furthermore, there was low certainty that omega-3 supplementation may assist in preventing chemotherapy-induced neurotoxic sensory loss, as demonstrated by the relative preservation of SNAP amplitudes. Data from 4 trials indicated there were no significant concerns about the safety of omega-3 supplementation, with the relative risk of adverse events and serious adverse events being similar to those observed with a placebo intervention. There is currently limited RCT data to confidently draw conclusions about the effects of omega-3 PUFA oral supplementation in systemic conditions other than chemotherapy-induced peripheral neuropathy, ie, conditions in which peripheral nerves are also vulnerable to damage (eg, diabetes mellitus).
The methodological rigor of the trials included in this systematic review varied considerably. The domain showing the most variability was “other sources of bias,” with approximately 30% of studies being judged at high risk of bias due to commercial funding. In a recent systematic review, industry sponsorship of interventional studies was shown to significantly bias results toward more favorable conclusions about the sponsor’s products in comparison with studies not funded by industry.46 This limitation reduces both the overall certainty of the evidence and the ability to draw definitive conclusions.
Overall completeness and applicability of evidence
Studies investigating omega-3 PUFA supplementation in chemotherapy-induced peripheral neuropathy. The peripheral nervous system is particularly vulnerable to damage from neurotoxicity induced by chemotherapeutic agents.2 Chemotherapy-induced peripheral neuropathy produces a dose-limiting effect, affecting 30% to 70% of people undergoing chemotherapy, and can severely affect quality of life, as symptoms can persist after cessation of chemotherapy.47 Two common chemotherapeutic compounds are paclitaxel, used to treat breast, ovarian, and lung cancers, and oxaliplatin, typically used to treat colorectal cancer.6 Paclitaxel, a taxane-derived agent, has been proposed to interfere with microtubule-based axonal transport in the peripheral nerves, affecting predominantly distal sensory responses.48 Oxaliplatin, a platinum-based compound, induces peripheral neuropathy as 2 distinct clinical presentations. The acute form is characterized by altered sensory and motor symptoms in patients exposed to cold temperatures. These symptoms are caused by the chelating effects of the oxalate group on calcium and magnesium ions, thereby interrupting sodium currents and channel kinetics.49 In contrast, platinum binding to neuronal DNA induces a cumulative, sensory-predominant form of neuropathy, leading to axonal degradation, neuronal degeneration, and dorsal root ganglion apoptosis.50 Although paclitaxel and oxaliplatin are proposed to have different underlying mechanisms of neurotoxicity leading to axonal dysfunction, both compounds induce forms of peripheral neuropathy that share pathophysiological similarities and involve interrupted axonal transport, neuronal injury and inflammation, oxidative stress, and mitochondrial damage.51,52 Furthermore, both compounds can induce peripheral neuropathy, which, in its chronic form, manifests as cumulative sensory neuropathy or neuronopathy that affects sensory cell bodies of the dorsal root ganglion; these neurons are particularly vulnerable because they are not protected by the blood–nerve barrier.47,50
Pooled data from 2 studies (n = 128 participants) that considered the effects of omega-3 PUFA supplementation in oxaliplatin- or paclitaxel-induced neuropathies showed that omega-3 supplements in doses less than 2000 mg/d (EPA and DHA combined), taken over the course of chemotherapy and for 1 month after cessation, significantly reduced the incidence of peripheral neuropathy (RR=0.58; 95%CI, 0.43–0.77; P = 0.0002).26,27 In both trials, the scale for measuring the incidence of peripheral neuropathy was the reduced Total Neuropathy Score, a reliable, validated psychometric tool for assessing peripheral neuropathy.45 The reduced Total Neuropathy Score considers multiple domains: subjective sensory symptoms, pin sensibility, vibration sensibility, muscle strength, deep tendon reflexes, and amplitudes measured by nerve conduction studies of the sural and peroneal nerves.
Oxaliplatin- and paclitaxel-induced neuropathies frequently present as a progressive reduction of SNAP amplitudes, with relatively smaller changes in results of motor nerve conduction studies or needle electromyography.51,53 A decrease in sural SNAP amplitudes of more than 50% from baseline is used as a criterion for diagnosing sensory neuropathy associated with chemotherapy.51,53 The data presented here suggest that omega-3 PUFA supplements reduce sensory axonal loss induced by neurotoxic chemotherapy, relative to placebo, as indicated by the pooled results for sural SNAP amplitudes (MD = 4.19 µV; 95%CI, 2.19–6.19; P < 0.0001). However, the capacity for this analysis to derive clear estimates of overall treatment effects may be affected by intergroup baseline differences in 1 trial (ie, the placebo group had lower baseline SNAP amplitudes). Despite this difference, in both RCTs, the omega-3 PUFA supplementation group had a lower relative change from baseline in SNAP amplitude compared with the placebo group (36.68% vs 100% with oxaliplatin)26 and no change vs 28.9% with paclitaxel.27 With regard to the motor nerve conduction studies, the omega-3 intervention group showed more favorable compound motor action potential amplitudes of the peroneal and tibial nerves and lower tibial distal motor latency at the end of the follow-up period compared with the placebo group. However, in 1 of the 2 studies,26 the baseline distal motor latencies throughout the study appeared very prolonged; these values are considered outside of the normal limits of any known techniques or laboratories. Additionally, the temperature of patient extremities, which can significantly impact the parameters recorded, was not mentioned, and relative differences between groups could potentially cause spurious results.
Currently, there are no therapeutic agents recommended for prophylaxis of chemotherapy-induced peripheral neuropathy. The modification of chemotherapy dose and the cessation of chemotherapy are considered the most successful approaches for preventing functional compromise.6,47 International clinical guidelines published by the American Society of Clinical Oncology Clinical Practice, Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers,54 report that, on the basis of a dearth of high-quality, consistent evidence, further research is essential to identify agents for preventing chemotherapy-induced peripheral neuropathy. These guidelines referenced the results of 1 of the RCTs included in the present review, which involved 57 participants with breast cancer,27 but did not recommend prescribing omega-3 fatty acid supplements for prevention of chemotherapy-induced peripheral neuropathy at that time, given that the “promising-appearing result [had] not been replicated.”54 The same investigator group recently published another RCT involving 71 participants undergoing chemotherapy for metastatic colon cancer.26 The results lend further support to the potential ability of omega-3 supplementation to reduce sensory nerve damage, although larger-scale, multicenter trials are still lacking.
Studies investigating omega-3 supplementation for other causes of peripheral neuropathy. It was not possible to quantitatively synthesize data from RCTs that investigated omega-3 PUFA oral supplementation for other causes of peripheral neuropathy, as studies considered different disease states, used alternate outcome measures, did not report data quantitatively, or reported data in different formats (eg, change from baseline vs endpoint).
Two trials assessed an omega-3 intervention in people with diabetes mellitus.41,44 Of these studies, 1 included individuals with type 2 diabetes and reported no significant difference in any routine parameter of nerve conduction studies between the omega-3 and placebo supplement groups.41 However, baseline data for these participants were not provided. The other trial included people with type 1 diabetes.44 In this study, the only statistically significant difference was a relatively preserved median nerve conduction velocity in the omega-3 intervention arm. However, all lower limb sensory and motor parameters, including sural SNAP amplitudes and conduction velocities, were comparable between groups. Given that diabetes-related neuropathy typically presents in a length-dependent manner, with sensory symptoms developing before motor signs of weakness, the clinical significance of an isolated difference in a forearm motor parameter in nerve conduction studies is questionable. Furthermore, in these studies, neither temperature nor baseline data were reported, which significantly limits the interpretation of these results.
Notably, few of the included trials quantified the structure or function of small nerve fibers. One study quantified small nerve fiber structure in the cornea,29 and 2 trials reported on small nerve fiber function in the skin, measured using quantitative sensory testing.28,41 While routine diagnostic procedures such as nerve conduction studies are validated, reliable methods for quantifying the function of large nerve fibers,55 these techniques have limited capacity to detect changes in the integrity of small nerve fibers, which may precede involvement of large nerve fibers in peripheral neuropathy.56 Monitoring changes to small nerve fibers, which have regenerative capacity, may also provide a more accurate reflection of the efficacy of therapeutic interventions.57,58 In this regard, corneal confocal microscopy allows for the visualization of small nerve fibers in vivo and is a sensitive, noninvasive marker of peripheral neuropathy.59 Moreover, increased corneal nerve fiber length is a marker for nerve regeneration.60 In patients with type 1 diabetes, corneal nerve fiber length was shown to improve after kidney and pancreas transplantation, suggesting it could be an indicator of early nerve repair that may be undetected by conventional neuropathy assessments.61
While it was not possible to pool data from RCTs on the effect of omega-3 fatty acid supplementation in a population with diabetes, another open-label study involving 40 participants investigated the effect of 12 months of omega-3 supplementation in the form of seal oil.62 The authors reported a 29% improvement, from baseline, in corneal nerve fiber length (change from baseline: 8.3±2.9 mm/mm2 to 10.1±3.7 mm/mm2; P = 0.002) after participants were given seal oil containing 2330 mg of long-chain omega-3 fatty acids (EPA, 750 mg/d; DPA, 560 mg/d; and DHA, 1020 mg/d) at a dosage of 10 mL/d. This study was not included in the current systematic review, as it is a single-arm, open-label trial with no control or placebo group. Peripheral nerve function was also assessed comprehensively in this study by means of nerve conduction studies, quantitative sensory testing, and autonomic testing; none of these parameters showed a significant change from baseline over 12 months.
The 2 studies in this review that used quantitative sensory testing adopted this technique as the only measure of small fiber function.28,41 One of these studies assessed participants with musculoskeletal pain and fibromyalgia.28 Although there is some evidence that individuals with fibromyalgia have abnormal C-fiber nociceptors with spontaneous activity and increased mechanosensitivity,63 as well as abnormal in vivo confocal microscopy morphology,64 the role of small fiber neuropathy in this condition is poorly understood. Furthermore, the use of quantitative sensory testing alone as a measure of small fiber function requires caution, as abnormalities detected with this psychophysical test can be caused by central sensitization, rather than by peripheral nerve dysfunction.
Finally, only 3 studies included patient-reported outcomes,26–28 2 of which were incorporated into a composite score.26,27 Patient-reported outcomes may not only reflect early-onset changes in peripheral neuropathy6 but also may provide valuable information about how treatments affect an individual or impact quality of life6,56 and thus should be considered in future research.
Dosage of omega-3 PUFA intervention
The daily dose of long-chain omega-3 fatty acids (ie, EPA and DHA combined) in the included studies was highly heterogeneous. Three studies used dosages of less than 1000 mg/d,34,42,44 6 studies used dosages of 1000 to 3000 mg/d,26–29,35,36 and 6 studies used dosages equal to or exceeding 3000 mg/d.37–41,43 No studies exceeded a daily dosage of 5000 mg/d (EPA and DHA combined), which is considered the upper limit of safety for adults by the European Food Safety Authority.65 Dosage is likely an important consideration for the potential therapeutic effects of omega-3 supplements on peripheral nerves. For example, systematic reviews show that dosages below 3000 mg/d are largely ineffective for improving cardiovascular outcomes.66,67 The currently recommended dosing regimen of omega-3 ethyl esters for hypertriglyceridemia is 4000 mg/d.68 In contrast, dosages in the range of approximately 1500 mg/d have been shown to modulate ocular inflammation.69 Thus, further research is essential to clarify the optimal dosage for the specific applications of preventing or treating peripheral neuropathy.
Another consideration is the form of the omega-3 supplement. Of the 6 articles using the highest doses, 4 specified that the omega-3 fatty acids were supplemented in ethyl ester form (Omacor or Lovaza).37–39,41 Only 1 other study specified the form of omega-3 supplements as triglyceride or phospholipid subtypes.29 The incorporation of DHA and EPA into plasma, and the subsequent bioavailability of these fatty acids, may vary, depending on the preparation and formulation.70 Intestinal absorption of fatty acids is further influenced by the concentration of fatty acids in the diet; this is particularly relevant for ethyl ester forms, which have enhanced absorption in the presence of high-fat diets.71 Diet may not only influence the bioavailability of fatty acids70 but can also play a role in determining the overall effects of the intervention, given that therapeutic efficacy may differ in omega-3–sufficient compared with omega-3–deficient populations.72 Capturing participant dietary information (including changes in consumption of foods rich in omega-3 PUFAs), as well as information related to the form of omega-3 supplement, is therefore recommended for future RCTs.
Participant compliance was monitored in 8 of the 15 studies.27–29,35,36,40,42,44 In 1 study, serum concentrations of EPA and DHA were measured before and after the supplementation period and were reported by the authors to be significantly different between study groups at the end of the follow-up period.27 One study registered in a clinical trial registry reported that compliance would be monitored by measuring the concentrations of omega-3 fatty acids in serum phospholipids, using gas chromatography; this outcome measure, however, was not reported in the published version of the study.26 Five studies did not report whether compliance was monitored.34,37–39,43 Treatment adherence not only potentially influences treatment effect(s) but also may be an indicator of tolerability, particularly for higher doses.73 None of the included studies reported concerns about the safety or tolerability of omega-3 PUFA supplementation in adult study populations. Data from studies that reported adverse events show no significant difference in the incidence of adverse events or serious adverse events between intervention arms (RR = 0.98; 95%CI, 0.56–1.70; P = 0.94; and RR = 0.50; 95%CI, 0.10–2.41; P = 0.39, respectively).
Quality of the evidence
Overall, the certainty of the evidence related to the prespecified outcome measures was graded as low or very low, using the GRADE approach.30 The most frequent reason for downgrading, which was relevant to all of the prespecified outcomes, was imprecision. The certainty of evidence was downgraded for imprecision and risk of bias for the primary outcome. The certainty of evidence was downgraded for the same reasons for the secondary outcomes on SNAP amplitudes in the sural nerve, and motor nerve conduction velocity in the peroneal nerve. It must be acknowledged that data were pooled from 2 clinically similar studies, conducted by the same research group, involving a small sample of participants (n = 57) (thus not meeting the optimal information size as defined in the GRADE handbook).30 Moreover, 1 of studies was funded by industry.26
The certainty of evidence for effects on pain was downgraded to very low, because of imprecision, as data were derived from 1 small study28; for indirectness, because the pain outcome was not measured within the context of peripheral neuropathy; and for risk of bias, because of industry funding. The certainty of evidence for the effects of treatment on corneal nerve fiber length was downgraded to low, because of imprecision, as data were derived from 1 very small study (n = 12 participants)29 The certainty of evidence for the effects of omega-3 supplementation on symptoms of peripheral neuropathy and on intraepidermal nerve fiber density could not be determined, as no data were available for these outcomes.
CONCLUSION
In conclusion, the clinical management of peripheral neuropathy remains a challenge. Novel neuroprotective agents to prevent progressive nerve damage and improve health outcomes for patients are clearly needed. To date, several RCTs have assessed the effects of systemic omega-3 supplementation on peripheral neuropathy outcomes in a range of acute and chronic conditions. This systematic review finds, with low certainty, that omega-3 oral supplementation (at a daily dose of < 2000 mg of EPA + DHA combined, for 12 to 15 weeks), reduces the incidence of chemotherapy-induced peripheral neuropathy. Currently, there is insufficient evidence to establish with any certainty if omega-3 oral interventions can improve peripheral nerve structure or function in chronic diseases such as diabetes mellitus.
Peripheral neuropathy is a potential complication of several systemic conditions, most commonly diabetes. Progressive peripheral neuropathy is a leading cause of pain and disability and can have a profound negative impact on quality of life. While immunomodulatory therapies are available for some specific conditions, such as chronic inflammatory demyelinating polyneuropathy, Guillain-Barré syndrome, and vasculitic neuropathies, there are no disease-modifying treatments for preventing progressive nerve damage in most other conditions that cause peripheral neuropathy. Thus, there is an urgent need for clinical research to identify novel therapeutic strategies.
The findings from this review demonstrate a need to conduct appropriately powered, well-designed RCTs to investigate the potential therapeutic effects of omega-3 PUFA oral supplements on peripheral nerve integrity. Such trials should incorporate outcomes that are sensitive to the detection of changes in both small and large peripheral nerve fibers. The design of future trials should also consider participants’ baseline omega-3 PUFA intake and patient-reported outcomes, so that the impact of the intervention and the disease on quality of life can be assessed.
There are currently no universally accepted outcome measures to assess peripheral nerve integrity. The recommendations provided by the European Neuromuscular Centre’s International Workshop, “Selection of Outcome Measures for Peripheral Neuropathy Clinical Trials,” were considered in selecting the outcome measures for this review.56 These outcomes focus on the results of sensory and motor functional tests. The development of a core outcome set would enable outcomes reported in different RCTs to be more readily compared (and meta-analyzed to enable a comprehensive synthesis of the evidence), as recommended by the Core Outcome Measures in Effectiveness Trials (COMET) initiative.74
Acknowledgments
The authors acknowledge the advice of Iris Gordon, Cochrane Eyes and Vision Group, who provided feedback on the search strategies. The authors thank Irina Churilov for her assistance in translating the Russian papers as part of assessing studies for eligibility.
Author contributions. All authors made contributions to the conception and/or the design of the work. A.C.Z. conducted the literature search; A.C.Z., M.E.H.D.S., and L.E.D. assessed studies for eligibility and risk of bias and extracted data from included studies. A.C.Z. and L.E.D. performed the statistical analysis; J.K., L.R., and L.B. contributed to the interpretation of study data. A.C.Z. and L.E.D. drafted the manuscript; and J.K., L.R., R.J.M., L.B., J.P.C., and M.E.H.D.S. revised the content of the final review. All authors approved the final version of the manuscript.
Funding/support. This work was supported by the 2018 Melbourne Neuroscience Institute (MNI) Interdisciplinary Seed Fund grant (L.E.D., R.J.M., L.R., and J.K.); the funder had no role in the undertaking, data analyses, or reporting of this systematic review.
Declaration of interest. The authors have no relevant interests to declare but wish to note that L.E.D. is an author on one study included in this review (Chinnery et al.29). The risk of bias assessment and data extraction for this study were performed by A.C.Z. and M.E.H.D.S.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Figure S1 Risk of bias summary
Figure S2 Forest plots of comparison for sensory nerve conduction velocity
Figure S3 Forest plots of comparison for distal compound motor action potential latencies
Figure S4 Forest plots of comparison for motor nerve conduction velocity
Figure S5 Forest plot of comparison for adverse events
Table S1 Full characteristics of included studies
Table S2 Prespecified primary and secondary outcome measures
Table S3 Characteristics of excluded studies
Table S4 Adverse events
Appendix S1 PRISMA checklist
Appendix S2 Search strategies
References
National Research Council (US) Committee on Neurotoxicology and Models for Assessing Risk.
Covidence Systematic Review Software [computer program]. Melbourne, Australia: Veritas Health Innovation.
Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration;
Lovaza® and microvascular function in type 2 diabetes. ClinicalTrials.gov identifier: NCT00931879. https://clinicaltrials.gov/ct2/show/NCT00931879. Last updated May 9, 2017. Accessed November 21, 2017.
European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition, Allergies.
LOVAZA (omega-3-acid ethyl esters) [prescribing information].