Article Text
Abstract
The immune system protects the host from pathogenic organisms (bacteria, viruses, fungi, parasites). To deal with this array of threats, the immune system has evolved to include a myriad of specialised cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules, which are all ultimately derived from the diet. A number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important. Each of the nutrients named above has roles in supporting antibacterial and antiviral defence, but zinc and selenium seem to be particularly important for the latter. It would seem prudent for individuals to consume sufficient amounts of essential nutrients to support their immune system to help them deal with pathogens should they become infected. The gut microbiota plays a role in educating and regulating the immune system. Gut dysbiosis is a feature of disease including many infectious diseases and has been described in COVID-19. Dietary approaches to achieve a healthy microbiota can also benefit the immune system. Severe infection of the respiratory epithelium can lead to acute respiratory distress syndrome (ARDS), characterised by excessive and damaging host inflammation, termed a cytokine storm. This is seen in cases of severe COVID-19. There is evidence from ARDS in other settings that the cytokine storm can be controlled by n-3 fatty acids, possibly through their metabolism to specialised pro-resolving mediators.
- malnutrition
- infectious disease
- microbiome
- nutrient deficiencies
- pulmonary disease
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
The immune system exists to protect the host from noxious environmental agents especially pathogenic organisms, which may be in the form of bacteria, viruses, fungi or parasites. To deal with such an array of threats, the human immune system has evolved to include a myriad of cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules. These energy sources, substrates and regulatory molecules are ultimately derived from the diet. Hence an adequate supply of a wide range of nutrients is essential to support the immune system to function optimally.1 2 At the time of writing, the world is in the grip of a pandemic caused by infection with a new coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); the illness associated with infection by SARS-CoV-2 is called coronavirus disease discovered in 2019 or COVID-19.3 4 The aim of this article is to summarise the role of specific nutrients in supporting the immune system, particularly, but not exclusively, with regard to antiviral defences. The roles of nutrition in overcoming gut microbial dysbiosis and in calming a so-called ‘cytokine storm’ will also be discussed. First, some features of coronaviruses and of the immune system will be described.
Coronaviruses
Coronaviruses are a large group of single-stranded RNA viruses that are common among mammals and birds.5 6 Coronaviruses cause respiratory and, less frequently, gastrointestinal diseases.5 The respiratory symptoms caused by coronaviruses can range from common cold-like or mild influenza-like symptoms to severe pneumonia. In December 2019, a new type of coronavirus causing pneumonia and death was identified in Wuhan, China3 4; this new coronavirus is called SARS-CoV-2 because it is genetically similar to SARS-CoV which caused the 2002 outbreak of severe acute respiratory distress syndrome (ARDS). In fact, SARS-CoV-2 is the seventh known human coronavirus.7 However, SARS-CoV-2 is new to the human immune system and so there was no underlying existing natural immunity against it. This is probably why SARS-CoV-2 has spread so rapidly. SARS-CoV-2 infects respiratory epithelial cells causing the symptoms described above, and in severe cases requires ventilatory support. Older people, especially those with existing morbidities like diabetes, cardiovascular disease, respiratory disease and hypertension, are particularly susceptible to severe symptoms and mortality, as are individuals with suppressed immune systems.3 4 There is currently no treatment for infection with SARS-CoV-2 or for COVID-19. Current strategies aim to limit the spread of the virus by preventing contact between people. The search for vaccines to offer immune protection against SARS-CoV-2 and for pharmacological treatments to prevent the virus from replicating is underway. In the meantime, approaches to ensure that individuals’ immune systems are well supported should be taken. Nutrition should be at the forefront of these approaches.
The immune system
Introductory comments
The immune system becomes vital once an individual is exposed to an infectious agent. However, the nature of infectious agents varies and so different approaches are required by the immune system to deal with different types of infectious agent. These different approaches follow similar general strategies, which aim to seek out and destroy, but the precise immune mechanisms involved can differ. For example, most bacteria do not invade host cells and remain accessible to the host’s immune system; often these bacteria will be engulfed by innate phagocytic cells (typically neutrophils, monocytes, macrophages, dendritic cells), killed within intracellular phagocytic vacuoles and then digested. Remnants of the digested bacteria (antigens) can then be displayed via major histocompatibility class (MHC) II on the surface of the phagocyte. These antigens are recognised by antigen-specific CD4+ helper T lymphocytes and this triggers the acquired (also called adaptive) immune response to the bacteria, which involves the orchestrating T lymphocytes, B lymphocytes (which produce antigen-specific antibodies) and the further activation of innate immune cells. This response to extracellular bacteria is clearly targeted at killing those bacteria. Viruses (and some bacteria) invade host cells rather than remaining exclusively extracellular; this can trigger presentation of antigens via MHC I on the surface of the infected cells. Recognition of these antigens by CD8+ cytotoxic T lymphocytes results in killing of the host cell that is presenting the antigen. Natural killer cells also recognise virally infected cells and act in an analogous way to cytotoxic T lymphocytes by killing the infected cells. Thus, this response to virally infected cells is targeted at killing the host cells that harbour viruses. Killing host cells of course liberates viruses and the battle between host immune cells and virally infected cells continues.
There are four general functions of the immune system that enable effective host defence:
Creating a barrier to prevent pathogens from entering the body.
Identifying pathogens if they breech a barrier.
Eliminating pathogens.
Generating an immunological memory.
Barrier function
The barrier function of the immune system acts to prevent pathogens from entering the body from the external environment. This includes physical barriers like the skin and mucosal layers (gastrointestinal tract, respiratory tract, genitourinary tract); chemical barriers like the acid pH of the stomach; and biological barriers like the presence of commensal organisms on the skin and in the intestinal tract, secretions like IgA and antimicrobial proteins in saliva and tears, and the complement system.
Identification of pathogens
Pathogens are recognised by cells of the innate immune system, such as macrophages, monocytes and dendritic cells. This is achieved through the presence of pattern recognition receptors (PRRs) that recognise general molecular structures that are broadly shared by groups of pathogens. These structures are termed microbe-associated molecular patterns or MAMPs. When PRRs recognise MAMPs, the first line of host defensive responses is activated. PRRs include Toll-like receptors (TLRs). More than 10 functional TLRs have been identified in humans, each one detecting distinct MAMPs from bacteria, viruses, fungi and parasites. The best described of these are TLR4 which recognises the lipopolysaccharides from the cell wall of Gram-negative bacteria and TLR2 which recognises the lipoteichoic acid from the cell wall of Gram-positive bacteria. Several TLRs are expressed on the cell surface of innate immune cells because the pathogens they recognise, mainly bacteria, are extracellular. Because viruses enter host cells, it is important that there are also intracellular TLRs. Indeed, intracellular TLRs that recognise viral DNA, viral double-stranded RNA and viral single-stranded RNA exist. Among these, TLR7 and TLR8 are found in macrophages, monocytes, dendritic cells and some other cell types and are likely to be important in innate recognition of the single-stranded RNA of coronaviruses. However, proteins, including the spike glycoprotein, of the coronavirus coat are also likely to be recognised by both intracellular and extracellular PRRs.8–11
Elimination of pathogens
As mentioned earlier, extracellular bacteria can be engulfed by phagocytic cells that include macrophages and dendritic cells. After digestion of internalised bacteria, peptide fragments, termed antigens, are presented on the surface of the phagocytic cells (via MHC II) to antigen-specific CD4+ helper T lymphocytes. The activated helper T lymphocytes (specifically the T helper 1 phenotype) proliferate and produce cytokines including interleukin (IL)-2 and interferon (IFN)-γ. IFN-γ promotes antigen-specific antibody production by B lymphocytes. These antibodies coat the bacteria, neutralising them and making the process of phagocytosis more efficient.
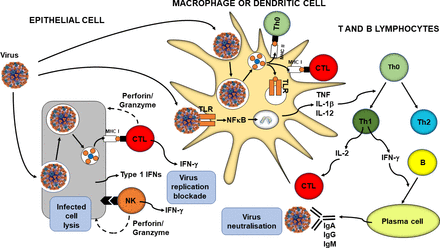
In parallel with phagocytosis, innate immune cell recognition of pathogens via PRRs triggers inflammatory signalling, activation of transcription factors like nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), inflammasome assembly, and production of classic inflammatory cytokines like tumour necrosis factor (TNF), IL-1β and IL-12. Viral infection of some cell types promotes release of type 1 IFNs (IFN-α and IFN-β) and these induce antiviral resistance, in part through activation of natural killer cells.12 13 Furthermore, as explained earlier, virally infected cells directly activate natural killer cells which act to kill the infected cell. In addition, PRR signalling induces maturation of dendritic cells which are responsible for viral antigen processing and presentation, so initiating acquired immunity. Upregulation of MHC I on virally infected cells including both respiratory epithelial cells and dendritic cells results in presentation of viral antigens to CD8+ cytotoxic T lymphocytes. This activates them to kill virally infected cells through the release of pore forming proteins like perforin. Presentation of viral antigens via MHC II and the cytokine milieu lead to the activation of CD4+ helper T lymphocytes with switching to the T helper 1 phenotype. These cells produce IL-2, which promotes cytotoxic T lymphocyte activity, and IFN-γ, which promotes differentiation of B lymphocytes to plasma cells which produce antiviral antibodies. These antibodies can bind to free viruses neutralising them. The processes involved in antiviral immunity are summarised in figure 1.
Overview of antiviral immunity. The events in the figure are explained in the text. B, B lymphocyte; CTL, cytotoxic T lymphocyte; IFN, interferon; Ig, immunoglobulin; IL, interleukin; MHC, major histocompatibility class; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer cell; Th, helper T lymphocyte; TLR, Toll-like receptor; TNF, tumour necrosis factor.
Immunological memory
Immunological memory refers to the ability of the immune system to quickly and specifically recognise an antigen that the body has previously encountered and initiate a corresponding immune response. There are two aspects of immunological memory. First, antibodies can persist in the circulation for many months to many years, providing protection against reinfection. Second, after the cessation of an active immune response, a small number of memory T (both CD4+ and CD8+) and B lymphocytes remain; they are in a resting state but if they encounter the same antigen that triggered their formation they are able to respond immediately and lead to rapid elimination of the source of the antigen. Memory cells have a long life (up to several decades). Immunological memory is the basis of vaccination.
Effect of ageing on the immune system
Ageing can be associated with a loss of immune competence, a process called immunosenescence.14–18 The features of immunosenescence are shown in box 1. One factor linked to immunosenescence is decreased output of immune cells from bone marrow, the site of origin of all immune cells. In addition, involution of the thymus with age decreases output of naive T lymphocytes, resulting in reduced capacity to respond to new antigens. Immunosenescence means that, compared with younger adults, older people have increased susceptibility to infections including respiratory tract infections and pneumonia and poorer responses to vaccination.14 15 19 20 The gut mucosa is the largest site of immune tissue in humans and senescence of the gut mucosal immune system has been demonstrated in murine models, with reductions in secretory IgA responses, impaired oral tolerance to new antigens and impaired mucosal dendritic cell function, as reviewed elsewhere.21 22 Immunosenescence may be one factor that predisposes older people to more severe COVID-19. Paradoxically, ageing is also linked to an increase in blood concentrations of many inflammatory mediators, a situation termed inflammageing.23 This state is considered to contribute to an increased risk of chronic conditions of ageing like cardiovascular disease, metabolic disease (diabetes, non-alcoholic fatty liver disease), neurodegeneration and some cancers23 and may predispose to mounting an excessive inflammatory response when infected. Although inflammation is part of the innate immune response and innate and acquired immunity should work in a coordinated and integrated way (see figure 1), an excessive inflammatory response can lead to impairments in acquired immunity.23
Some key features of age-related immune decline (immunosenescence)
T lymphocytes
Decreased numbers in the circulation.
Imbalances among different phenotypes.
Decline in naive T lymphocyte production and numbers.
Accumulation of non-functional memory T lymphocytes.
Diminished antigen receptor diversity.
Impaired responsiveness.
Impaired proliferation.
Impaired production of cytokines like interleukin (IL) 2 and interferon (IFN)-γ.
B lymphocytes
Decline in naive B lymphocyte numbers.
Accumulation of non-functional memory B lymphocytes.
Impaired responsiveness.
Altered balance of immunoglobulins.
Dendritic cells
Decreased phagocytosis.
Decreased Toll-like receptor (TLR) expression.
Decreased responsiveness.
Decreased type 1 IFN production.
Neutrophils
Numbers in the circulation are preserved.
Impaired chemotaxis.
Impaired oxidative burst and bacterial killing.
Impaired phagocytosis.
Decreased TLR expression.
Decreased production of neutrophil extracellular traps.
Decreased responsiveness.
Monocytes
Altered TLR expression.
Decreased responsiveness.
Altered pattern of cytokine production.
Macrophages
Impaired phagocytosis.
Altered TLR expression.
Natural killer cells
Increased numbers in the circulation.
Imbalances among different phenotypes.
Impaired cytotoxicity.
Impaired responsiveness.
Impaired production of cytokines.
Effect of obesity on the immune system
Obesity can be associated with a loss of immune competence,24 25 with impairments of the activity of helper T lymphocytes, cytotoxic T lymphocytes, B lymphocytes and natural killer cells,26–28 and reduced antibody and IFN-γ production.26 27 This means that, compared with healthy weight individuals, the obese have increased susceptibility to a range of bacterial, viral and fungal infections,24 29–31 and poorer responses to vaccination.26 31 The impact of obesity has been well explored in relation to influenza infection and vaccination against influenza. During the 2009 H1N1 influenza A virus pandemic, obese individuals showed delayed and weakened antiviral responses to infection and showed poorer recovery from disease compared with healthy weight individuals.26 Animal studies and case studies in humans show that obesity is associated with prolonged shedding of influenza virus, indicating an impairment in viral control and killing, and the emergence of virulent minor variants.26 Green and Beck32 note that compared with healthy weight individuals, vaccinated obese individuals have twice the risk of influenza or influenza-like illness, indicating poorer protection from vaccination in the obese. Sheridan et al 33 investigated the responses of immune cells from the blood of healthy weight, overweight and obese individuals to the influenza vaccine in vitro. Exposure of the blood immune cells to the vaccine increased the number of activated cytotoxic T lymphocytes, the number of granzyme expressing cytotoxic T lymphocytes and the number of IFN-γ producing cytotoxic T lymphocytes. However, the responses of cells from obese individuals were blunted by 40%, almost 60% and 65%, respectively. Cells from overweight individuals showed responses intermediate between those from healthy weight and obese individuals. Similar findings for the response of blood cells to the pandemic H1N1 influenza A virus were reported by Paich et al.34 Paradoxically, obesity is also linked to an increase in blood concentrations of many inflammatory mediators, a state of chronic low-grade inflammation.35 This state is considered to contribute to an increased risk of chronic conditions of ageing35 and may predispose to mounting an excessive inflammatory response when infected. Thus, obesity may be one factor that predisposes to more severe COVID-19; in support of this, a French report found that 85.7% of SARS-CoV-2 infected obese individuals required mechanical ventilation compared with 47.1% of infected healthy weight individuals.36
Nutrition, immunity and infection
Introductory comments
The immune system is functioning at all times, but cells become activated by the presence of pathogens (see The immune system). This activation results in a significant increase in the demand of the immune system for energy yielding substrates (glucose, amino acids and fatty acids). Activation of the immune response induces the production of lipid-derived mediators such as prostaglandins and leukotrienes and of many different types of protein including immunoglobulins, chemokines, cytokines, cytokine receptors, adhesion molecules and acute-phase proteins. This requires availability of the substrate fatty acids and amino acids, respectively. The immune response involves significant cellular proliferation, so increasing the number of immune cells available for defence: this requires DNA, RNA, protein and complex lipid synthesis and the ready availability of substrates to support this. The metabolic machinery involved in energy generation and biosynthesis requires many different vitamins and minerals as cofactors. Amino acids (eg, arginine) are precursors for the synthesis of polyamines, which have roles in the regulation of DNA replication and cell division. Various micronutrients (eg, iron, folate, zinc, magnesium) are also involved in nucleotide and nucleic acid synthesis. Some nutrients, such as vitamins A and D, and their metabolites are direct regulators of gene expression in immune cells and play a key role in the maturation, differentiation and responsiveness of immune cells. Creation of a pro-oxidant environment through generation of damaging reactive oxygen species is one element of innate immunity; the host needs protection against these through classic antioxidant vitamins (vitamins C and E) and the antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase); the latter require manganese, copper, zinc, iron and selenium. Thus, the roles for nutrients in supporting the function of the immune system are many and varied and it is easy to appreciate that an adequate and balanced supply of these is essential if an appropriate immune response is to be mounted. In essence, good nutrition creates an environment in which the immune system is able to respond appropriately to challenge, irrespective of the nature of the challenge. Conversely poor nutrition creates an environment in which the immune system cannot respond well. This is amply illustrated in conditions of nutrient deficiency (either ‘real life’ or experimentally induced) which are accompanied by impairments of both innate and acquired immunity and increased susceptibility to, and severity of, infections. Both the immune impairments and the susceptibility to infection can be reversed by correcting the deficiency(ies) showing a causal relationship between availability of specific nutrients and immune defences. This is recognised by the European Food Safety Authority which permits claims of ‘maintenance of functions of the immune system’ for vitamins A, B6, B12, C, D and folate (vitamin B9) and for the trace elements zinc, iron, selenium and copper.37 There are a number of comprehensive reviews of aspects of nutrition and immunity,1 2 38–42 mainly focusing on the role of micronutrients, as well as useful single-author and multiauthor books on the topic.43–46 In addition, there are many comprehensive nutrient-specific reviews, which are cited in the relevant sections below. Some of the text of the following sections is updated from a previous publication.1
Vitamin A, immunity and infection
There are a number of reviews of the role of vitamin A and its metabolites (eg, 9-cis-retinoic acid) in immunity and in host susceptibility to infection.47–54 These reviews contain citations to the many studies of vitamin A, immunity and infection that will be summarised here. Vitamin A is important for normal differentiation of epithelial tissue and for immune cell maturation and function. Thus, vitamin A deficiency is associated with impaired barrier function, altered immune responses and increased susceptibility to a range of infections. Vitamin A-deficient mice show breakdown of the gut barrier and impaired mucus secretion (due to loss of mucus-producing goblet cells), both of which would facilitate entry of pathogens. Many aspects of innate immunity, in addition to barrier function, are modulated by vitamin A and its metabolites. Vitamin A controls neutrophil maturation and in vitamin A deficiency blood neutrophil numbers are increased, but they have impaired phagocytic function. Therefore, the ability of neutrophils to ingest and kill bacteria is impaired. Vitamin A also supports phagocytic activity and oxidative burst of macrophages, so promoting bacterial killing. Natural killer cell activity is diminished by vitamin A deficiency, which would impair antiviral defences. The impact of vitamin A on acquired immunity is less clear and may depend on the exact setting and the vitamin A metabolite involved. Vitamin A controls dendritic cell and CD4+ T lymphocyte maturation and its deficiency alters the balance between T helper 1 and T helper 2 lymphocytes. Studies in experimental model systems indicate that the vitamin A metabolite 9-cis retinoic acid enhances T helper 1 responses. Retinoic acid promotes movement (homing) of T lymphocytes to the gut-associated lymphoid tissue. Interestingly, some gut-associated immune cells are able to synthesise retinoic acid. Retinoic acid is required for CD8+ T lymphocyte survival and proliferation and for normal functioning of B lymphocytes including antibody generation. Thus, vitamin A deficiency can impair the response to vaccination, as discussed elsewhere.55 In support of this, vitamin A-deficient Indonesian children provided with vitamin A showed a higher antibody response to tetanus vaccination than seen in vitamin A-deficient children.56 Vitamin A deficiency predisposes to respiratory infections, diarrhoea and severe measles. Systematic reviews and meta-analyses of trials in children with vitamin A report reduced all-cause mortality,57 reduced incidence, morbidity and mortality from measles57 and from infant diarrhoea,57 and improved symptoms in acute pneumonia58 (table 1).
Summary of selected recent meta-analyses of micronutrients and respiratory infections
B-group vitamins, immunity and infection
There is a recent comprehensive review of B vitamins and immunity.59 This review contains citations to the many studies of B-group vitamins and immunity that will be summarised here. B vitamins are involved in intestinal immune regulation, thus contributing to gut barrier function. Folic acid deficiency in animals causes thymus and spleen atrophy, and decreases circulating T lymphocyte numbers. Spleen lymphocyte proliferation is also reduced but the phagocytic and bactericidal capacity of neutrophils appears unchanged. In contrast, vitamin B12 deficiency decreases phagocytic and bacterial killing capacity of neutrophils, while vitamin B6 deficiency causes thymus and spleen atrophy, low blood T lymphocyte numbers and impaired lymphocyte proliferation and T lymphocyte-mediated immune responses. Vitamins B6 and B12 and folate all support the activity of natural killer cells and CD8+ cytotoxic T lymphocytes, effects which would be important in antiviral defence. Patients with vitamin B12 deficiency had low blood numbers of CD8+ T lymphocytes and low natural killer cell activity.60 In a study in healthy older humans, a vitamin B6-deficient diet for 21 days resulted in a decreased percentage and total number of circulating lymphocytes, and a decrease in T and B lymphocyte proliferation and IL-2 production.61 Repletion over 21 days using vitamin B6 at levels below those recommended did not return immune function to starting values, while repletion at the recommended intake (22.5 µg/kg body weight per day, which would be 1.575 mg/day in a 70 kg individual) did. Providing excess vitamin B6 (33.75 µg/kg body weight per day, which would be 2.362 mg/day in a 70 kg individual) for 4 days caused a further increase in lymphocyte proliferation and IL-2 production.
Vitamin C, immunity and infection
There are reviews of the role of vitamin C in immunity and in host susceptibility to infection.62 63 These reviews contain citations to the many studies of vitamin C, immunity and infection that will be summarised here. Vitamin C is required for collagen biosynthesis and is vital for maintaining epithelial integrity. It also has roles in several aspects of immunity, including leucocyte migration to sites of infection, phagocytosis and bacterial killing, natural killer cell activity, T lymphocyte function (especially of CD8+ cytotoxic T lymphocytes) and antibody production. Jacob et al 64 showed that a vitamin C-deficient diet in healthy young adult humans decreased mononuclear cell vitamin C content by 50% and decreased the T lymphocyte-mediated immune responses to recall antigens. Vitamin C deficiency in animal models increases susceptibility to a variety of infections.63 People deficient in vitamin C are susceptible to severe respiratory infections such as pneumonia. A meta-analysis reported a significant reduction in the risk of pneumonia with vitamin C supplementation, particularly in individuals with low dietary intakes65 (table 1). Vitamin C supplementation has also been shown to decrease the duration and severity of upper respiratory tract infections, such as the common cold, especially in people under enhanced physical stress.63 66
Vitamin D, immunity and infection
There are a number of reviews of the role of vitamin D and its metabolites in immunity and in host susceptibility to infection.67–78 These reviews contain citations to the many studies of vitamin D, immunity and infection that will be summarised here. The active form of vitamin D (1,25-dihydroxyvitamin D3) is referred to here as vitamin D. Vitamin D receptors have been identified in most immune cells and some cells of the immune system can synthesise the active form of vitamin D from its precursor, suggesting that vitamin D is likely to have important immunoregulatory properties. Vitamin D enhances epithelial integrity and induces antimicrobial peptide (eg, cathelicidin) synthesis in epithelial cells and macrophages,79 80 directly enhancing host defence. However, the effects of vitamin D on the cellular components of immunity are rather complex. Vitamin D promotes differentiation of monocytes to macrophages and increases phagocytosis, superoxide production and bacterial killing by innate immune cells. It also promotes antigen processing by dendritic cells although antigen presentation may be impaired. Vitamin D is also reported to inhibit T-cell proliferation and production of cytokines by T helper 1 lymphocytes and of antibodies by B lymphocytes, highlighting the paradoxical nature of its effects. Effects on T helper 2 responses are not clear and vitamin D seems to increase number of regulatory T lymphocytes. Vitamin D seems to have little impact on CD8+ T lymphocytes. A systematic review and meta-analysis of the influence of vitamin D status on influenza vaccination (nine studies involving 2367 individuals) found lower seroprotection rates to influenza A virus subtype H3N2 and to influenza B virus in those who were vitamin D deficient.81 Berry et al 82 described an inverse linear relationship between vitamin D levels and respiratory tract infections in a cross-sectional study of 6789 British adults. In agreement with this, data from the US Third National Health and Nutrition Examination Survey which included 18 883 adults showed an independent inverse association between serum 25(OH)-vitamin D and recent upper respiratory tract infection.83 Other studies also report that individuals with low vitamin D status have a higher risk of viral respiratory tract infections.84 Supplementation of Japanese schoolchildren with vitamin D for 4 months during winter decreased the risk of influenza by about 40%.85 Meta-analyses have concluded that vitamin D supplementation can reduce the risk of respiratory tract infections86–89 (table 1).
Vitamin E, immunity and infection
There are a number of reviews of the role of vitamin E in immunity and host susceptibility to infection.90–92 These reviews contain citations to the many studies of vitamin E, immunity and infection that will be summarised here. In laboratory animals, vitamin E deficiency decreases lymphocyte proliferation, natural killer cell activity, specific antibody production following vaccination and phagocytosis by neutrophils. Vitamin E deficiency also increases susceptibility of animals to infectious pathogens. Vitamin E supplementation of the diet of laboratory animals enhances antibody production, lymphocyte proliferation, T helper 1-type cytokine production, natural killer cell activity and macrophage phagocytosis. Vitamin E promotes interaction between dendritic cells and CD4+ T lymphocytes. There is a positive association between plasma vitamin E and cell-mediated immune responses, and a negative association has been demonstrated between plasma vitamin E and the risk of infections in healthy adults over 60 years of age.93 There appears to be particular benefit of vitamin E supplementation for the elderly.94–97 Studies by Meydani et al 94 95 demonstrated that vitamin E supplementation at high doses (one study94 used 800 mg/day and the other95 used doses of 60, 200 and 800 mg/day) enhanced T helper 1 cell-mediated immunity (lymphocyte proliferation, IL-2 production) and improved vaccination responses, including to hepatitis B virus. Supplementation of older adults with vitamin E (200 mg/day) improved neutrophil chemotaxis and phagocytosis, natural killer cell activity and mitogen-induced lymphocyte proliferation.97 Secondary analysis of data from the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study identified that daily vitamin E supplements for 5 to 8 years reduced the incidence of hospital treated, community-acquired pneumonia in smokers.98 One study reported that vitamin E supplementation (200 IU/day~135 mg/day) for 1 year decreased risk of upper respiratory tract infections in the elderly,99 but another study did not see an effect of supplemental vitamin E (200 mg/day) on the incidence, duration or severity of respiratory infections in an elderly population.100
Zinc, immunity and infection
There are a number of reviews of the role of zinc in immunity and host susceptibility to infection.101–109 These reviews contain citations to the many studies of zinc, immunity and infection that will be summarised here. Of note, Read et al 110 have recently provided a very insightful evaluation of the role of zinc in antiviral immunity. Zinc inhibits the RNA polymerase required by RNA viruses, like coronaviruses, to replicate,111 suggesting that zinc may play a key role in host defence against RNA viruses. In vitro replication of influenza virus was inhibited by the zinc ionophore pyrrolidine dithiocarbamate,112 and there are indications that zinc might inhibit replication of SARS-CoVs in vitro.113 In addition, as discussed by Read et al,110 the zinc-binding metallothioneins seem to play an important role in antiviral defence.114 Zinc deficiency has a marked impact on bone marrow, decreasing the number immune precursor cells, with reduced output of naive B lymphocytes and causes thymic atrophy, reducing output of naive T lymphocytes. Therefore, zinc is important in maintaining T and B lymphocyte numbers. Zinc deficiency impairs many aspects of innate immunity, including phagocytosis, respiratory burst and natural killer cell activity. Zinc also supports the release of neutrophil extracellular traps that capture microbes.115 There are also marked effects of zinc deficiency on acquired immunity. Circulating CD4+ T lymphocyte numbers and function (eg, IL-2 and IFN-γ production) are decreased and there is a disturbance in favour of T helper 2 cells. Likewise, B lymphocyte numbers and antibody production are decreased in zinc deficiency. Zinc supports proliferation of CD8+ cytotoxic T lymphocytes, key cells in antiviral defence. Many of the in vitro immune effects of zinc are prevented by zinc chelation.116 Moderate or mild zinc deficiency or experimental zinc deficiency in humans result in decreased natural killer cell activity, T lymphocyte proliferation, IL-2 production and cell-mediated immune responses which can all be corrected by zinc repletion.117 118 In patients with zinc deficiency related to sickle cell disease, natural killer cell activity is decreased, but can be returned to normal by zinc supplementation.119 Patients with the zinc malabsorption syndrome acrodermatitis enteropathica display severe immune impairments120 and increased susceptibility to bacterial, viral and fungal infections. Zinc supplementation (30 mg/day) increased T lymphocyte proliferation in elderly care home residents in the USA, an effect mainly due to an increase in numbers of T lymphocytes.121 The wide ranging impact of zinc deficiency on immune components is an important contributor to the increased susceptibility to infections, especially lower respiratory tract infection and diarrhoea, seen in zinc deficiency. Correcting zinc deficiency lowers the likelihood of diarrhoea and of respiratory and skin infections, although some studies fail to show benefit of zinc supplementation in respiratory disease.105 Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair zinc.122 Recent systematic reviews and meta-analyses of trials with zinc report shorter duration of common cold in adults,123 124 reduced incidence and prevalence of pneumonia in children125 and reduced mortality when given to adults with severe pneumonia126 (table 1).
Copper, immunity and infection
There are a number of reviews of the role of copper in immunity and host susceptibility to infection.127–129 These reviews contain citations to the many studies of copper, immunity and infection that will be summarised here. Copper itself has antimicrobial properties. Copper supports neutrophil, monocyte and macrophage function and natural killer cell activity. It promotes T lymphocyte responses such as proliferation and IL-2 production. Copper deficiency in animals impairs a range of immune functions and increases susceptibility to bacterial and parasitic challenges. Human studies show that subjects on a low copper diet have decreased lymphocyte proliferation and IL-2 production, with copper administration reversing these effects.130 Children with Menke’s syndrome, a rare congenital disease with complete absence of the circulating copper-carrying protein caeruloplasmin, show immune impairments and have increased bacterial infections, diarrhoea and pneumonia.131 Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair copper.122
Selenium, immunity and infection
There are a number of reviews of the role of selenium in immunity and host susceptibility to infection.132–138 These reviews contain citations to the many studies of selenium, immunity and infection that will be summarised here. Selenium deficiency in laboratory animals adversely affects several components of both innate and acquired immunity, including T and B lymphocyte function including antibody production and increases susceptibility to infections. Lower selenium concentrations in humans have been linked with diminished natural killer cell activity and increased mycobacterial disease. Selenium deficiency was shown to permit mutations of coxsackievirus, polio virus and murine influenza virus increasing virulence.139–142 These latter observations suggest that poor selenium status could result in the emergence of more pathogenic strains of virus, thereby increasing the risks and burdens associated with viral infection. Selenium supplementation (100 to 300 µg/day depending on the study) has been shown to improve various aspects of immune function in humans,143–145 including in the elderly.146 147 Selenium supplementation (50 or 100 µg/day) in adults in the UK with low selenium status improved some aspects of their immune response to a poliovirus vaccine.148
Iron, immunity and infection
There are a number of reviews of the role of iron in immunity and host susceptibility to infection.149–159 These reviews contain citations to the many studies of iron, immunity and infection that will be summarised here. Iron deficiency induces thymus atrophy, reducing output of naive T lymphocytes, and has multiple effects on immune function in humans. The effects are wide ranging and include impairment of respiratory burst and bacterial killing, natural killer cell activity, T lymphocyte proliferation and production of T helper 1 cytokines. T lymphocyte proliferation was lower by 50% to 60% in iron-deficient than in iron-replete housebound older Canadian women.160 These observations would suggest a clear case for iron deficiency increasing susceptibility to infection. However, the relationship between iron deficiency and susceptibility to infection remains complex.150 154–158 Evidence suggests that infections caused by organisms that spend part of their life-cycle intracellularly, such as plasmodia and mycobacteria, may actually be enhanced by iron. In the tropics, in children of all ages, iron at doses above a particular threshold has been associated with increased risk of malaria and other infections, including pneumonia. Thus, iron intervention in malaria-endemic areas is not advised, particularly high doses in the young, those with compromised immunity and during the peak malaria transmission season. There are different explanations for the detrimental effects of iron administration on infections. First, iron overload causes impairment of immune function.149–159 Second, excess iron favours damaging inflammation. Third, micro-organisms require iron and providing it may favour the growth of the pathogen. Perhaps for the latter reasons several host immune mechanisms have developed for withholding iron from a pathogen.154–157 159 In a recent study giving iron (50 mg on each of 4 days a week) to iron-deficient schoolchildren in South Africa increased the risk of respiratory infections161; coadministration of n-3 fatty acids (500 mg on each of 4 days a week) mitigated the effect of iron. Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair iron.122
Gut microbiota, immunity and infection
Human gut microbiota
The human body is host to a significant number of bacteria and other organisms which colonise internal and external areas, such as the skin, mouth and gut. The community of organisms in a particular location is referred to as the microbiota. The gut microbiota shows a high degree of variability among individuals,162 reflecting differing exposures to environmental factors and the influence of host phenotype such as age and ethnicity. The large intestine is the site of the greatest number and diversity of bacterial species, with recent estimates of 1011 bacteria/g of colon contents.163 The gut microbiota is strongly influenced by habitual diet.164–167 Furthermore, both ageing and the presence or absence of disease significantly influence the composition of the microbiota.168 For example, with ageing, the abundance and diversity of bifidobacteria decline,169 while bacteria including streptococci, staphylococci, enterococci and enterobacteria increase.170 Changes seen within the gut microbiota with age are environment specific, with significant differences observed between populations from different countries. Within countries there are significant differences in the microbiota of free-living older adults and those residing in residential care.171 172 An abnormal gut microbiota, termed dysbiosis, is seen in obesity and in individuals with chronic age-related conditions.166 167 173–175 It is interesting to note that Xu et al 176 comment that some Chinese patients with COVID-19 showed intestinal dysbiosis with low numbers of lactobacilli and bifidobacteria.
Gut microbiota, probiotics and the immune system
Indigenous commensal bacteria within the gastrointestinal tract are believed to play a role in host immune defence by creating a barrier against colonisation by pathogens. Disease and the use of antibiotics can disrupt this barrier, creating an environment that favours the growth of pathogenic organisms. There is now evidence that providing exogenous, live, ‘desirable’ bacteria, termed probiotics, can contribute to maintenance of the host’s gastrointestinal barrier.177 Probiotic organisms are found in fermented foods including traditionally cultured dairy products and some fermented milks; the most commonly used commercial organisms are various lactobacilli and bifidobacteria.178 These organisms are able to colonise the gut temporarily, making their regular consumption necessary. In addition to creating a physical barrier, some of the products of the metabolism of both endogenous commensal bacteria and probiotic bacteria, including lactic acid and antimicrobial proteins, can directly inhibit the growth of pathogens.179 Probiotic bacteria also compete with some pathogenic bacteria for available nutrients. In addition to these direct interactions between commensal and probiotic organisms on the one hand and pathogens on the other, commensal and probiotic organisms can interact with the host’s gut epithelium and gut-associated immune tissues.179–181 These communications with the host may occur through chemicals released from the bacteria or through direct cell-to-cell contact and it is through these interactions that probiotics are thought to be able to influence immune function, even at sites distant from the gut.181 Nevertheless, the precise nature of these interactions is not very well understood.181 A large number of studies have examined the influence of various probiotic organisms, either alone or in combination, on immune function, infection and inflammatory conditions in human subjects.182 Certain probiotic organisms appear to enhance innate immunity (particularly phagocytosis and natural killer cell activity), but they seem to have a less pronounced effect on acquired immunity.182 Studies show improved vaccination responses in individuals taking probiotics,183 184 as reviewed elsewhere.185 Recent systematic reviews and meta-analyses confirm that probiotics or prebiotics (these are usually non-digestible oligosaccharides that act as fuels for some types of bacteria enhancing their growth; many probiotics are bifidogenic) enhance the antibody response to seasonal influenza vaccination in adults.186 187 The studies with probiotics have most often used lactobacilli or bifidobacteria.
Probiotic bacteria and gastrointestinal infections
A number of studies in children report lower incidence and duration of diarrhoea with certain probiotics. Recent systematic reviews and meta-analyses report that Lactobacillus paracasei CBA L74 reduces the risk of diarrhoea,188 that Lactobacillus acidophilus LB reduces duration of diarrhoea,188 that probiotics and synbiotics (combinations of probiotics and prebiotics) reduce durations of diarrhoea and hospitalisation and hasten recovery,189 that Lactobacillus rhamnosus GG reduces duration of diarrhoea,190 that Lactobacillus reuteri DSM 17938 reduces durations of diarrhoea and hospitalisation and increases early cure rate,191 192 and that Bacillus clausii reduces durations of diarrhoea and hospitalisation.193 In adults, there is now good evidence that probiotics protect against antibiotic-associated diarrhoea.194–198 Recent systematic reviews and meta-analyses report that probiotics reduce the risk of antibiotic-associated diarrhoea in adults aged 18 to 64 years but not in older adults (>65 years),199 that probiotics reduce the risk of Clostridium difficile-associated diarrhoea,200 that probiotics reduce the incidence and duration of antibiotic-associated diarrhoea and C. difficile-associated diarrhoea, with lactobacilli especially Lactobacillus casei being most effective,201 that L. rhamnosus GG may be most effective at treating antibiotic-associated diarrhoea,202 and that L. casei may be most effective at treating C. difficile-associated diarrhoea.202 What is evident from this research is that, although probiotics are effective in preventing and treating diarrhoea in both children and adults, there are considerable differences in the effects of different probiotic species and strains and the effects observed with one type of probiotic cannot be extrapolated to another. The recently released Handbook of COVID-19 Prevention and Treatment 203 comments that ‘some COVID-19 patients have gastrointestinal symptoms (such as abdominal pain and diarrhoea) due to direct viral infection of the intestinal mucosa or anti-viral and anti-infective drugs’. The handbook goes on to say that the dysbiosis seen in these patients176 ‘may lead to bacterial translocation and secondary infection, so it is important to maintain the balance of intestinal microecology [i.e. microbiota] by microecological modulator and nutritional support’ and that ‘microecologics [probiotics?] can reduce bacterial translocation and secondary infection … inhibit intestinal harmful bacteria, reduce toxin production and reduce infection caused by gut microflora dysbiosis … improve the gastrointestinal symptoms of patients … improve faecal character and defaecation frequency, and reduce diarrhoea by inhibiting intestinal mucosal atrophy … antibiotics can be adjusted timely and probiotics can be prescribed … these can reduce the chances of intestinal bacterial translocation and gut-derived infection’. These statements seem to be based on the existing literature rather than evidence from patients with COVID-19, and there is no clear description that such interventions have been successfully (or even unsuccessfully) performed in these patients. Indeed, Gao et al 204 state that ‘there is no direct clinical evidence that the modulation of gut microbiota plays the (sic) therapeutic role in the treatment of COVID-19’. Nevertheless, the observations suggest a potential for probiotic administration to have clinical relevance in patients with COVID-19.
Probiotic bacteria and respiratory infections
The gut microbiota seems to be protective against respiratory infection, as its depletion or absence in mice leads to impaired immune responses and worsens outcomes following bacterial or viral respiratory infection.205 206 These observations suggest a gut–lung axis of some importance in maintaining respiratory fitness during infection. There are a number of studies of probiotics in human respiratory disease, mainly in children, and mainly using different lactobacilli and bifidobacteria. Many of these studies find benefits of probiotics in terms of reduced incidence or severity of respiratory tract infections. These studies have been subject to a number of systematic reviews and meta-analyses over recent years207–215; the findings of these are summarised in table 2. Taken together, these findings provide some evidence that probiotics, in particular some lactobacilli and bifidobacteria, reduce the incidence, and improve the outcomes, of respiratory infections in humans. Thus, the observation that some Chinese patients with COVID-19 showed intestinal dysbiosis with low numbers of lactobacilli and bifidobacterial176 is important, but whether this dysbiosis is a predisposing factor to COVID-19 in those patients is not known. However, the totality of the evidence demonstrating that lactobacilli and bifidobacteria may improve immune function, enhance the response to seasonal influenza vaccination (which mimics a viral infection), reduce the incidence of respiratory infections, including those caused by viruses, and improve outcomes in those with respiratory infections would favour the use of these organisms as a strategy to reduce the risk and severity of viral respiratory infections.
Summary of selected systematic reviews and meta-analyses reporting on probiotics and respiratory infections
Nutritional intervention to control a cytokine storm
Coronaviruses cause respiratory disease and can lead to substantial lung damage.3–7 In trying to deal with this damage, cells of the immune system infiltrate the lungs initiating a significant inflammatory reaction. This can cause small blood vessels in the lung to leak fluid and fill up the alveoli, which makes it difficult for oxygen to enter the bloodstream for delivery to the body’s organs. This is when a patient will need ventilatory support. In the course of the battle between the host immune system and coronaviruses, excessive stimulation of the inflammatory response can occur. This is manifested as substantial production of reactive oxygen species, inflammatory eicosanoids and inflammatory chemokines and cytokines such as TNF-α, IL-1β and IL-6. This pro-oxidative, proinflammatory state is referred to as a ‘cytokine storm’; this is because this response of the innate immune system becomes damaging to host tissue and actually contributes to lung injury and respiratory failure. This condition is ARDS. The link between coronavirus infection, a cytokine storm and ARDS is elegantly described elsewhere.216 Mortality from ARDS is typically high. Patients with advanced COVID-19 are described to have markedly elevated inflammatory markers in their bloodstream,217 218 and ferritin, C reactive protein and IL-6 levels were significantly higher in non-survivors than survivors reports from Wuhan, China.219 220 As well as being directly damaging to host tissue, the excessive inflammatory response (ie, the cytokine storm) suppresses the acquired immune response; for example, numbers of CD4+ and CD8+ T lymphocytes are reduced,217 218 and the ability of CD4+ T lymphocytes to produce IFN-γ is impaired.217 This impairment of acquired immunity means that the individual’s ability to deal with the virus is seriously hindered.
Because of its pro-oxidant, proinflammatory state, ARDS may be amenable to treatment with nutrients that target oxidative stress and inflammation. The former would include classic antioxidants like vitamin C221 222 and vitamin E and also trace elements223 224 to support the activity of antioxidant enzymes, while the latter would include the bioactive n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). EPA and DHA have anti-inflammatory properties acting to decrease the production of inflammatory eicosanoids produced from arachidonic acid, to inhibit NFκB signalling and to reduce production of inflammatory cytokines, as reviewed elsewhere.225–227 EPA is metabolised to weakly inflammatory eicosanoids,228 and both EPA and DHA are metabolised to metabolites known as specialised pro-resolving mediators (SPMs) which are known to resolve (turn off) ongoing inflammatory processes.229–231 Using an injury model with the isolated rabbit lung, EPA was shown to decrease arachidonic acid-derived inflammatory eicosanoids, arterial pressure and vascular leakage.232 Perfusion of isolated rabbit lungs with a fish oil-containing lipid emulsion markedly attenuated the vascular inflammatory reaction.233 Animal models of lung injury have shown that fish oil attenuates pulmonary accumulation of neutrophils,234 reduces lung permeability,235 reduces pulmonary oedema236 and attenuates cardiopulmonary dysfunction.237 The pro-resolving effects of EPA- and DHA-derived SPMs seem to be highly relevant to the effect of n-3 fatty acids in lung injury: Hecker et al 238 reported that the beneficial effects of fish oil in a murine model of acute lung injury were abrogated in mice lacking ChemR23, a receptor for some SPMs. Hence, the effects of n-3 fatty acids in lung injury might be due to their conversion to SPMs. In accordance with this, a number of studies of individual SPMs in various animal models of lung injury report reduced lung inflammation, increased bacterial killing and less or resolved lung injury.239–250 The active SPMs described in these animal studies include resolvin D1, aspirin-triggered resolvin D1, resolvin D3, aspirin-triggered resolvin D3, all produced from DHA, and resolvin E1, produced from EPA.
A number of trials of n-3 fatty acids in patients with ARDS have been performed. The earliest trials251–253 used the same enteral formulation which provided a high dose of EPA and DHA along with antioxidants and γ-linolenic acid. All three of these trials reported favourable effects on multiple inflammatory, respiratory and clinical outcomes, and a meta-analysis of these trials found significant improvements in ventilator-free days, new organ failures, length of stay in the intensive care unit and mortality.254 A more recent Cochrane meta-analysis255 pooled data from 10 randomised controlled trials of n-3 fatty acids in patients with ARDS. Six of these trials used the n-3 fatty acid, antioxidant and γ-linolenic acid formulation, while three used other enteral formulas dominated by n-3 fatty acids and one used parenteral n-3 fatty acids. The findings of the meta-analysis are summarised in table 3. The meta-analysis concluded that administration of n-3 fatty acids usually in combination with other bioactive nutrients led to ‘reductions in duration of mechanical ventilation and intensive care unit length of stay, along with improved oxygenation’. Putting all these observations together, it seems that patients with ARDS can be treated favourably with n-3 fatty acids, perhaps in combination with antioxidants, which act to reduce inflammation and the cytokine storm most likely through their conversion to SPMs, although EPA and DHA do have anti-inflammatory effects in their own right.
Summary of the findings of the meta-analysis of Dushianthan et al 255 of the effects of n-3 fatty acid-rich formulas in patients with ARDS
Steps to take to support the immune system through good nutrition
The foregoing discussion highlights that a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) are vital for supporting immune function. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important in this regard. The understanding of the importance of these nutrients in immunity and in ensuring the host is better able to cope with pathogen exposure comes from states of deficiency (either experimental or ‘real world’) and their reversal. Thus, it is clear that situations of frank essential nutrient deficiency impair immune function and increase susceptibility to infections and that these two outcomes can both be prevented or reversed by treating the deficiency(ies). This may be through diet or in some cases may require supplementation or some other form of therapeutic administration, depending on the nutrient, the extent of the deficiency and the setting. Moving away from frank deficiency, there will be individuals in all populations who have ‘suboptimal’ intakes and status of one or more essential nutrients. It is not entirely clear the extent to which immune function in those individuals will be compromised. However, it seems likely that individuals with suboptimal intakes of a range of essential nutrients are likely to show suboptimal immune responses; this probably contributes to the variation in immune outcomes that is seen in the general population.256 257 In the interests of assuring the best possible immune response if an individual becomes infected, it would seem prudent to consume sufficient amounts of essential nutrients, although in most cases these amounts are not explicitly defined. Table 4 lists good dietary sources of key nutrients that support the immune system. This listing conveys that the best diet to support the immune system is one with a diverse and varied intake of vegetables, fruits, berries, nuts, seeds, grains and pulses along with some meats, eggs, dairy products and oily fish. This diet is consistent with those regarded as generally healthy258 and is consistent with current dietary guidelines.259 Such a diet would preclude too much processed and ‘junk’ food and excessive amounts of saturated fat and sugar. A randomised controlled trial of >5 servings of fruits and vegetables per day compared with <2 servings per day in older people (age 65 to 85 years) reported a better response to pneumococcal vaccination in the group consuming the higher amount of fruits and vegetables, although the response to tetanus vaccination was not different between the two groups.260 Human trials suggest that the intakes of some micronutrients needed to optimally support the immune system are likely to be in excess of intakes that can easily be achieved through diet alone. This is the case for vitamins C, D and E and zinc and selenium. There may be a role for immune-targeted supplements to achieve the intakes of these nutrients necessary to fully support the immune system. In addition to considering the ‘direct’ effects of nutrition on the immune system, many plant foods, fibre and fermented foods play a role in creating and maintaining a healthy gut microbiota167 175 that will also help to support the immune system.179–181
Important dietary sources of nutrients that support the immune system
Summary and conclusions
The immune system protects the host from pathogenic organisms (bacteria, viruses, fungi, parasites). To deal with such an array of threats, the human immune system has evolved to include a myriad of specialised cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules, which are all ultimately derived from the diet. Through experimental research and studies of people with deficiencies, a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important in this regard. All of nutrients named above have roles in supporting antibacterial and antiviral defences but zinc and selenium seem to be particularly important for the latter. It would seem prudent for individuals to consume sufficient amounts of essential nutrients to support their immune system to help them to deal with pathogens should they become infected. Consumption of a diet of diverse and varied plant-based and animal-based foods that is consistent with current healthy eating guidelines would be best to support the immune system. However, human trials suggest that the intakes of some micronutrients (vitamins C, D and E and zinc and selenium) needed to optimally support the immune system are in excess of intakes that can easily be achieved through diet alone and in this case supplementation might be considered. The gut microbiota plays a role in educating and regulating the immune system and gut dysbiosis is a feature of disease including many infectious diseases. Therefore, dietary approaches to achieve a healthy microbiota can also benefit the immune system. There is evidence that probiotic bacteria, particularly some lactobacilli and bifidobacteria, can modify the microbiota, modulate the immune response and protect against infections, including of the respiratory tract. Many plant foods, fibre and fermented foods play a role in creating and maintaining a healthy gut microbiota and so will also help to support the immune system. Thus, specific nutrients and the foods that provide them can play a role in supporting the immune system in order that the host can better defend against bacteria and viruses if infected. Therefore, having a healthy diet could be an important factor, but one of many, in determining outcome in individuals should they become infected with coronavirus. However, it is important to note that there are no published nutrition studies in the context of SARS-CoV-2 or COVID-19. Chinese researchers have noted dysbiosis in patients with severe COVID-19 and have recommended treatment with probiotics, but it is not clear whether this was done and, if it was, whether it was successful in improving clinical outcome. Severe infection of the respiratory epithelium can lead to ARDS, characterised by excessive and damaging host inflammation, termed a cytokine storm. This is seen in cases of severe COVID-19. There is evidence from ARDS in other settings that the cytokine storm can be controlled by the n-3 fatty acids EPA and DHA, possibly through their metabolism to SPMs. This therapeutic approach has not been attempted in severe COVID-19 and warrants investigation.
Acknowledgments
PCC is supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre.
References
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
Footnotes
Contributors The author was solely responsible for all aspects of preparation of the manuscript.
Funding The author has not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests PCC has research funding from Bayer Consumer Care; acts as an advisor/consultant to BASF AS, DSM, Cargill, Smartfish, Nutrileads, Bayer Consumer Care and Pfizer (now GSK) Consumer Healthcare; has received reimbursement for travel and/or speaking from Danone, Fresenius Kabi, Baxter Healthcare, B Braun Melsungen, Pfizer (now GSK) Consumer Healthcare, Abbott, Smartfish, Biogredia and the California Walnut Commission; and is President and member of the Board of Directors of the European Branch of the International Life Sciences Institute.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.